Introduction
Glioma represents the most prevalent and lethal
primary brain tumor (1). A number of
glioma cases have demonstrated low responses to conventional
cytotoxic chemotherapy. Despite great efforts to improve
therapeutics, the clinical outcome of glioma remains poor (1). Therefore, there is an urgent need to
develop novel treatment to improve the prognosis of glioma
(2).
Dysregulated signaling pathways have been considered
to be involved in cancer progression; the Raf/mitogen-activated and
extracellular signal-regulated kinase kinase (MEK)/extracellular
signal-regulated kinase (ERK) cascade and the phosphatidylinositol
3-kinase (PI3K)/Akt cascade are commonly activated pathways in a
number of types of cancer (3).
The Raf/MEK/ERK pathway is one of the most common
oncogenic pathways and is critical in driving cell proliferation,
survival and preventing apoptosis. Upon activation by growth
factors, serum, polypeptide hormones, neurotransmitters or
chemokines, activated ERK phosphorylates and regulates multiple
cytoplasmic signaling proteins and transcription factors (4). These events result in gene expression
changes and alterations in cell survival, division and metabolism.
A previous study demonstrated that dysregulation of this pathway is
involved in the oncogenesis of various types of human cancer
(5). Therefore, inhibition of the
Raf/MEK/ERK pathway is a promising strategy for cancer therapy
(6).
The PI3K/AKT pathway is another crucial signaling
pathway for cancer development (7).
Growth factors and cytokines activate the lipid kinase PI3K and
then generate phosphatidylinositol 3,4,5-triphosphate (PIP3), which
activates the recruitment of protein kinase AKT to cell membrane.
Activated AKT phosphorylates Ser/Thr residues in proteins that are
involved in cell survival, proliferation, and metabolic pathways.
Abberrant AKT activation has been documented in a number of types
of human cancer (8). Therefore,
inhibition of the PI3K/AKT pathway is also a promising strategy for
cancer therapy (9).
A previous study indicated that the Raf/MEK/ERK and
PI3K/AKT signaling pathways cooperatively link with each other to
enhance the proliferation and apoptotic resistance capacity in
cancer cells (10). Both signaling
pathways are activated by growth factor receptors and their
complicated crosstalk at multiple points greatly increases the
therapeutic effects of single signaling inhibition in cancer cells
(11). In addition, these two
pathways can exert complementary and redundant functions when only
one single pathway is inhibited (12). Therefore, more clinical benefit may be
obtained by simultaneously targeting both cascades (13).
Dihydroartemisinin (DHA) is a semi-synthetic
derivative of the anti-malarial drug artemisinin. Previous studies
have demonstrated that DHA may exert its anti-proliferation and
apoptosis-inducing effects on a number of types of cancer cells,
including leukemia, prostate cancer, ovarian cancer and colorectal
cancer cells (14–17). In addition, it has been reported that
DHA induces apoptosis in C6 glioma cells by increasing the
generation of reactive oxygen species (ROS) and inhibiting
activation of hypoxia inducible factor-1α (HIF-1α) (18). However, the underlying molecular
mechanisms by which DHA exerts its anti-glioma effects remain
unclear. In the present study, the effects of DHA treatment of
glioma cells and the potential molecular mechanisms were
investigated, particularly focusing on the impact on Raf/MEK/ERK
and PI3K/AKT pathways.
Materials and methods
Cells and reagents
The BT325 cell line was obtained from Beijing
Neurosurgical Institute Collection (Beijing, China) and the C6 cell
line was purchased from American Type Culture Collection (Manassas,
VA, USA). All cells were cultured at 37°C in Dulbecco's modified
Eagle medium (DMEM) (Gibco Life Technologies, Carlsbad, CA, USA)
containing 10% fetal bovine serum (FBS) (Gibco), and 100 U/ml
penicillin-streptomycin (Gibco). Dihydroartemisinin (DHA) was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in
dimethylsulfoxide (DMSO) with cell medium resulting in a final DMSO
concentration of 1%.
