Introduction
Primary mediastinal large B-cell lymphoma (PMLBCL),
which originates from thymic B cells, was once recognized as a
distinctive clinical-pathological subtype of diffuse large B-cell
lymphoma (DLBCL) according to the World Health Organization (WHO)
in 2008 (1,2). PMLBCL affects young individuals, with a
female prevalence. Patients present with a bulky mediastinal mass,
which is commonly associated with adjacent organ infiltration and
superior vena cava syndrome (3–5).
The optimal treatment for PMLBCL remains undefined.
The cyclophosphamide, doxorubicin, vincristine and prednisone
(CHOP) regimen is considered to be inferior to other more intensive
regimens (6–10), such as the methotrexate, cytarabine,
cyclophosphamide, vincristine, prednisone and bleomycin regimen
(MACOP-B), dose-dense regimens, or even front-line consolidation
high-dose therapy and autologous stem cell transplantation.
However, none of these intensified approaches is now expected to
provide results superior to those observed with rituximab plus CHOP
(RCHOP). Rituximab, as a monoclonal antibody, has revolutionized
the treatment of aggressive B-cell lymphomas (11,12). A
number of studies have confirmed that RCHOP improves the outcome of
PMLBCL patients (13–15). Although the majority of patients
initially respond to this therapeutic approach, certain patients
relapse and eventually succumb to the disease. Therefore studies
are currently focused on supplemental treatments such as radiation
therapy (RT). The issue of whether the administration of RT after
chemotherapy is beneficial to patients with PMLBCL remains
unresolved, particularly in the rituximab era. Therefore, the
present study summarized the clinical data of 63 PMLBCL patients,
who were treated in affiliated hospitals (Xiangya hospital, The
Second Xiangya Hospital and the Affiliated Cancer Hospital of
Xiangya School of Medicine) of Central South University (Changsha,
China) between January 2000 and January 2013, in an attempt to
investigate the role of radiotherapy in PMLBCL.
Patients and methods
Patients
Patients with a histologically confirmed diagnosis
of PMLBCL who were treated in the affiliated hospitals (Xiangya
hospital, The Second Xiangya Hospital and the Affiliated Cancer
Hospital of Xiangya School of Medicine) of Central South University
between January 2000 and January 2013 were included in this
analysis. The diagnosis of PMLBCL was based on the WHO criteria
(2). All the patients were previously
untreated and recruited without a history of previous malignant
tumors, primary central nervous system involvement, severe
coincident illnesses, second primary tumors or a positive human
immunodeficiency virus status.
Stage was defined according to the Ann Arbor staging
system (16). The International
Prognostic Index (IPI) was also evaluated (17). Bulky disease was defined as a
mediastinal mass >10 cm in diameter.
All patients included in the study completed 6–8
cycles of CHOP or CHOP-like chemotherapy with or without rituximab.
All patients underwent imaging studies [positron emission
tomography-computed tomography (PET/CT) or CT] to assess the
response to chemotherapy (during and/or after completion of
chemotherapy) (18). Treatment
response was evaluated based on the International Working Group
Recommendations for Response Criteria for Non-Hodgkin's Lymphoma,
with complete remission (CR), partial remission (PR), stable
disease (SD) and progressive disease (PD) statuses (19). At the completion of chemotherapy,
involved field radiotherapy (IFRT) was allowed, at the treating
physician's discretion. It was assumed that RT was more likely to
be administered to patients with previously bulky disease, and
disease that failed to achieve CR upon chemotherapy.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). The
continuous characteristics, such as age, were presented as the
median/range and were compared with the Wilcoxon rank-sum test.
Other characteristics, including gender, Ann Arbor stage, lactate
dehydrogenase level, bulky disease status, IPI score and
chemotherapy regimen, were counted as categorical variables and
compared by χ2 test or Fisher's exact test.
The major endpoint of the analysis was overall
survival (OS) calculated from the date of diagnosis until the date
of mortality or final follow-up. Progress-free survival (PFS) was
defined as survival from the date of diagnosis until the date of i)
progression of disease, ii) relapse, iii) mortality from any cause
or iv) final follow-up.
A univariate analysis was performed using the
Kaplan-Meier method to assess 5-year OS and 5-year PFS. Statistical
differences between survival curves were evaluated by log-rank
test. Multivariate Cox proportional hazards modeling was conducted
using the enter selection technique. In all tests, P<0.05 was
used to indicate a significant difference. Hazard ratios (HRs) and
their 95% confidence intervals (CIs) were estimated to assess the
magnitude of risk.
