Introduction
Myxoma is a true benign neoplasm of mesenchymal
origin that produces mucopolysaccharide and immature collagen.
Although it is more commonly found in the left atrium of the heart,
a myxoma can arise in any region of the body, such as the
genitourinary tract, bones, retroperitoneum, skin, joints and
skeletal muscles (1,2). When arising from muscle, the tumor is
termed an intramuscular myxoma (IMM) (2). IMM is a rare lesion with incidence rates
varying between 0.1 and 0.13 per 100,000 individuals per year
(3). The benign lesion favors a
female predilection, occurring between the fourth and sixth decades
of life (4). Wide surgical resection
is the most successful form of management, and the prognosis is
good (5). Lung cancer is the leading
cause of cancer mortality in the United States, with an estimated
224,210 novel cases and 159,260 mortalities in 2014 (6). The malignant tumor can metastasize to
almost every region of the body, including skeletal muscle,
although this is rather rare (7).
There is no consensus on the optimal therapeutic strategy for
muscle metastasis, as it may be the end-stage presentation of lung
cancer (8). IMM has certain
characteristic features, however, when concomitant with a malignant
neoplasm, particularly those which possess a high incidence of
distant metastases, it may be misdiagnosed as muscle metastasis due
to its rare occurrence and particularly rare concurrence (9,10). The
current study presents, to the best of our knowledge, the first
case of non-small cell lung cancer (NSCLC) concomitant with IMM
arising in the right psoas mimicking intramuscular metastasis and
briefly reviews the literature concerning this subject.
Case report
A 64-year-old, non-smoking, female was referred to
Shandong Cancer Hospital and Institute (Shandong, Jinan, China) on
May 2013 due to a progressive cough with little sputum, dyspnea,
left chest pain and right lumbar pain that had persisted for four
months. The lumbar pain was aggravated upon walking and was
relieved spontaneously upon resting. A physical examination
revealed a few moist rales, decreased breath sounds over the left
lung, and pressing and percussive pain in the right lumbar region.
The patient experienced no numbness or tingling in the legs.
Examination of the lumbar spine showed normal lumbar lordosis.
Sensation in the lower extremity was intact. There was no muscular
contracture or palpable mass. Reflexes in the patella and ankle
were all symmetrical and normal.
A series of further medical examinations were
required for accurate diagnosis. Written informed consent was
obtained from the family of the patient. No abnormality was shown