Cell viability assay
Cell viability was measured by
3-[4,5-dimethylthiazol-2-thiazolyl]-2,5-diphenyl-tetrazolium
bromide (MTT) assay. Logarithmically growing glioma cells were
seeded in 96-well culture clusters (Costar, Cambridge, MA, USA) at
a density of 5,000–6,000 cells/well with culture medium and
incubated for 24 h. On the following day, cells were treated with
desired concentrations of DHA. Four hours before the desired time
points, 10 µl of 10 mg/ml MTT was added, and incubated for 4 h, the
medium was removed and 200 µl DMSO was added to each well. The
optical density (OD) value at 570 nm was measured using MRX II
absorbance reader (DYNEX Technologies, Chantilly, VA, USA). The
cell viability was expressed as the percentage of absorbance in
cells with DHA treatment versus the control group. The experiment
was replicated in triplicate. Results were expressed as the mean ±
standard deviation (SD) values.
Apoptosis analysis by flow
cytometry
Apoptotic cells were measured with Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (BD
Biosciences, Sparks, MD, USA). Briefly, cells were cultured in
6-well plates at 3×105 cells per well. After
DHA-treatment for 10 h, a cell suspension was prepared by
trypsinization, and was centrifuged at 800 × g for 5 min at 4°C,
and then the cells were resuspended in 500 µl of binding buffer.
The cells were incubated with 10 µl Annexin V-FITC solution and 5
µl PI at 37°C for 30 min away from the light. FACs analysis was
performed by flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA). For each sample, 10,000 cells were analyzed. All of the
experiments were performed in triplicate.
Western blot analysis
Cells were plated in tissue culture dishes overnight
and treated with DHA at the given concentrations for 24 h.
Following harvest, adherent cells were washed with cold PBS and
resuspended in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 2
mM ethylenediaminetetra-acetic acid (EDTA), 1% NP-40) containing
protease inhibitor cocktail (Amresco Inc., Framingham, MA, USA).
Protein levels in the extracts were quantified using BCA assay
(Pierce Biotechnology, Inc., Rockford, IL, USA). Equal amounts of
total protein extracts were resolved by 10% standard sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a polyvinylidene fluoride (PVDF) membrane (0.45
mm; EMD Millipore, Billerica, MA, USA). Membranes were blocked with
5% fat-free dry milk/Tris-buffered saline (TBS)-Tween 20 (TBST;
cat. no. 9997, Cell Signaling Technology Inc., Danvers, MA, USA) at
room temperature for 1 h, then incubated with the following
anti-human primary antibodies to MEK (monoclonal rabbit; 1:1,000;
cat. no. 8727), ERK (monoclonal rabbit; 1:1,000; cat. no. 4348),
AKT (monoclonal mouse; 1:1,000; cat. no. 12694), phospho-MEK
(monoclonal rabbit; 1:1,000; cat. no. 2338), phospho-ERK
(monoclonal rabbit; 1:1,000; cat. no. 4377), phospho-AKT
(polyclonal rabbit; 1:1,000; cat. no. 9272), Bcl-2 (monoclonal
mouse; 1:500; cat. no. 15071), Mcl-1 (monoclonal rabbit; 1:500;
cat. no. 5453), Bcl-xL (monoclonal rabbit; 1:500; cat. no. 13835),
Bax (monoclonal rabbit; 1:500; cat. no. 5023) and GAPDH (monoclonal
rabbit; 1:2,000; cat. no. 2118; Cell Signaling Technology Inc.)
overnight at 4°C. The membranes were then washed three times with
TBST for 5 min at room temperature. Secondary horseradish
peroxidase-linked mouse anti-sheep (1:1,000; cat. no. 7076) or
rabbit anti-sheep IgG (1:1,000; cat. no. 5127) antibodies (Cell
Signaling Technology Inc.) were incubated for 1 h at room
temperature. The membranes were then washed three times with TBST
for 5 min at room temperature, and the immunoblots were visualized
by enhanced chemiluminescence reagent (GE Healthcare Life Sciences,
Logan, UT, USA).