Results
Patients and treatment
characteristics
A total of 82 patients with a histologically
confirmed diagnosis of PMLBCL who were who were treated in the
affiliated hospitals of Central South University between January
2000 and January 2013 were identified. Among these, 19 patients
were excluded, as they did not have detailed treatment or follow-up
information and were referred only for a second opinion. A total of
63 patients were therefore included in the final analysis. The
clinical characteristics and a comparison between the patients with
RT and without RT are summarized in Table
I. The cohort consisted of 36 males and 27 females. The median
age at diagnosis was 28 years (range, 12–78 years). According to
the Ann Arbor staging system, 36 patients were in stages I–II
(57.14%) and 27 patients were in stages III–IV (42.86%). Bulky
disease was present in 28 patients (44.4%). The median follow-up
time was 49 months (range, 7–132 months).
 | Table I.Patient clinical characteristics and a
comparison between patients with RT and without RT. |
Table I.
Patient clinical characteristics and a
comparison between patients with RT and without RT.
| Variable | Total | RT | No RT | P-value |
|---|
| Total, n | 63 (100.00) | 35 (55.6) | 28 (44.4) | NA |
| Gender, n (%) |
|
|
| 0.620 |
| Male | 36 (57.1) | 21 (60.0) | 15 (53.6) |
|
|
Female | 27 (42.9) | 14 (40.0) | 13 (46.4) |
|
| Age, n (%) |
|
|
| 0.800 |
| ≤30
years | 34 (54.0) | 18 (51.4) | 16 (57.1) |
|
| >30
years | 29 (46.0) | 17 (48.6) | 12 (42.9) |
|
| Median age (range),
years | 28 (12–78) | 28
(15–67) | 29
(12–78) | 0.708 |
| AA stage, n (%) |
|
|
| 0.000a |
| I–II | 36 (57.1) | 29 (82.9) | 7
(25.0) |
|
|
III–IV | 27 (42.9) | 6
(17.1) | 21 (75.0) |
|
| Bulky disease, n
(%) |
|
|
| 0.002a |
| No | 35 (55.6) | 13 (37.1) | 22 (78.6) |
|
| Yes | 28 (44.4) | 22 (62.9) | 6
(21.4) |
|
| Treated with
rituximab, n (%) |
|
|
| 0.213 |
| No | 28 (44.4) | 13 (37.1) | 15 (53.6) |
|
| Yes | 35 (55.6) | 22 (62.9) | 13 (46.4) |
|
| LDH, n (%) |
|
|
| 0.136 |
| ≤UNL | 36 (57.1) | 23 (65.7) | 13 (42.4) |
|
|
>UNL | 27 (42.9) | 12 (34.3) | 15 (57.6) |
|
| IPI, n (%) |
|
|
| 0.195 |
| 0–1 | 38 (60.3) | 24 (68.6) | 14 (50.0) |
|
| ≥2 | 25 (39.7) | 11 (31.4) | 14 (50.0) |
|
All patients received 6–8 cycles of CHOP or a
CHOP-like regimen, with 35 patients (55.6%) receiving RCHOP.
Post-chemotherapy imaging consisted of PET/CT in 60.3% of patients
and CT in 39.7%. A CR was achieved in 38 patients (60.3%), PR/SD in
18 (28.6%) and PD in 7 (11.1%).
A total of 35 patients who achieved CR or PR
following chemotherapy received IFRT to the mediastinum or
mediastinum and supraclavicular area. The dosage of IFRT was 40–45
Gy with 6 MV X-rays. IFRT was delivered to 29 out of 36 (80.6%)
patients with stage I–II disease and 6 out of 27 (22.2%) patients
with stage III–IV disease. Comparisons between the characteristics
of patients who received IFRT and those who did not showed that the
patients with bulky disease and stage I–II disease received IFRT
more frequently (Table I).
