Introduction
In numerous high-income countries with
well-developed health care systems, regulatory bodies and other
stakeholders participate in efforts towards improving the system,
by optimizing access for patients requiring treatment, while also
avoiding over-treatment and the use of non-cost-effective
interventions (1). Adequate access
for minorities and/or underserved regions and populations requires
considerable health resources; such resources must therefore not be
misspent by doctors prescribing therapeutic measures with unproven
or doubtful benefit (2). Cancer
treatment provides an example of the difficulties that arise when
attempting to select the most appropriate treatment for a patient
(3). Ideally, every patient should
receive the optimum treatment and number of chemotherapy cycles or
radiation fractions during the phase of disease where such
treatment is meaningful to prolong life or maintain good functional
status. By contrast, in the phase of unavoidable progression of the
disease, the focus should be placed on easily tolerable palliative
measures and avoidance of hospitalization or active anticancer
therapy (4).
While palliative radiotherapy undoubtedly benefits
patients with cancer in various stages of the disease, in terms of
improving symptoms and potentially prolonging survival, its use
during the last month of life has recently been questioned
(5–7).
Clinicians are often excessively optimistic when estimating the
life expectancy of patients with advanced cancer, which may lead to
treatment decisions that eventually become a burden for patients
and caregivers, without improving the quality of life of the
patients during the terminal phase of the disease (8,9). A recent
audit of clinical practice in Nordland Hospital (Bodø, Nordland,
Norway) between 2007 and 2009 revealed that 9% of all palliative
radiotherapy was administered to patients during their last month
of life (10). In other words, the
physician's decision-making was appropriate in ~9 out of 10
patients, based on the assumption that patients with extremely
short survival time are unlikely to experience any significant
improvement in symptoms or quality of life (11). Due to the lack of patient-reported
outcomes, the clinical benefit of palliative radiotherapy during
the last month of life was not analyzed in the present study.
The appropriateness of using an arbitrary cut-off to
define short survival/end of life (such as the 1 month cut-off used
in previous publications) may also be debated, as a number of
patients may survive only few days longer than this period
(12). In order to avoid
administering palliative radiotherapy during the last month of
life, or to make informed decisions in cases where patients request
treatment, despite limited expectation of survival, a prediction
tool was previously developed and validated by Angelo et al
(10). This prediction model was
based on the following baseline parameters: i) Patients with lung
or bladder cancer of any histological type; ii) those with an
Eastern Cooperative Oncology Group performance status score of 3 or
4 (ECOG PS 3/4); iii) those presenting progressive disease outside
the target volume(s) of radiotherapy; iv) those exhibiting levels
of hemoglobin below the institutional limit of normal; v) those who
received opioid analgesics at the start of radiotherapy; and vi)
those who received steroids at the start of the radiotherapy
treatment.
Based on this model, 75% of patients with all six of
the aforementioned characteristics received radiotherapy during
their last month of life. This percentage reduced to 74% in the
validation dataset. However, the disadvantage of this model is that
it is solely applicable to patients with lung or bladder cancer,
and not to those suffering from other malignancies. Therefore, for
the present study, a larger database was created, containing
information from 2007 to 2011, which provides a higher likelihood
of identifying predictive factors that may lead to a more powerful
prediction model. The primary aim of the present study was to
analyze the prognostic factors of survival time for patients with
advanced cancer, and to evaluate in detail the clinical records of
patients who had received palliative radiotherapy during the last
month of life.
Patients and methods
Patients
The records of 873 consecutive patients with
metastatic or otherwise incurable cancer receiving palliative
radiotherapy at a single hospital were retrospectively reviewed.
Patients with hematological or primary brain malignancies were not
included in the analysis, due to the different biological behavior
of these types of cancer.
The patients included in the study had commenced
treatment during the period from June 20th, 2007 (the
opening date of the radiotherapy facility at Nordland Hospital) to
December 31st, 2011. Curative radiotherapy was not
administered at Nordland Hospital during this period. All of the
medical records of the patients, details of their treatment, and
date of mortality were available on the electronic patient record
(EPR) system of Nordland Hospital.
The survival status and date of mortality or last
follow-up of the these patients were obtained from their
corresponding EPRs during September 2014, resulting in ≥2.5 years
of follow-up for the surviving patients. The survival time was
measured from day 1 of palliative radiotherapy. If a patient was
subjected to a second course of radiotherapy, the follow-up was
censored at that time, and another record was created for the next
radiation treatment, as each course of radiotherapy carries its own
probability of being administered during the last month of life.