Statistical analysis
Data are expressed as the mean ± SD, and evaluated
for significant differences using one-way analysis of variance
(ANOVA) via SPSS software, version 13.0 (SPSS Inc., Chicago, IL,
USA). A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
DHA inhibited glioma cell
proliferation
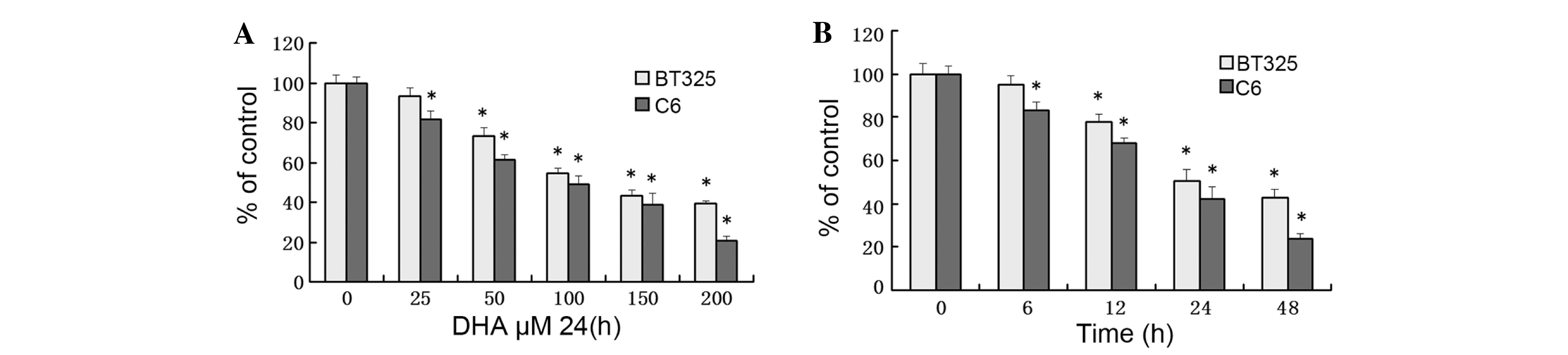
The effects of DHA on inhibition of proliferation of
BT325 and C6 cells at dosages between 25–200 µM, were examined. MTT
assay demonstrated that the effects of DHA on the viability of the
cell lines were dose- and time-dependent (Fig. 1). Treatment of BT325 and C6 cells with