Prognostic analysis
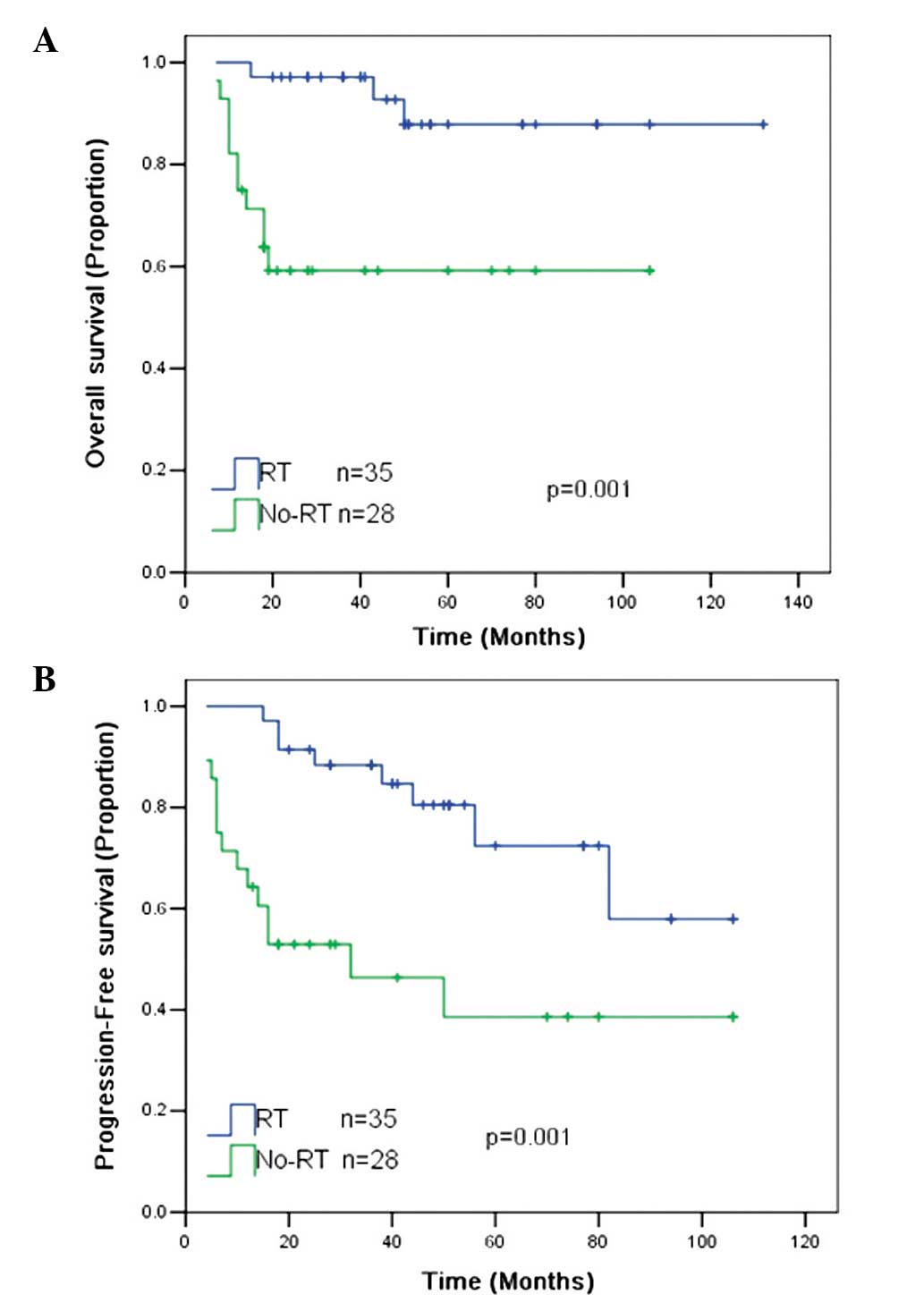
The 5-year OS and PFS rates for all patients were 74
and 59%, respectively. RT was associated with significantly
improved 5-year OS (87 vs. 58%; P=0.001) and 5-year PFS (75 vs.
39%; P=0.001) rates compared with the patients without RT (Fig. 1).