This methodology was used in our previous prediction model
(10).
Statistical analysis
IBM SPSS Statistical software, version 21 (IBM SPSS,
Armonk, NY, USA), was used to evaluate the association between
survival and potential prognostic factors, including blood
biochemical and hematological parameters, such as levels of
C-reactive protein (CRP), leukocytes, thrombocytes, hemoglobin,
creatinine, lactate dehydrogenase (LDH), albumin, alkaline
phosphatase and calcium. The institutional upper and lower limits
of the normal values were applied: CRP, <10 mg/l; leukocytes
3.5–11.0×109/l; thrombocytes, 130–400×109/l;
hemoglobin, 11.7–15.3 g/dl (females) and 13.4–17.0 g/dl (males);
creatinine, 45–90 µmol/l; LDH, <205 U/l; albumin, 36–48 g/l;
alkaline phosphatase, <105 U/l; and calcium 2.15–2.55 mmmol/l.
Only those blood test results that were obtained within 1 week
prior to the start of radiotherapy were included in the analysis.
The ECOG PS of the patients at the time of consultation regarding
radiotherapy, characteristics of the radiotherapy treatment
received, and other baseline factors are presented in Table I.
 | Table I.Baseline characteristics of patients
(n=873). |
Table I.
Baseline characteristics of patients
(n=873).
|
| Patients |
|---|
|
|
|
|---|
| Parameter | n | % |
|---|
| Gender |
|
|
|
Female | 310 | 35.5 |
| Male | 563 | 64.5 |
| Age, years |
|
|
|
<65 | 315 | 36.1 |
|
65–79 | 404 | 46.3 |
| ≥80 | 154 | 17.6 |
| ECOG PS |
|
|
| 0 | 97 | 11.1 |
| 1 | 262 | 30.0 |
| 2 | 295 | 33.8 |
| 3 | 189 | 21.6 |
| 4 | 30 | 3.4 |
| Type of cancer |
|
|
|
Prostate | 222 | 25.4 |
|
Breast | 108 | 12.4 |
| Non-small
cell lung | 194 | 22.2 |
| Small
cell lung | 48 | 5.5 |
|
Colorectal | 54 | 6.2 |
|
Pancreatic | 11 | 1.3 |
|
Bladder | 48 | 5.5 |
|
Kidney | 61 | 7.0 |
| Malignant
melanoma | 23 | 2.6 |
| Other
primary tumor | 104 | 11.9 |
| Metastases |
|
|
|
Bonea | 572 | 65.5 |
|
Braina | 159 | 18.2 |
|
Livera | 170 | 19.5 |
|
Lunga | 214 | 24.5 |
| Adrenal
glanda | 90 | 10.3 |
| Pleural
and/or effusion | 94 | 10.8 |
| History of previous
cancer diagnosis | 89 | 10.2 |
| Previous systemic
therapy | 443 | 50.7 |
| RT
characteristics |
|
|
|
Progressive disease outside RT
volume(s) | 447 | 51.2 |
| Opioid
analgesics used at the start of RT | 458 | 52.5 |
| Steroids
used at the start of RT | 435 | 49.8 |
|
Incomplete RT | 46 | 5.3 |
Actuarial survival curves were generated using the
Kaplan-Meier method and compared by log-rank test (univariate
analysis performed for all baseline factors). For the multivariate
analysis of survival from the start of the radiotherapy treatment,
a Cox regression analysis was used (forward stepwise method). All
factors with a significant P-value identified by the univariate
log-rank test were considered in the subsequent multivariate
regression analysis. The associations between the different
variables of interest were assessed with the χ2 and
Fisher's exact probability tests. P<0.05 was considered to
indicate a statistically significant difference in two-sided
tests.