100 µM DHA for 48 h caused 62.5±3.6% and 77.3±6.4% reduction in
cell viability, respectively (Fig.
1B; P<0.05).
DHA results in apoptotic death in
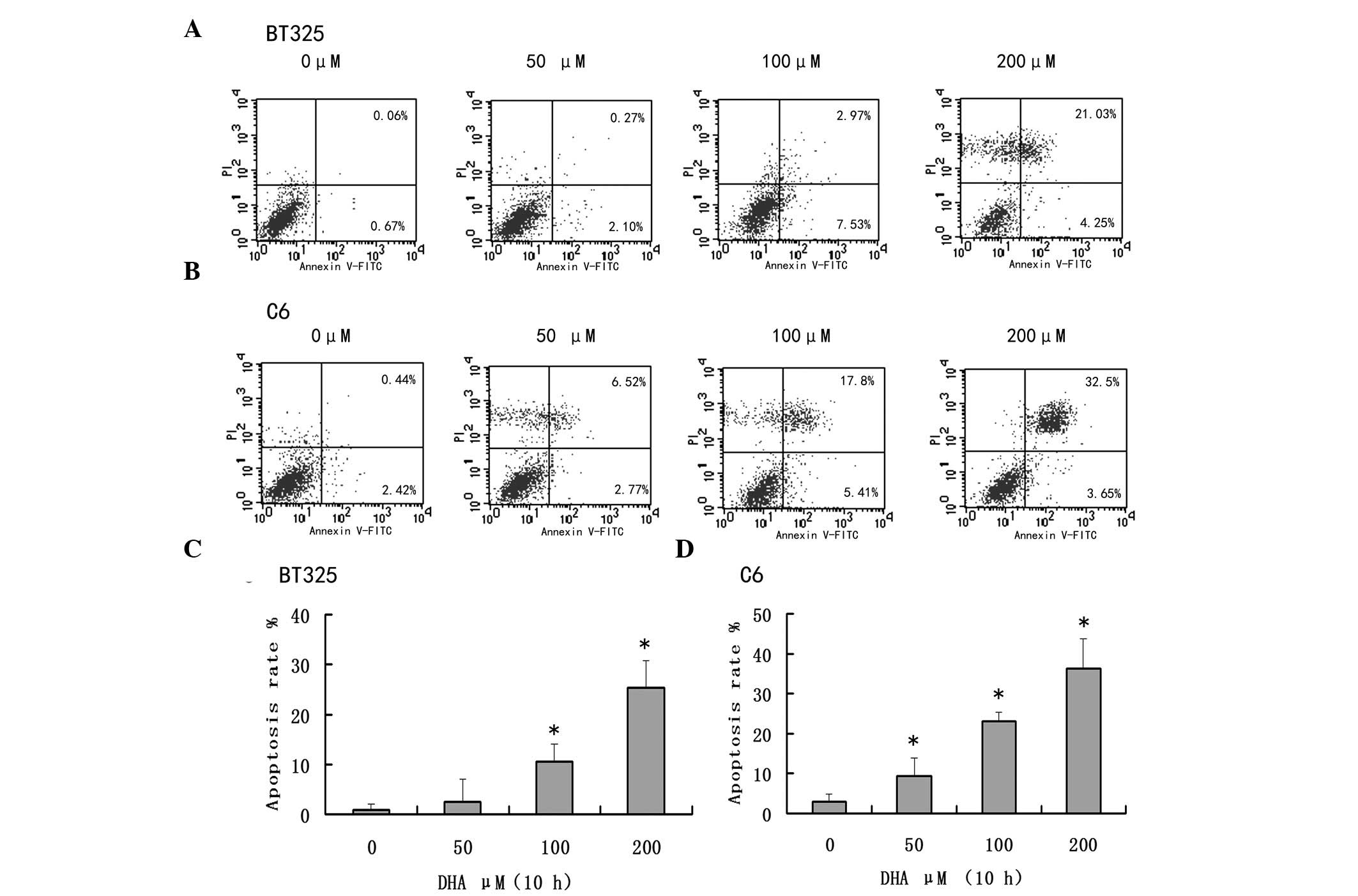
glioma cells
The proapoptotic effects of DHA were examined in
BT325 and C6 cells. As presented in Fig.
2, DHA promoted apoptosis in both cell lines in a
dose-dependent manner (P<0.05; Fig 2C
and D).
DHA inhibits Raf/MEK/ERK signaling
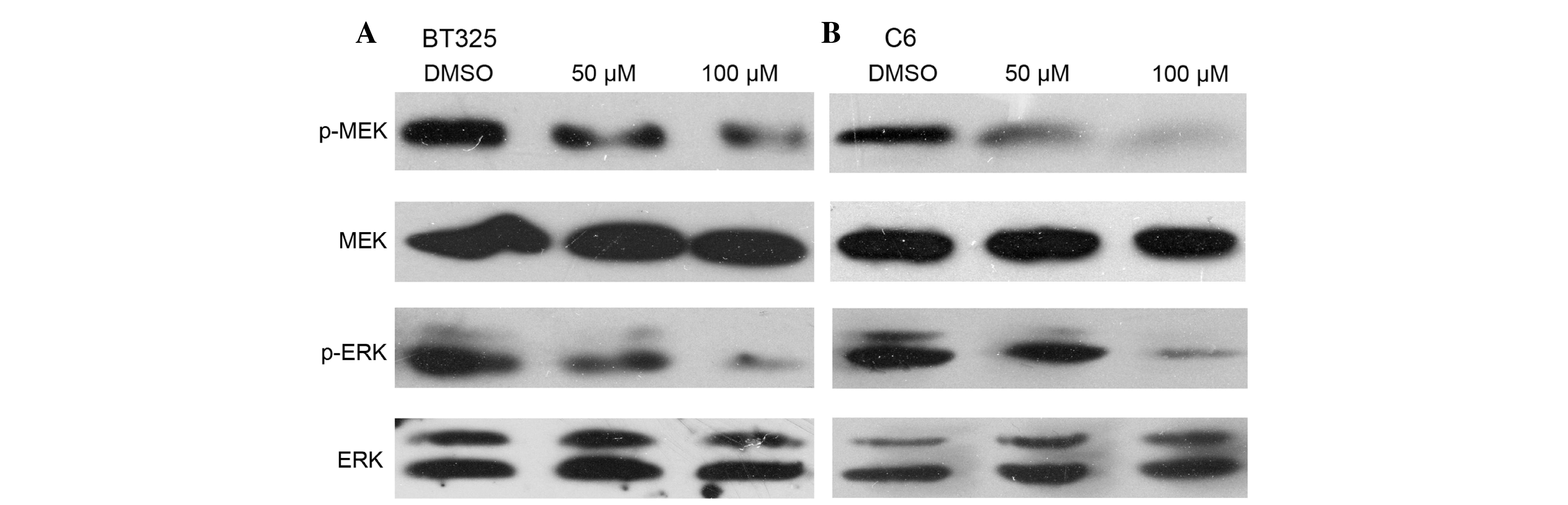
pathway in glioma cells
A previous study reported that DHA inhibits
Raf/MEK/ERK pathway in a number of types of cancer (14). Therefore the impact on the
phosphorylation of these signaling molecules was examined in glioma