on routine laboratory investigations. An initial chest X-ray
revealed left lung opacity. Further examinations were also
conducted. The serum cytokeratin-19 fragment 21-1 (Cyfra21-1) level
was elevated to 7.59 ng/ml (normal range, 0–3.3 ng/ml). A computed
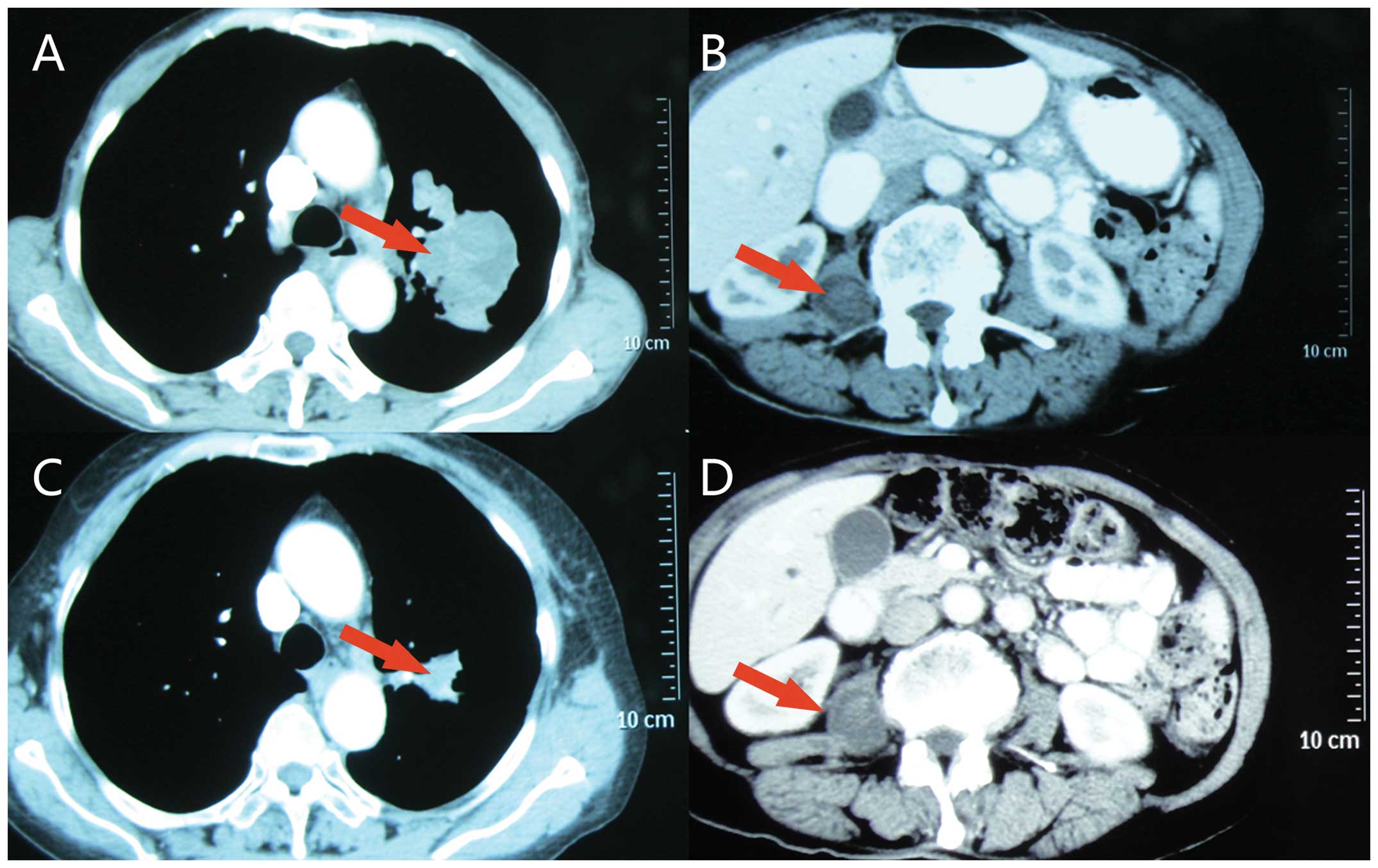
tomography (CT) scan of the chest and abdomen revealed a
heterogeneously enhanced 4×5-cm soft-tissue mass with a lobular and
spiculate boundary in the left upper lobe, numerous enlarged lymph
nodes in the left hilar region and aortopulmonary window. A
low-density, well-circumscribed and homogeneous 2.0×2.8-cm mass
with mild enhancement was also observed inside the right psoas
(Fig. 1A and B). No evidence of
distant metastasis was found upon brain magnetic resonance imaging
(MRI) or skeletal scintigraphy. Based on the aforementioned
examination results, a presumptive clinical diagnosis was
compatible with left NSCLC and right psoas muscle metastasis
(cT2aN3M1b, stage IV) according to American Joint Committee on
Cancer (AJCC) 7th edition staging system for lung cancer (11). The subsequent treatment would be
mainly palliative for the end-stage cancer. However, the diagnosis
of muscle metastasis was uncertain when considering that the
low-density mass with slight enhancement shown by CT did not
correspond with the rich vascularization and rapid metabolism of a
metastatic lesions, and the fact that there were no abnormalities
in the organs susceptible to metastasis, such as the liver and
adrenal glands.
To ensure the correct diagnosis for the two lesions,
a bronchoscopy and CT-guided puncture biopsy was conducted
sequentially. The histology of the lung specimen showed
characteristic features of squamous cell lung carcinoma, including
mitosis, nuclear hyperchromatism, keratin pearls and intercellular
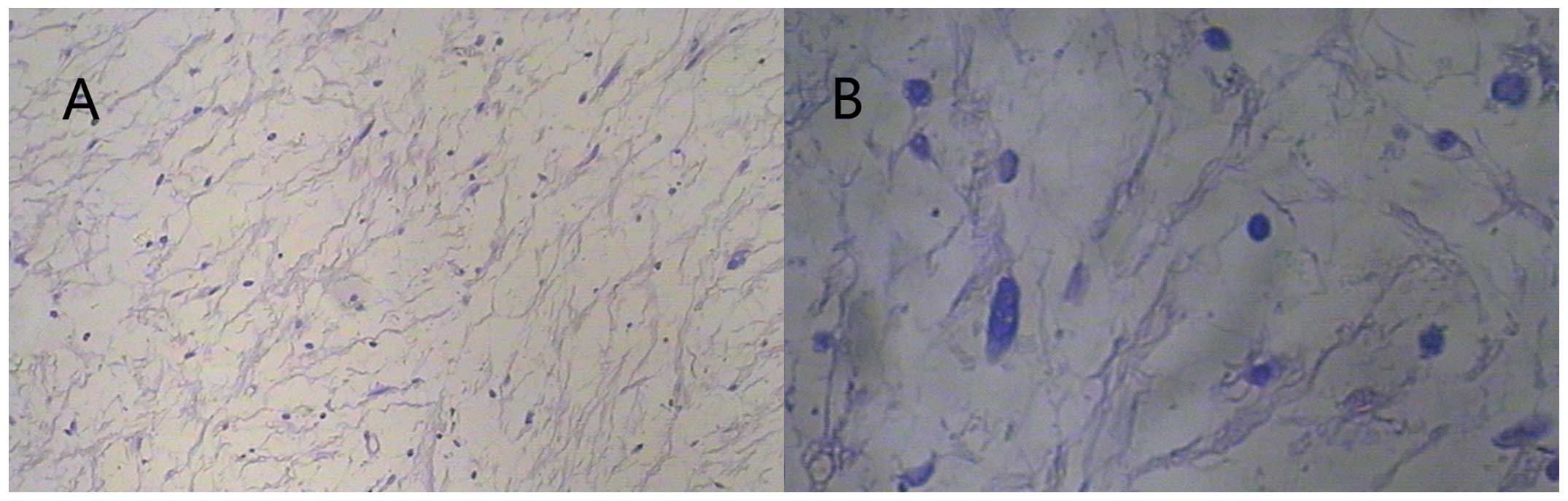
bridges, as expected. However, microscopic analysis of the right
psoas muscle specimen showed delicate, monotonous spindle-cells
loosely arranged in an abundant myxoid matrix, without evidence of
mitotic activity, hemorrhage or necrosis, consistent with IMM
(Fig. 2A and B). Thus, the definitive
diagnosis was left lung squamous cell carcinoma (cT2aN3M0, stage
IIIB) with concomitant IMM arising in the right psoas (11).
For the IMM, although a wide local resection is the
most effective treatment and the prognosis for this treatment is
excellent; since the patient was elderly, the lesion was benign and
there was the presence of highly malignant comorbidity,
conservative treatment, consisting analgesics for oral use with
regular follow-up was recommended. Concurrent chemoradiotherapy