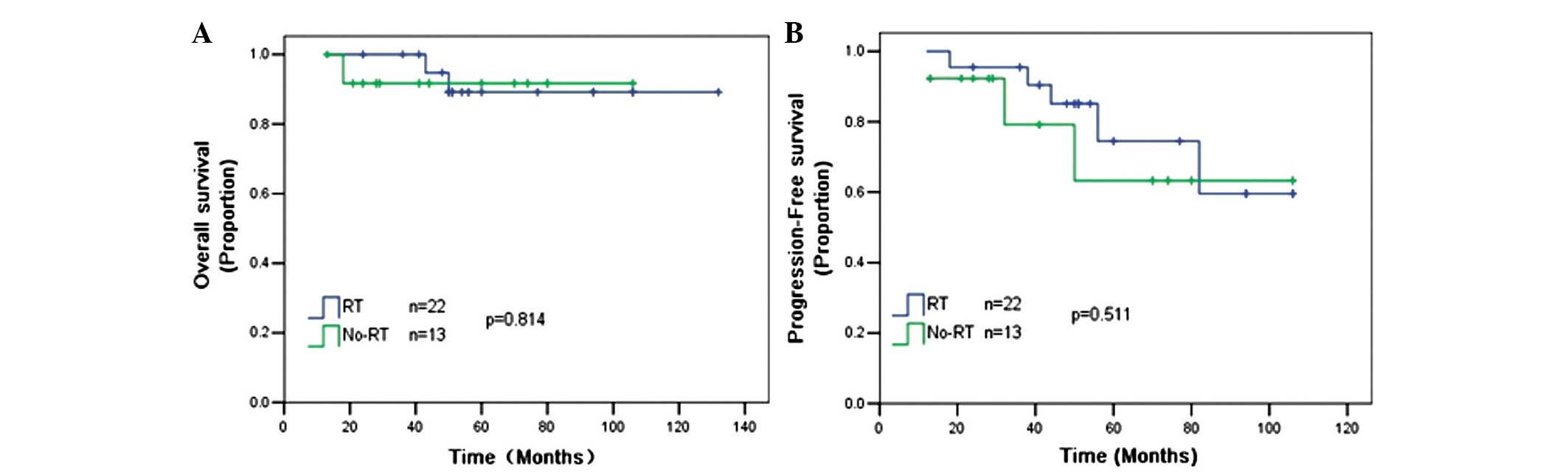
The role of RT in the setting of R-CHOP chemotherapy
remains unclear. An analysis was performed on 35 patients who
received 6–8 cycles of R-CHOP separately, and it was found that RT
could not improve the 5-year OS (88 vs. 92%; P=0.814) and 5-year
PFS (78 vs. 65%; P=0.511) rates compared with patients without RT
(Fig. 2).
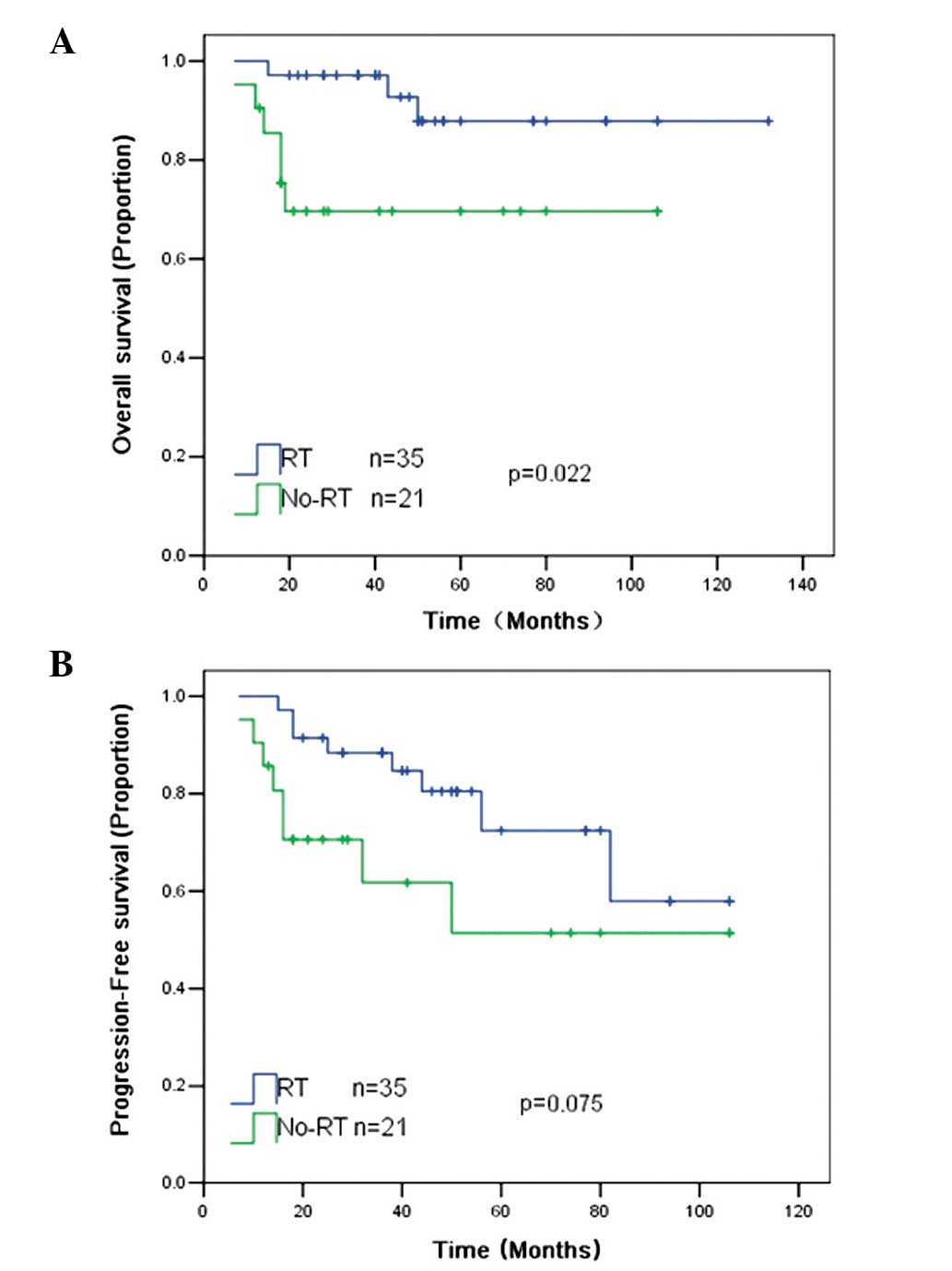
An analysis was further performed on 56 patients who
achieved CR, PR or SD after chemotherapy. RT was associated with a
significantly improved 5-year OS rate (87 vs. 69%; P=0.022) and
exhibited a trend towards an improved 5-year PFS rate (75 vs. 52%;
P=0.075) compared with patients without RT (Fig. 3).
On univariate analysis of all patients, five
factors, namely stage, the addition of rituximab, treatment with
RT, IPI score and response to chemotherapy, were found to
significantly affect the OS and PFS rates (Table II).
 | Table II.Univariate analysis of OS and PFS
rates for all patients. |
Table II.
Univariate analysis of OS and PFS
rates for all patients.
| Variable | 5-year OS rate
(%) | P-value | 5-year PFS rate
(%) | P-value |
|---|
| Total | 74 |
| 59 |
|
| Gender |
| 0.852 |
| 0.934 |
| Male | 74 |
| 57 |
|
|