cells. Western blot analysis demonstrated that after 24 h of DHA
exposure, the expression levels of Raf/MEK/ERK signaling pathway
members were reduced in both cell lines in a dose-dependent manner
(Fig. 3).
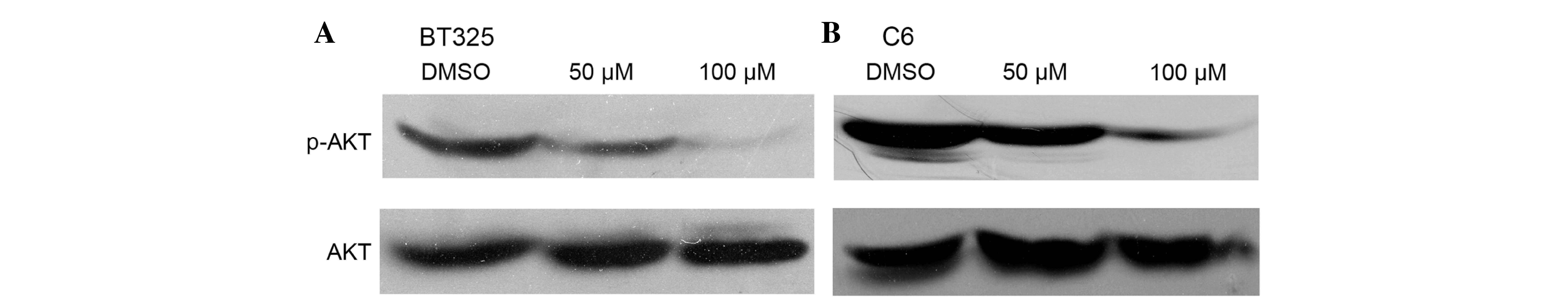
DHA inhibits AKT activation in glioma
cells
Dysregulation of the PI3K/AKT pathway is commonly
observed in glioma cells (19). The
effects of DHA on the activation of PI3K/AKT signaling were
examined in glioma cells. Western blot analysis demonstrated that
after 24 h of DHA exposure, DHA treatment markedly inhibited the
phophorylation of AKT in a dose-dependent manner (Fig. 4).