Results
Baseline characteristics and risk in
metastatic vs. non-metastatic disease
The median age of the patients included in the
analysis was 68 years (range, 23–97 years). The median time from
diagnosis of cancer to receipt of palliative radiotherapy was 24
months (range, 0–386 months). In patients with distant metastases,
the median time from the detection of the first metastasis to the
administration of palliative radiotherapy was 6 months (range,
0–149 months). Further details are presented in Table I. The majority of patients received
radiotherapy for painful bone (54%) or brain metastases (15%), or
for thoracic symptoms resulting from lung cancer (12%). The most
common fractionation regimen was 3 Gy × 10 (43%), followed by 5–7
fractions of 4 Gy (22%). Stereotactic radiotherapy was not
available. Depending on the anatomical site of the tumor and the
total dose received, 2- or 3-dimensional treatment planning was
used. As indicated in Table II,
fewer patients with non-metastatic cancer received radiotherapy
during their last month of life compared with patients with
metastatic cancer (7 vs. 13%, respectively; P=0.12).
 | Table II.Rate of palliative radiotherapy during
the last month of life according to the type of cancer. |
Table II.
Rate of palliative radiotherapy during
the last month of life according to the type of cancer.
| Primary cancer type
(total cases, n) | Metastatic, n | Non-metastatic,
n |
|---|
| All combined
(873) | 764 (87.5%) | 109 (12.5%) |
| Prostate (222) | 217 | 5 |
| Breast (108) | 104 | 4 |
| Thyroid (7) | 7 | 0 |
| Non-small cell lung
(194) | 138 | 56 |
| Small cell lung
(48) | 41 | 7 |
| Colorectal
(54) | 45 | 9 |
| Small bowel
(2) | 2 | 0 |
| Pancreatic
(11) | 9 | 2 |
| Gastric (4) | 4 | 0 |
| Esophageal
(20) | 10 | 10 |
| Bladder (48) | 35 | 13 |
| Kidney (61) | 61 | 0 |
| Head and neck
(15) | 12 | 3 |
| Sarcoma (5) | 5 | 0 |
| Hepatocellular
(6) | 6 | 0 |
| Gynecological
(11) | 11 | 0 |
| Malignant melanoma
(23) | 23 | 0 |
| Squamous cell skin
(2) | 2 | 0 |
| Unknown primary
tumor (32) | 32 | 0 |
Prognostic factors for survival
The median survival time for the patients included
in the analysis was calculated to be 6 months, and the 1- and
2-year survival rates were 35 and 27%, respectively. Multivariate
analysis identified a number of significant prognostic factors,
including ECOG PS; presence of brain, liver or bone metastases;
disease progression outside the irradiated field, despite receiving
systemic therapy; unavailability of systemic treatment due to age
or comorbidity; history of >1 type of cancer diagnosis; use of
opioid analgesics; and levels of CRP >30 mg/l (P=0.0001). Other
factors also contributed to the regression model, including