Female | 74 |
| 63 |
|
| Age, years |
| 0.471 |
| 0.447 |
| ≤30 | 81 |
| 51 |
|
|
>30 | 70 |
| 66 |
|
| AA stage |
| 0.001a |
| 0.012a |
|
I–II | 88 |
| 69 |
|
|
III–IV | 57 |
| 50 |
|
| Radiotherapy |
| 0.001a |
| 0.001a |
| No | 58 |
| 39 |
|
|
Yes | 87 |
| 75 |
|
| Treated with
rituximab |
| 0.001a |
| 0.000a |
| No | 58 |
| 44 | 0.000a |
|
Yes | 88 |
| 74 |
|
| Response |
| 0.000a |
|
|
| CR | 89 |
| 75 |
|
|
PR+SD | 63 |
| 38 |
|
| PD | 21 |
| 0 |
|
| Bulky disease |
| 0.960 |
| 0.747 |
| No | 72 |
| 58 |
|
|
Yes | 76 |
| 61 |
|
| LDH |
| 0.743 |
| 0.225 |
|
≤UNL | 74 |
| 61 |
|
|
>UNL | 76 |
| 61 |
|
| IPI |
|
0.017a |
|
0.023a |
|
0–1 | 82 |
| 65 |
|
| ≥2 | 63 |
| 52 |
|
On multivariate analysis, RT (HR, 0.157; P=0.018)
and the addition of rituximab to CHOP chemotherapy (HR, 0.156;
P=0.009) were predictive of an increased OS rate. Similarly, RT
(HR, 0.111; P=0.001) and the addition of rituximab to CHOP
chemotherapy (HR, 0.231; P=0.002) were predicative of an increased
PFS rate. Furthermore, there was a trend towards a decreased PFS
rate with bulky disease (HR, 2.994; P=0.058), however, this was not
statistically significant (Table
III).
 | Table III.Multivariate analysis for clinical
outcomes. |
Table III.