(CRT) is recommended as the best treatment strategy for NSCLC in
National Comprehensive Cancer Network guidelines (12), however, it may not have been well
tolerated by the patient when considering the poor performance
status (Karnofsky performance status score, 70). In fact, the
patient was administered sequential CRT for the primary lung cancer
consisting of 1,250 mg/m2 gemcitabine on days 1 and 8,
plus 75 mg/m2 cisplatin on day 1, every 21 days for 4
cycles, followed by definitive 3-dimensional conformal radiotherapy
with a total dose of 60 Gy in 2 Gy daily fractions. The primary
lung cancer lesion and metastatic lymph nodes were reduced
slightly, and the symptoms associated with lung cancer were
relieved after 2 cycles of chemotherapy and further improved once
the chemotherapy plan was completed. The efficacy of treatment was
assessed as partial remission. Subsequent to half a year of
follow-up, the symptoms of coughing, dyspnea and left chest pain
were completely resolved. The right lumbar pain still existed, but
was relieved by analgesics. The serum Cyfra21-1 level was decreased
to normal at 1.15 ng/ml. The CT reexamination demonstrated that the
lesion size in the left lung had significantly decreased to 1.5×2.0
cm compared with pre-treatment and that there was no marked change
of the IMM (Fig. 1C and D). The
patient received supportive care and was followed up every 3
months. Ten months later, a skeletal scintigraphy revealed the
presence of bone metastases. One month later, 17 months after
diagnosis, the patient succumbed to the disease.
Discussion
In the present case, the chest discomfort and lumbar
pain appeared almost at the same time. The abnormal lesions in the
left lung and right psoas were detected by CT simultaneously. The
CT manifestation of the lung lesion and the serum specific
biomarker of the tumor supported the diagnosis of NSCLC. However,
the nature of the psoas lesion could not be ascertained. Although
the CT presentation did not conform to the typical skeletal muscle
metastasis, which is rather rare, this suspicious diagnosis could
not be ruled out solely relying on the imaging findings,
particularly considering that NSCLC is prone to distant metastases
(7). The differential diagnosis
should include intramuscular metastasis and primary soft-tissue
tumors, as each can arise from the psoas muscle and cause lumbar
pain (13–15). Furthermore, the definition of the
nature of the muscle abnormality would be of great value, as the
correct diagnosis would affect the following clinical management
and vary the prognosis of the patient. The aspects mentioned next
may be beneficial in making a differential diagnosis.