leukocytosis (based on the institutional upper limit of normal,
11.0×109 cells/l; P=0.006), use of steroids (P=0.007),
and pleural metastases and/or effusion (P=0.01). Notably, the
number of metastatic sites and the age of the patient were not
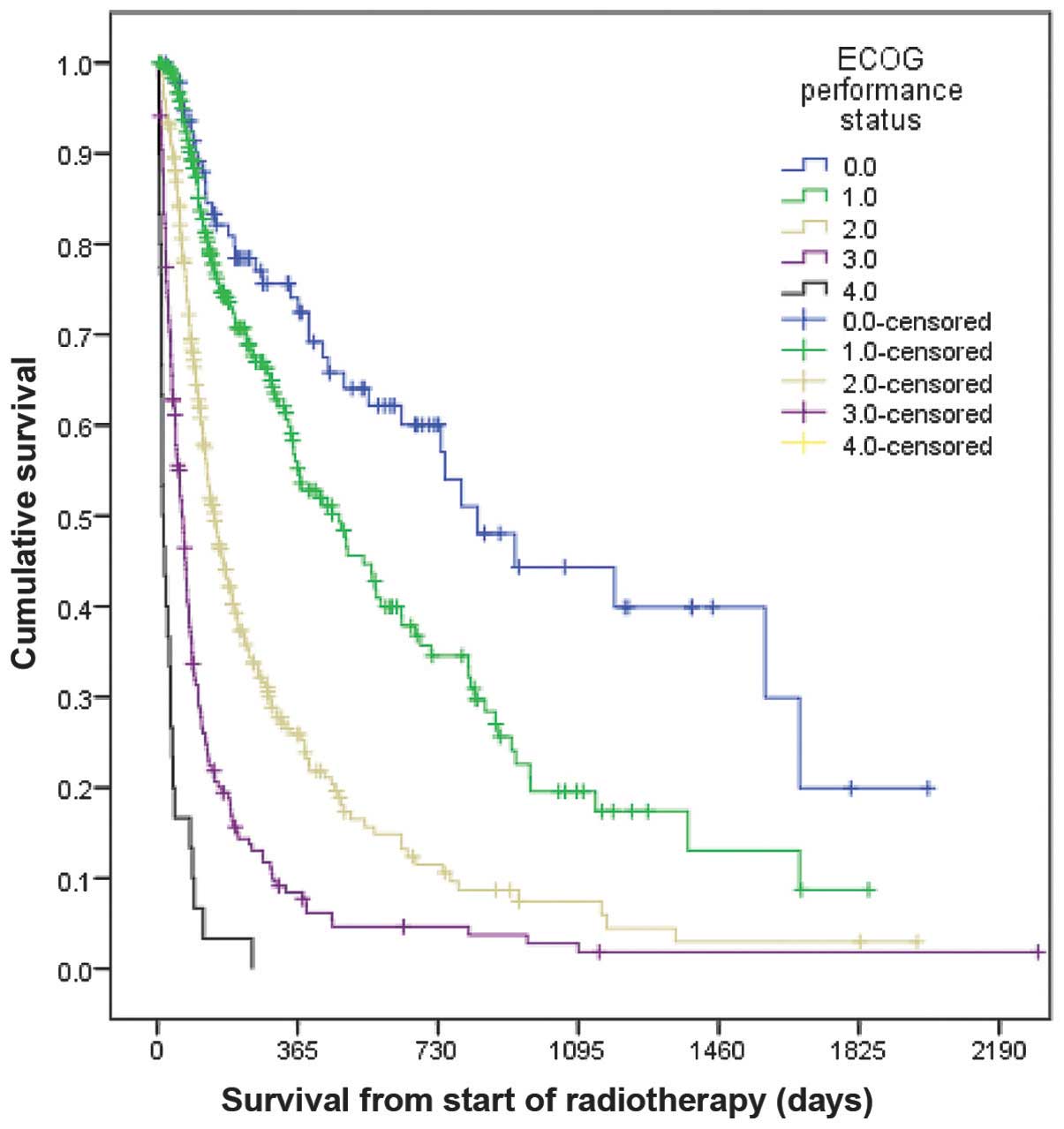
observed to be significant. The most important risk factor for
predicting short survival time was ECOG PS 4 (median survival time,
14 days; 1-year survival rate, 0%), followed by ECOG PS 3 (median
survival time, 64 days; 1-year survival rate, 8%), as represented
in Fig. 1 (P=0.0001). All patients
with ECOG PS 4 were hospitalized when receiving radiotherapy. Of
the 30 patients with ECOG PS 4, 22 received radiotherapy during the
last month of life (73%). This rate was lower (30%) for patients
with ECOG PS 3.
Similarly to the previously reported method
(10), a scoring system was created
that included all identified significant independent prognostic
factors, weighted according to their influence on survival
(Tables III and IV). Due to their significantly different
hazard ratios for survival, ECOG PS 3 was assigned 3 points, while
ECOG PS 4 was assigned 5 points. The other parameters were assessed
in a present/absent format. Patients with metastatic cancer and
points sum ≥21 were at high risk of receiving radiotherapy during
their last month of life (77%). Comparable results were observed in
patients without metastases and points sum ≥12 (75%). However, this
score did not clearly outperform our previous prediction model
(10) (77/75 vs. 75%, respectively).
Based on this previous model, which requires a diagnosis of lung or
bladder cancer, among other selection criteria, avoiding
radiotherapy would have been recommended in 15% of patients with
lung or bladder cancer, as this proportion of patients fulfilled
all of the criteria. In the present study, 290 patients presented
lung or bladder cancer, and therefore 43.5 patients (15%) should
not be considered for treatment, if adhering to the old model. By
contrast, the score calculated in the present study would lead to a
recommendation against treatment in only 34 patients (4.6%) with
lung or bladder cancer, which includes patients with metastatic
disease and point sum of ≥21 and patients with non-metastatic
disease and point sum of ≥12. Considering that 105 patients
received radiotherapy during the last month of life, the majority
of patients would still proceed to treatment, according to this
model. These findings emphasize the limitations of the scoring
method in the present study.
 | Table III.Score predicting radiotherapy during
the last month of life in 641 patients with metastatic cancer. |
Table III.