Multivariate analysis for clinical
outcomes.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Factor P-value | HR | 95% CI |
|
|---|
| OS |
|
|
|
| RT,
yes | 0.157 | 0.034–0.731 | 0.018a |
|
Rituximab, yes | 0.156 | 0.039–0.622 | 0.009a |
| Bulky,
yes | 1.634 | 0.432–6.177 | 0.469 |
| AA
stage | 2.216 | 0.434–11.319 | 0.339 |
|
IPI | 1.549 | 0.368–6.523 | 0.551 |
| PFS |
|
|
|
| RT,
yes | 0.111 | 0.032–0.384 | 0.001a |
|
Rituximab, yes | 0.231 | 0.092–0.575 | 0.002a |
| Bulky,
yes | 2.994 | 0.963–9.313 | 0.058 |
| AA
stage | 0.718 | 0.221–2.332 | 0.582 |
|
IPI | 2.181 | 0.707–6.728 | 0.175 |
Discussion
The role of RT in PMLBCL is always controversial. In
the pre-rituximab era, numerous studies have evaluated the role of
RT. Data from a study on 138 PMLBCL patients who were treated in 13
Italian institutions showed that IFRT plus chemotherapy improved
the outcome compared with chemotherapy alone (P=0.04), while
consolidation IF-RT to the mediastinum further improved the outcome
of CR patients (8). A retrospective
study on 53 PMLBCL patients treated with MACOP-B (methotrexate,
doxorubicin, cyclophosphamide, vincristine, prednisone,
bleomycin)/VACOP-B (etoposide, doxorubicin, cyclophosphamide,
vincristine, prednisone, bleomycin) plus RT from Padova, Italy,
showed that the 5-year disease-free survival and OS rates were
93.42 and 86.6%, respectively. A total of 37.7% of the patients
achieved a CR and 56.6% of the patients achieved a PR after IFRT
plus chemotherapy, while 92% of the patients who had already
obtained a PR improved to a CR following radiotherapy (20). Another retrospective study conducted
by a group from Rome, Italy, included 85 patients with PMLBCL, all
of whom received third-generation regimen MACOP-B plus IFRT.
Following a median follow-up time of 81 months, progression or
relapse was observed in 15 out of 84 patients (17.9%). The 5-year
OS and PFS rates were 87 and 81%, respectively. This study showed
that MACOP-B and IFRT induced high response and lymphoma-free
survival rates (10). In the present
study, RT was associated with significantly improved PFS and OS
rates compared with patients without RT, and furthermore, the
analysis performed on patients who achieved more than PD following
chemotherapy showed that RT was associated with a significantly
improved 5-year OS rate and exhibited a trend for an improved
5-year PFS rate, which is similar to the results of the
aforementioned studies.
However, there are several studies that do not
support this view. A study from the University of British Columbia
consisting of 151 patients showed that when comparing the eras
prior to and following the routine administration of radiotherapy,
there was no significant difference in 5-year PFS (74 vs. 62%;
P=0.09) or OS (78 vs. 69%; P=0.14) rates (21). Similarly, a study conducted in France
suggested that by comparing dose-intensified CHOP and CHOP plus RT,
RT may not be necessary in PMLBCL when a CR or a CR, unconfirmed,
is achieved with dose-intensified chemotherapy (6).
Indeed, the aforementioned studies were all
performed in the era prior to rituximab treatment. Rituximab, as a
cluster of differentiation 20 antibody, has revolutionized the
treatment of aggressive B-cell lymphomas. The current National
Comprehensive Cancer Network 2013 guidelines recommended rituximab
as the first-line treatment for PMLBCL. Rituximab combined with
chemotherapy has been confirmed to be very effective and safe for
PMLBCL in multiple studies (14,15,22), and
the superiority of certain intensive regimens over CHOP for
treatment of PMBCL disappeared once rituximab was added (23).
However, several studies suggested that RCHOP was
associated with a high rate of primary refractory disease. A study
on 37 PMLBCL patients found that ~50% of PMBCL patients showed
residual disease on PET scan following rituximab plus chemotherapy
(24). A recent retrospective study
on 63 PMLBCL patients treated with RCHOP showed that primary
induction failure occurred in 13 (20.6%) patients (25).
Furthermore, the question of whether IFRT is still
required for PMLBCL in the rituximab era has been raised. In an
attempt to clarify the role of RT under the current standard of
care, a couple of studies have recently been published. A study
from Beijing, China, consisting of 79 patients with PMLBCL
indicated that RT plus RCHOP chemotherapy was associated with
excellent survival and local control rates. The 5-year OS, PFS and
local control rates for early-stage patients were 73.6, 69.9 and
92.6% for chemotherapy and RT, and 50.8% (P=0.076), 36.9% (P=0.008)
and 56.4% (P<0.001) for chemotherapy alone, respectively
(5). However, another study conducted
by a group from Greece suggested that the addition of RT to RCHOP
chemotherapy did not improve the 5-year PFS (92 vs. 93%; P>0.2)
and 5-year OS (96 vs. 100%; P>0.2) rates compared with RCHOP
alone (15). The National Cancer
Institute group has presented encouraging data with regard to 51
PMLBCL patients who were treated with dose-adjusted etoposide,
doxorubicin, cyclophosphamide, vincristine, prednisone and
rituximab (DA-EPOCH-R) chemotherapy, without the routine use of RT.