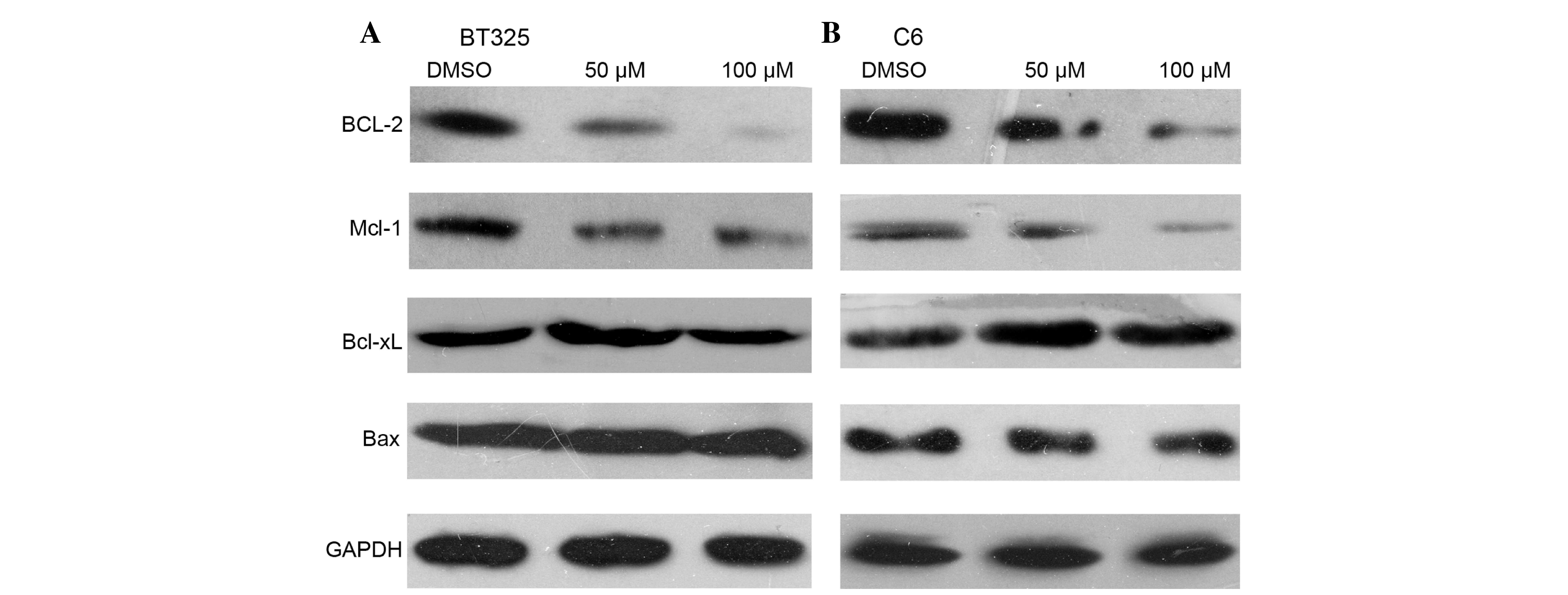
Involvement of Bcl-2 family proteins
in DHA-induced glioma cell apoptosis
The Bcl-2 family proteins serve important roles in
the regulation of cell apoptosis (20). The effects of DHA treatment on Bcl-2
protein expression levels were examined. Western blot analysis
demonstrated that DHA treatment markedly inhibited the expression
of Bcl-2 and Mcl-1, two antiapoptotic Bcl-2 proteins (Fig. 5). However, DHA treatment had no impact
on the protein expression levels of Bcl-xL or Bax.