Score predicting radiotherapy during
the last month of life in 641 patients with metastatic cancer.
| A, Characteristics
of patients |
|
|
|
|---|
|
|---|
| Parameter | Multivariate
P-value | Hazard ratio | Points |
|---|
| ECOG PS 3/4 | 0.0001 | 2.9/5.2 | 3/5 |
| Brain
metastases | 0.0001 | 2.8 | 3 |
| Liver
metastases | 0.0001 | 3.1 | 3 |
| Bone
metastases | 0.0001 | 2.7 | 3 |
| Progressive
diseasea | 0.0001 | 3.2 | 3 |
| >1 diagnosis of
cancer | 0.0001 | 2.7 | 3 |
| Opioid
analgesics | 0.0001 | 3.0 | 3 |
| CRP >30
mg/l | 0.0001 | 3.1 | 3 |
| Steroids | 0.0070 | 2.2 | 2 |
| Leukocytosis | 0.0060 | 2.0 | 2 |
| Pleural metastases
and/or effusion | 0.0100 | 1.4 | 1 |
|
| B, Points
score |
|
|
|
|
|
| Patients irradiated
in last month of life |
|
|
|
|
| Points sum | Patients
irradiated, n | n | Percentage |
|
| 21 | 6 | 5 | 83 |
| 22 | 12 | 10 | 83 |
| >22 | 12 | 8 | 67 |
| Total | 30 | 23 | 77 |
 | Table IV.Score predicting radiotherapy during
the last month of life in 98 patients with non-metastatic
cancer. |
Table IV.
Score predicting radiotherapy during
the last month of life in 98 patients with non-metastatic
cancer.
| A, Characteristics
of patients |
|
|
|
|---|
|
|---|
| Parameter | Multivariate
P-value | Hazard ratio | Points |
|---|
| ECOG PS 3/4 | 0.0001 | 3.1/4.9 | 3/5 |
| >1 diagnosis of
cancer | 0.0001 | 2.9 | 3 |
| Opioid
analgesics | 0.0001 | 3.2 | 3 |
| CRP >30
mg/l | 0.0001 | 2.8 | 3 |
| Steroids | 0.0070 | 2.0 | 2 |
| Leukocytosis | 0.0060 | 1.8 | 2 |
| Pleural
effusion | 0.0100 | 1.3 | 1 |
|
| B, Points
score |
|
|
|
|
|
| Patients irradiated
in last month of life |
|
|
|
|
| Points sum | Patients
irradiated, n | n | Percentage |
|
| 11 | 5 | 1 | 20 |
| 12 | 1 | 1 | 100 |
| 13 | 1 | 1 | 100 |
| >13 | 2 | 1 | 50 |
| Total (>11) | 4 | 3 | 75 |
Therefore, a pragmatic approach would be to extend
the score of the previous model, which includes patients with lung
or bladder cancer, and develop further disease-specific scores for
the remaining patients. This possibility was explored, and a
disease-specific score for patients with prostate or breast cancer
was considered, since these groups contained >100 cases each,
and presented comparable survival outcomes. The median survival
times were observed to be 12.3 and 13.8 months for patients with
prostate and breast cancer, respectively (P=0.57). None of the
other groups exhibited comparable survival figures (the maximum
survival time was 9.7 months in the case of patients with kidney
cancer). In the combined prostate/breast cancer dataset, there were
21 instances of palliative radiotherapy during the last month of
life, all among patients with metastatic disease (Table II). Multivariate analysis of
prognostic factors revealed as significant the following
parameters: ECOG PS (P=0.0001), elevated levels of serum LDH
(P=0.0001), and presence of liver (P=0.0001), adrenal gland
(P=0.01) and pleural metastases and/or effusion (P=0.036). The
number of metastatic sites and the age of the patient were not
observed to be significant. A score was assigned based on all of
the above significant factors (Table
V). However, due to the insufficient number of events, it was
not possible to obtain firm conclusions from the analysis.
Furthermore, despite the possibility of larger studies confirming
that patients with points sum ≥10 present extremely short survival
times and are poor candidates for radiotherapy, according to this
model, treatment would be withheld in 1% of patients with prostate
or breast cancer; therefore, the majority of patients with poor
prognosis would still proceed to radiotherapy.
 | Table V.Score predicting radiotherapy during
the last month of life in patients with breast or prostate
cancer. |
Table V.