During the median 5-year follow-up, the event-free survival and OS
rates were 93 and 97%, respectively. A total of 96% patients
achieved a CR (26). The result
indicated that DA-EPOCH-R had a high cure rate and removed the
requirement for radiotherapy in patients with PMLBCL.
Although the present findings indicated that RT was
associated with improved survival for all the patients, in the
subgroup analysis of 35 patients who received RCHOP, it was shown
that RT could not improve the 5-year OS (88 vs. 92%; P=0.814) and
5-year PFS (78 vs. 65%; P=0.511) rates compared with RCHOP alone.
However, these results were limited, as the study was retrospective
and had an imbalanced number of patients who received RT plus
chemotherapy vs. chemotherapy alone.
The present study cannot adequately address whether
dose RT can be safely omitted in the rituximab era in selected
patients, as the published experience of RT combined with RCHOP (or
rituximab plus another chemotherapy regimen) in PMLBCL patients
remains limited and is mainly derived from small patient series.
The substitution of rituximab for RT requires further
investigation.
In summary, the present study demonstrated that RT
plus chemotherapy could confer a survival benefit for all patients
with PMLBCL, but RT did not confer a survival benefit for patients
who were treated with rituximab. It could be considered an option
for PMLBCL patients. A number of unanswered questions remain with
regard to the management of PMLBCL. The role of RT in the era of
targeted therapy should be evaluated in future randomized clinical
trials.
References
|
1
|
Harris NL, Jaffe ES, Stein H, Banks PM,
Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter
KC, et al: A revised European-American classification of lymphoid
neoplasms: A proposal from the International Lymphoma Study Group.
Blood. 84:1361–1392. 1994.PubMed/NCBI
|
|
2
|
Steven H: Swerdlow IAfRoC, World Health
Organization: WHO classification of tumours of haematopoietic and
lymphoid tissues. International Agency for Research on Cancer.
2008.
|
|
3
|
No authors listed: A clinical evaluation
of the international lymphoma study group classification of
non-Hodgkin's lymphoma. The non-hodgkin's lymphoma classification
project. Blood. 89:3909–3918. 1997.PubMed/NCBI
|
|
4
|
Zhu YJ, Huang JJ, Xia Y, Zhao W, Jiang WQ,
Lin TY, Huang HQ and Li ZM: Primary mediastinal large B-cell
lymphoma (PMLBCL) in Chinese patients: Clinical characteristics and
prognostic factors. Int J Hematol. 94:178–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu LM, Fang H, Wang WH, et al: Prognostic
significance of rituximab and radiotherapy for patients with
primary mediastinal large B-cell lymphoma receiving
doxorubicin-containing chemotherapy. Leuk Lymphoma. 54:1684–1690.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massoud M, Koscielny S, Lapusan S, Bosq J
and Ribrag V: Primary mediastinal large B-cell lymphomas treated
with dose-intensified CHOP alone or CHOP combined with
radiotherapy. Leuk Lymphoma. 49:1510–1515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamlin PA, Portlock CS, Straus DJ, et al:
Primary mediastinal large B-cell lymphoma: Optimal therapy and
prognostic factor analysis in 141 consecutive patients treated at
Memorial Sloan Kettering from 1980 to 1999. Br J Haematol.
130:691–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Todeschini G, Secchi S, Morra E, et al:
Primary mediastinal large B-cell lymphoma (PMLBCL): Long-term
results from a retrospective multicentre Italian experience in 138
patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer.
90:372–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zinzani PL, Martelli M, Bertini M, et al:
Induction chemotherapy strategies for primary mediastinal large
B-cell lymphoma with sclerosis: A retrospective multinational study
on 426 previously untreated patients. Haematologica. 87:1258–1264.