Discussion
As important anti-apoptotic signaling pathways,
Raf/MEK/ERK and PI3K/AKT signaling cascades have attracted
considerable attention (3). These
signaling pathways have been demonstrated to serve critical roles
in the transduction of growth factor signals, which regulate gene
expression to control cell survival, proliferation, motility, and
metabolism. Mutations may occur in upstream receptors such as EGFR
and Flt-3, or downstream members such as Ras, Raf, PI3K, PTEN and
AKT (13). Accumulating evidence has
indicated that the crosstalk between Ras/Raf/MEK/ERK and the
PI3K/AKT pathways serves important roles in promoting cell
proliferation and inhibiting apoptosis of cancer, and pathway
cross-inhibition reduces the effectiveness of single agents
(12). A number of studies have
demonstrated synergistic anticancer effects of simultaneous
inhibition of both pathways. Treatment of basal-like cell lines
with a selective MEK inhibitor resulted in enhanced PI3K pathway
signaling, whereas supplementation of PI3K inhibitor induces
apoptosis both in vitro and in vivo (18,21). These
evidence indicates that dual inhibition of Raf/MEK/ERK and PI3K/AKT
signaling may result in more clinical benefit and therefore may
provide certain advantages over single pathway inhibitors (22).
Although the anti-cancer activity of DHA has been
demonstrated in a number of types of cancer (23–25), the
underlying mechanisms remain largely unknown. To optimize the
therapeutic effect of DHA against glioma cells and to explore its
molecular mechanisms, the BT325 and C6 cell lines were used in the
present study. Indeed, using cell viability and apoptosis analysis,
it was indicated that DHA is capable of inducing the apoptosis of
human glioma cells in a concentration- and time-dependent manner.
For further illuminating the possible mechanisms, the present study
was extended to investigate the effect of DHA on MEK/ERK and
PI3K/AKT signaling factors. The results demonstrated that DHA
suppressed MEK, ERK and AKT phosphorylation in the dose-dependent
manner, indicating that DHA may induce apoptosis through a process
that involves Raf/MEK/ERK and PI3K/AKT pathways inactivation. The
Raf/MEK/ERK and PI3K/AKT signal pathways have been implicated in a
wide variety of processes in cancer cells including the regulation
of cell proliferation, survival and apoptosis. Therefore, the
present study hypothesized that the combination of Raf/MEK/ERK and
PI3K/AKT pathway inhibition and DHA treatment would greatly
suppress the proliferation of glioma cells and markedly induce
apoptosis.
Inducing apoptosis is an attractive strategy for
cancer therapy, which is finally determined by the balance between
pro- and anti-apoptotic mechanisms (20). The expression of the Bcl-2 protein
family was also examined; this family of proteins serves important
roles in the regulation of mitochondria-dependent apoptosis. Bcl-2
protein is the prototype of this family, which inhibits cell
apoptosis through multiple mechanisms (26). Mcl-1 is another highly expressed
anti-apoptotic protein expressed in a number of types of
malignancies and has been demonstrated to mediate resistance to
chemotherapy, whereas Bax is a pro-apoptotic member. In the present
study, no significant differences in the expression of Bax protein
were observed after DHA treatment in glioma cells. However, the
expression of Bcl-2 and Mcl-1 proteins was significantly suppressed
by DHA in a dose-dependent manner. These results indicated that DHA
may exerts its anti-glioma effects through the inhibition of
pro-apoptotic proteins Bcl-2 and Mcl-1.
In summary, the data in the present study indicates
that DHA suppresses the Raf/MEK/ERK and PI3K/AKT pathways in the
glioma cells, which provides valuable information on the molecular
mechanism of its anticancer activity. Therefore, DHA may be a
promising molecule for the treatment of glioma.
Glossary
Abbreviations
Abbreviations:
|
DHA
|
dihydroartemisinin
|
|
MTT
|
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide
|
|
MEK
|
mitogen-activated and extracellular
signal-regulated kinase kinase)
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PIP3
|
phosphatidylinositol 3,4,5
triphosphate
|
References
|
1
|
Sathornsumetee S, Reardon DA, Desjardins
A, et al: Molecularly targeted therapy for malignant glioma.
Cancer. 110:13–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y and Jiang T: Understanding high
grade glioma: Molecular mechanism, therapy and comprehensive
management. Cancer Lett. 331:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saini KS, Loi S, de Azambuja E, et al:
Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the
treatment of breast cancer. Cancer Treat Rev. 39:935–946. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redman EK, Brookes PS and Karcz MK: Role
of p90(RSK) in regulating the crabtree effect: implications for
cancer. Biochem Soc Trans. 41:124–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hasskarl J: Sorafenib: Targeting multiple
tyrosine kinases in cancer. Recent Results Cancer Res. 201:145–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han L, Yang Y, Yue X, et al: Inactivation
of PI3K/AKT signaling inhibits glioma cell growth through
modulation of beta-catenin-mediated transcription. Brain Res.