Score predicting radiotherapy during
the last month of life in patients with breast or prostate
cancer.
| A, Characteristics
of patients |
|
|
|
|---|
|
|---|
| Parameter | Multivariate
P-value | Hazard ratio | Points |
|---|
| ECOG PS 3/4 | 0.0001 | 2.8/5.1 | 3/5 |
| Serum lactate
dehydrogenase | 0.0001 | 3.3 | 3 |
| Liver
metastases | 0.0001 | 2.9 | 3 |
| Adrenal gland
metastases | 0.0100 | 1.3 | 1 |
| Pleural metastases
and/or effusion | 0.0036 | 1.4 | 1 |
|
| B, Points
score |
|
|
|
|
|
| Patients irradiated
in last month of life |
|
|
|
|
| Points sum | Patients
irradiated, n | n | Percentage |
|
| 7 | 5 | 2 | 40 |
| 8 | 4 | 1 | 25 |
| 9 | 4 | 0 | 0 |
| >10 | 2 | 2 | 100 |
Frequency of unexpected
mortalities
Details of the clinical course were reviewed for the
105 patients included in the present study who had received
palliative radiotherapy during the last month of life. The analysis
identified a number of patients who had died unexpectedly, as
follows: i) A 57-year old male patient with ECOG PS 2, presenting
hepatocellular cancer with painful bone metastases and abdominal
progression following systemic therapy with sorafenib, who
succumbed to hepatic and renal failure due to erroneous drainage of
excessively large volumes of ascites; ii) a 76-year old male
patient with ECOG PS 2, affected by prostate cancer and painful
bone metastases, who succumbed to small bowel ischemia; iii) a
67-year old female patient with ECOG PS 2, exhibiting non-small
cell lung cancer with adrenal gland metastases and dyspnea from
thoracic progression, who was receiving second-line systemic
therapy with erlotinib, and died from a pneumothorax as a result of
pleurodesis; iv) a 38-year old female patient with ECOG PS 2,
presenting human epidermal growth factor receptor 2-positive breast
cancer and ulcerated skin metastases, who was receiving third-line
systemic therapy, and succumbed to right ventricular failure,
possibly due to extensive lymphangitis carcinomatosa; v) a 68-year
old male patient with ECOG PS 2, non-small cell lung cancer and
pulmonary metastasis, who was undergoing sequential
chemoradiotherapy, succumbed to sudden cardiac death; vi) a 51-year
old female patient with ECOG PS 2 and supposedly limited pelvic
relapse of small cell bladder cancer post-surgery, who died from
rapid progression of novel distant metastases that resulted in
hypercalcemia; and vii) a 72-year old male patient with ECOG PS 2,
prostate cancer and painful bone metastases, who succumbed to
myocardial infarction. All remaining cases presented a combination
of adverse prognostic features, including ECOG PS 3/4, extensive
metastatic disease, documented disease progression outside of the
irradiated field(s), or lack of further systemic treatment
options.
Discussion
The most important finding from the present
comprehensive retrospective chart review is that numerous instances
of palliative radiotherapy during the last month of life appear to
be preventable if physicians are aware of the prognosis of the
patient. Unexpected events, such as unforeseeable cardiac events or
iatrogenic complications were unusual, whereas adverse prognostic
factors indicating limited survival time were frequent. However, no
simple prognostic model is capable of predicting mortality within
30 days with high accuracy (13). As
discussed in the present study, numerous prognostic factors
identified as significant in univariate analysis remained
significant in the multivariate regression model. In the prediction
model, complex prediction scores displayed some potential; however,
the majority of patients who received radiotherapy during the last
month of life were not be identified a priori. It is
important to highlight the clinical dilemma of decision-making in
favor of or against palliative radiotherapy in patients with
limited survival expectations (14).
Considering that short-course regimens with no or low-grade side
effects exist, which often improve symptoms such as pain, dyspnea
and hemoptysis, clinicians are wary of withholding a meaningful
therapeutic measure for patients with terminal cancer (15–18).
Therefore, prediction tools must not predict short survival times
in patients who survive long enough to experience a reduced burden
of symptoms, and must also identify the majority of patients will
succumb to the disease too early to benefit from the treatment.