2002.PubMed/NCBI
|
|
10
|
De Sanctis V, Finolezzi E, Osti MF, et al:
MACOP-B and involved-field radiotherapy is an effective and safe
therapy for primary mediastinal large B cell lymphoma. Int J Radiat
Oncol Biol Phys. 72:1154–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sehn LH, Donaldson J, Chhanabhai M, et al:
Introduction of combined CHOP plus rituximab therapy dramatically
improved outcome of diffuse large B-cell lymphoma in British
Columbia. J Clin Oncol. 23:5027–5033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfreundschuh M, Trümper L, Osterborg A, et
al: CHOP-like chemotherapy plus rituximab vs. CHOP-like
chemotherapy alone in young patients with good-prognosis diffuse
large-B-cell lymphoma: A randomised controlled trial by the
MabThera International Trial (MInT) Group. Lancet Oncol. 7:379–391.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson PWM and Davies AJ: Primary
mediastinal B-cell lymphoma. Hematology/the Education Program of
the American Society of Hematology. Hematology Am Soc Hematol Educ
Program. 2008:349–358. 2008. View Article : Google Scholar
|
|
14
|
Rieger M, Osterborg A, Pettengell R, White
D, Gill D, Walewski J, Kuhnt E, Loeffler M, Pfreundschuh M and Ho
AD: Mab Thera International Trial (MInT) Group: Primary mediastinal
B-cell lymphoma treated with CHOP-like chemotherapy with or without
rituximab: Results of the Mabthera International Trial Group study.
Ann Oncol. 22:664–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vassilakopoulos TP, Pangalis GA,
Katsigiannis A, Papageorgiou SG, Constantinou N, Terpos E, Zorbala
A, Vrakidou E, Repoussis P, Poziopoulos C, et al: Rituximab,
cyclophosphamide, doxorubicin, vincristine and prednisone with or
without radiotherapy in primary mediastinal large B-cell lymphoma:
The emerging standard of care. Oncologist. 17:239–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
17
|
No authors listed: A predictive model for
aggressive non-hodgkin's lymphoma. The international non-hodgkin's
lymphoma prognostic factors project. N Engl J Med. 329:987–994.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Juweid ME, Wiseman GA, Vose JM, Ritchie
JM, Menda Y, Wooldridge JE, Mottaghy FM, Rohren EM, Blumstein NM,
Stolpen A, et al: Response assessment of aggressive non-Hodgkin's
lymphoma by integrated International Workshop Criteria and
fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin
Oncol. 23:4652–4661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazzarotto R, Boso C, Vianello F, Aversa
MS, Chiarion-Sileni V, Trentin L, Zambello R, Muzzio PC, Fiore D
and Sotti G: Primary mediastinal large B-cell lymphoma: results of
intensive chemotherapy regimens (MACOP-B/VACOP-B) plus involved
field radiotherapy on 53 patients. A single institution experience.
Int J Radiat Oncol Biol Phys. 68:823–829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Savage KJ, Al-Rajhi N, Voss N, Paltiel C,
Klasa R, Gascoyne RD and Connors JM: Favorable outcome of primary
mediastinal large B-cell lymphoma in a single institution: The
British Columbia experience. Ann Oncol. 17:123–130. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zinzani PL, Stefoni V, Finolezzi E,
Brusamolino E, Cabras MG, Chiappella A, Salvi F, Rossi A, Broccoli
A and Martelli M: Rituximab combined with MACOP-B or VACOP-B and
radiation therapy in primary mediastinal large B-cell lymphoma: A
retrospective study. Clin Lymphoma Myeloma. 9:381–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avigdor A, Sirotkin T, Kedmi M, Ribakovsy
E, Berkowicz M, Davidovitz Y, Kneller A, Merkel D, Volchek Y,
Davidson T, et al: The impact of R-VACOP-B and interim FDG-PET/CT
on outcome in primary mediastinal large B cell lymphoma. Ann
Hematol. 93:1297–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filippi AR, Piva C, Giunta F, Bellò M,
Chiappella A, Caracciolo D, Zotta M, Douroukas A, Ragona R, Vitolo
U, et al: Radiation therapy in primary mediastinal B-cell lymphoma
with positron emission tomography positivity after rituximab
chemotherapy. Int J Radiat Oncol Biol Phys. 87:311–316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soumerai JD, Hellmann MD, Feng Y, Sohani
AR, Toomey CE, Barnes JA, Takvorian RW, Neuberg D, Hochberg EP and
Abramson JS: Treatment of primary mediastinal B-cell lymphoma with
rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone is associated with a high rate of primary refractory
disease. Leuk Lymphoma. 55:538–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunleavy K, Pittaluga S, Maeda LS, Advani
R, Chen CC, Hessler J, Steinberg SM, Grant C, Wright G, Varma G, et
al: Dose-adjusted EPOCH-rituximab therapy in primary mediastinal
B-cell lymphoma. N Engl J Med. 368:1408–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|