1366:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chin YR and Toker A: Function of Akt/PKB
signaling to cell motility, invasion and the tumor stroma in
cancer. Cell Signal. 21:470–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghayad SE and Cohen PA: Inhibitors of the
PI3K/Akt/mTOR pathway: New hope for breast cancer patients. Recent
Pat Anticancer Drug Discov. 5:29–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Wu J, Zheng H, et al: Discovery of
3-(2-aminoethyl)-5-(3-phenyl-propylidene)-thiazolidine-2,4-dione as
a dual inhibitor of the Raf/MEK/ERK and the PI3K/Akt signaling
pathways. Bioorg Med Chem Lett. 20:4526–4530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steelman LS, Abrams SL, Whelan J, et al:
Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT
pathways to leukemia. Leukemia. 22:686–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCubrey JA, Steelman LS, Kempf CR, et al:
Therapeutic resistance resulting from mutations in Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol.
226:2762–2781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee J, Zhang G, Wu X, Xu F, Zhou J and
Zhang X: Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+
chronic myeloid leukemia K562 cells involve, AKT, ERK and NF-kappaB
modulation. J Cancer Res Clin Oncol. 138:2095–2102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang XM, Zhang L, Ding GF and Wang QZ:
Inhibitory effect of dihydroartemisinin on the growth of human
prostate cancer PC-3 M cells and its mechanism. Zhonghua Nan Ke
Xue. 18:590–594. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Feng X, Li L, Jiang H, Jiang K, Jin Y and
Zheng J: Dihydroartemisinin potentiates the anticancer effect of
cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer
cells: involvement of apoptosis and autophagy. Biochem Biophys Res
Commun. 444:376–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu M, Sun L, Zhou J and Yang J:
Dihydroartemisinin induces apoptosis in colorectal cancer cells
through the mitochondria-dependent pathway. Tumour Bio.
35:5307–5314. 2014. View Article : Google Scholar
|
|
18
|
Hoeflich KP, O'Brien C, Boyd Z, et al: In
vivo antitumor activity of MEK and phosphatidylinositol 3-kinase
inhibitors in basal-like breast cancer models. Clin Cancer Res.
15:4649–4664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubiatowski T, Jang T, Lachyankar MB,
Salmonsen R, Nabi RR, Quesenberry PJ, Litofsky NS, Ross AH and
Recht LD: Association of increased phosphatidylinositol 3-kinase
signaling with increased invasiveness and gelatinase activity in
malignant gliomas. J Neurosurg. 95:480–488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mirzoeva OK, Das D, Heiser LM, et al:
Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase
feedback signaling determine susceptibility of breast cancer cells
to MEK inhibition. Cancer Res. 69:565–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheppard KE, Cullinane C, Hannan KM, et
al: Synergistic inhibition of ovarian cancer cell growth by
combining selective PI3K/mTOR and RAS/ERK pathway inhibitors. Eur J
Cancer. 49:3936–3944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang XJ, Li CT, Zhang WP, Lu YB, Fang SH
and Wei EQ: Dihydroartemisinin potentiates the cytotoxic effect of
temozolomide in rat C6 glioma cells. Pharmacology. 82:1–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu YY, Chen TS, Qu JL, Pan WL, Sun L and
Wei XB: Dihydroartemisinin (DHA) induces caspase-3-dependent
apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed
Sci. 16:162009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Wang W, Xu J, et al:
Dihydroartemisinin inhibits tumor growth of human osteosarcoma
cells by suppressing Wnt/beta-catenin signaling. Oncol Rep.
30:1723–1730. 2013.PubMed/NCBI
|
|
26
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: Role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|