According to the results of the present study,
disease-specific models may possess promising potential. In
addition to patients with lung, bladder, prostate and breast
cancer, relatively high rates of palliative radiotherapy during the
last month of life were also observed in patients with metastatic
kidney, colorectal and pancreatic cancer (Table II). However, further studies of
disease-specific models will require larger databases.
Previous studies have reported data in agreement
with the findings presented in the current study. In this regard,
Anshushaug et al (19) also
observed that ECOG PS 3/4 was strongly associated with palliative
radiotherapy during the last month of life. The impact of
performance status as a prognostic factor in patients with brain
metastases is well known (20). In a
Canadian study, palliative patients with cancer presenting ECOG PS
4 or 3 exhibited a median survival time of 25.5 or 55.0 days,
respectively (21). These figures are
in agreement with the results obtained in the present study, in
which ECOG PS 4 was observed to perform almost as well as the
complex scores in the prediction model. In the current study, 73%
of patients with ECOG PS 4 had received treatment during the last
month of life. However, the majority of patients who received
radiotherapy during the last month of life presented ECOG PS 3, and
a number of patients presented ECOG PS 2. As described in Fig. 1, certain patients with ECOG PS 3 may
experience beneficial effects from radiotherapy, including
prolonged survival. Consequently, they should be regarded possible
candidates for therapy. However, clinicians should assess these
patients comprehensively and thoroughly, and make individual
decisions accordingly. A clear definition and communication to the
patient of the goals of the treatment is mandatory, in order to
avoid inaccurate beliefs about the effects of the treatment
(22). Other factors associated with
the treatment, including the toxic side-effects of the therapy,
time for the patient to travel to the hospital, cost of the therapy
and absence of family members to provide support for the patient,
must also be considered (23).
Furthermore, if radiotherapy is to be prescribed, it is important
to select easily tolerable regimens and avoid lengthy treatment
courses (24). The policy of Nordland
Hospital is to assess the above mentioned scores for patients with
lung/bladder and breast/prostate cancer. In this sense, patients at
high-risk, and all those patients with other primary tumors and
ECOG PS 3/4, receive information about the assessment and
participate in the decision-making process for or against
radiotherapy.
The limitations of the present study include
incomplete baseline information in certain cases, particularly
regarding the results of blood tests, and the limited number of
events in the disease-specific analyses. In addition, not all
patients included in the study underwent complete restaging during
the month prior to radiotherapy and, therefore, the metastatic
burden may have been greater than suspected in certain cases.
Furthermore, data on the palliative efficacy of radiotherapy was
not collected. Regardless, the present study provides important
stimuli for further research towards the development of
decision-making tools that may reduce subjectivity in the daily
clinical assessment of the suitability of palliative radiotherapy
for patients with terminal cancer. Therefore, future studies which
assess the clinical benefit of palliative radiotherapy in patients
with terminal cancer and stratify patients by primary tumor type
are required. The additional inclusion of quality of life
parameters and symptom severity into prognostic models may also be
useful.
References
|
1
|
Scalo JF and Rascati KL: Trends and issues
in oncology costs. Expert Rev Pharmacoecon Outcomes Res. 14:35–44.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enewold L, Horner MJ, Shriver CD and Zhu
K: Socioeconomic disparities in colorectal cancer mortality in the
United States, 1990–2007. J Community Health. 39:760–766. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dienstmann R, Salazar R and Tabernero J:
Personalizing colon cancer adjuvant therapy: Selecting optimal
treatments for individual patients. J Clin Oncol. 33:1787–1796.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Randén M, Helde-Frankling M, Runesdotter S
and Strang P: Treatment decisions and discontinuation of palliative
chemotherapy near the end-of-life, in relation to socioeconomic
variables. Acta Oncol. 52:1062–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guadagnolo BA, Liao KP, Elting L, Giordano
S, Buchholz TA and Shih YC: Use of radiation therapy in the last 30
days of life among a large population-based cohort of elderly
patients in the United States. J Clin Oncol. 31:80–87. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy JD, Nelson LM, Chang DT, Mell LK
and Le QT: Patterns of care in palliative radiotherapy: A
population-based study. J Oncol Pract. 9:e220–e227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapadia NS, Mamet R, Zornosa C, Niland JC,
D'Amico TA and Hayman JA: Radiation therapy at the end of life in
patients with incurable nonsmall cell lung cancer. Cancer.
118:4339–4345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gripp S, Mjartan S, Boelke E and Willers
R: Palliative radiotherapy tailored to life expectancy in end-stage
cancer patients: Reality or myth? Cancer. 116:3251–3256. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartsell WF, Desilvio M, Bruner DW,
Scarantino C, Ivker R, Roach M III, Suh J, Demas WF, Movsas B,
Petersen IA and Konski AA: Can physicians accurately predict
survival time in patients with metastatic cancer? Analysis of RTOG
97–14. J Palliat Med. 11:723–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Angelo K, Norum J, Dalhaug A, Pawinski A,
Aandahl G, Haukland E, Engljähringer K and Nieder C: Development
and validation of a model predicting short survival (death within
30 days) after palliative radiotherapy. Anticancer Res. 34:877–885.
2014.PubMed/NCBI
|
|
11
|
Langley RE, Stephens RJ, Nankivell M, Pugh
C, Moore B, Navani N, Wilson P, Faivre-Finn C, Barton R, Parmar MK
and Mulvenna PM: QUARTZ Investigators: Interim data from the
Medical Research Council QUARTZ Trial: Does whole brain
radiotherapy affect the survival and quality of life of patients
with brain metastases from non-small cell lung cancer? Clin Oncol
(R Coll Radiol). 25:e23–e30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nieder C, Andratschke N, Angelo K,
Haukland E and Grosu AL: Development of a score predicting survival
after palliative reirradiation. J Oncol. 2014:1282402014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramchandran KJ, Shega JW, Von Roenn J,
Schumacher M, Szmuilowicz E, Rademaker A, Weitner BB, Loftus PD,
Chu IM and Weitzman S: A predictive model to identify hospitalized
cancer patients at risk for 30-day mortality based on admission
criteria via the electronic medical record. Cancer. 119:2074–2080.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieder C, Norum J, Dalhaug A, Aandahl G
and Pawinski A: Radiotherapy versus best supportive care in
patients with brain metastases and adverse prognostic factors. Clin
Exp Metastasis. 30:723–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cameron MG, Kersten C, Vistad I, Fosså S
and Guren MG: Palliative pelvic radiotherapy of symptomatic
incurable rectal cancer - a systematic review. Acta Oncol.
53:164–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laugsand TS, Kaasa S, Romundstad P,
Johannesen TB and Lund JÅ: Radiotherapy for bone metastases:
Practice in Norway 1997–2007. A national registry-based study. Acta
Oncol. 52:1129–1136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Oorschot B, Schuler M, Simon A,
Schleicher U and Geinitz H: Patterns of care and course of symptoms
in palliative radiotherapy: A multicenter pilot study analysis.
Strahlenther Onkol. 187:461–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Oorschot B, Rades D, Schulze W,
Beckmann G and Feyer P: Palliative radiotherapy - new approaches.
Semin Oncol. 38:443–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anshushaug M, Gynnild MA, Kaasa S,
Kvikstad A and Grønberg BH: Characterization of patients receiving
palliative chemo- and radiotherapy during end of life at a regional
cancer center in Norway. Acta Oncol. 54:395–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leth T, von Oettingen G, Lassen-Ramshad
YA, Lukacova S and Høyer M: Survival and prognostic factors in
patients treated with stereotactic radiotherapy for brain
metastases. Acta Oncol. 54:107–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang RW, Caraiscos VB, Swami N, Banerjee
S, Mak E, Kaya E, Rodin G, Bryson J, Ridley JZ, Le LW and
Zimmermann C: Simple prognostic model for patients with advanced
cancer based on performance status. J Oncol Pract. 10:e335–e341.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen AB, Cronin A, Weeks JC, Chrischilles
EA, Malin J, Hayman JA and Schrag D: Expectations about the
effectiveness of radiation therapy among patients with incurable
lung cancer. J Clin Oncol. 31:2730–2735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma S, Hertan L and Jones J: Palliative
radiotherapy: Current status and future directions. Semin Oncol.
41:751–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lutz ST, Jones J and Chow E: Role of
radiation therapy in palliative care of the patient with cancer. J
Clin Oncol. 32:2913–2919. 2014. View Article : Google Scholar : PubMed/NCBI
|