With regard to the epidemiology, a large portion of
patients suffering from NSCLC are diagnosed at an advanced stage
with distant metastases. The most commonly involved sites are the
liver (33–40%), abdominal lymph nodes (29%), adrenal glands
(18–38%), bones (19–33%), brain (15–43%) and kidneys (16–23%)
(16). In general, hematogenous
skeletal muscle metastasis is rare and the metastasis of lung
cancer is even rarer, although it is the most common source
(17). The accurate incidence of
skeletal muscle metastasis is uncertain, although has been reported
as 0.8% in patients with cancer and 1% in patients with lung cancer
through autopsy findings. Psoas metastases are extremely rare, with
only few cases having been reported (17,18). The
trunk musculature (particularly the paravertebral and iliopsoas
muscles) and the thigh muscles are the most frequent sites
(19).
IMM, however, is much more rare (3). IMM generally occur in the large muscles
of the thighs, shoulders, buttocks or upper arm, in order of
descending frequency (20). These
rare lesions mainly occur between the fourth and sixth decades of
life, with a slight predominance in women due to unknown reasons
(4). As no capsule encompasses them,
the lesions may infiltrate the adjacent tissues occasionally
without metastasis (21). Only four
cases of psoas IMM have been reported in the literature to date
(14,22–24), and
no case has been recorded to be concomitant with malignancy. So,
when this does occur, the abnormality is difficult to diagnose as
IMM rather than a metastasis.
The most frequent clinical manifestation of muscle
metastasis is local pain, with or without a palpable mass (15). In individual cases, skeletal muscle
metastasis from lung cancer has even presented as the initial
clinical manifestation, so it may be easily misdiagnosed,
particularly when the primary tumor is unknown (25). However, the symptoms of IMM depend
largely on the location and size of the lesion, with presentation
as a solitary slow-growing painless mass, or secondary to
compression of adjacent structures, mainly nerves and vessels,
although ~80% of cases are asymptomatic. Two of the reported cases
in the literature complained of pain in the region associated with
the psoas (14).
IMM and intramuscular metastasis can each arise from
the psoas muscle and cause lumbar pain, as reported in the
literature. Therefore, it is not possible to distinguish between
them based on the presentation only. Their imaging characteristics
are also required for the differential diagnosis. As the tumors are
rare in the clinic, experience to diagnose them is clearly lacking.
Relevant knowledge is mostly obtained from previous case studies.
Intramuscular metastasis usually manifests as a rim-enhancing focus
with a central hypoattenuation likely due to necrosis (26). CT findings of IMM, as shown in
Fig. 1, are a cystic,
well-circumscribed, low-attenuated and homogeneous mass within the
skeletal muscle (20). The mass
exhibits a high content of mesenchyme, with few vascular and no
internal calcifications, and as a result will show mild or no
enhancement following intravenous administration of contrast
medium. The two lesions show similar MRI features of a high signal
intensity on T2-weighted images, and a low or isointense signal
intensity on T1-weighted images (23,27).
Published studies on the use of CT or MRI suggest that an imaging
examination cannot accurately determine the psoas muscle pathology
(28), and the diagnosis of these
lesions is not usually straightforward, even with radiological
imaging. Muscle metastasis can, for example, mimic a benign lesion
such as an abscess, as previously reported (29). As a result, the value of
histopathological evaluation is highlighted in the differential
diagnosis.
For histopathological evaluation, image-guided
biopsy and fine-needle aspiration cytology are frequently required
to confirm the nature of the lesions. For IMM, microscopic
examination shows a hypocellular and hypovascular tumor with
undifferentiated bland stellate or spindle-shaped cells, separated
by reticulin fibers in abundant myxoid matrix of hyaluronic acid
sparse. It shows the benign characteristics with the absence of
mitosis, nuclear atypia and necrosis (20,24). The
majority of lesions infiltrate into the adjacent muscles, as the
delicate pseudocapsules are incomplete on close inspection
(30). On immunohistochemical
staining, IMM cells stain positively for vimentin, and variably for
CD34 and actin; immunostain for S-100 protein is typically
negative, unlike in myxoid liposarcoma and neurothekeoma (nerve
sheath myxoma) (30). As the
metastatic lesion is transferred hematogenously, it will show
malignant characteristics, such as mitosis, nuclear atypia,
necrosis and specific immunohistochemical results corresponding
with the primary tumor site (31). In
the present study, a rather rare lesion, namely an IMM, was
unexpectedly found in the psoas, with left lung squamous cell
carcinoma.
If a diagnosis of muscle metastasis is ascertained,
it may be the end-stage presentation of lung cancer portending a
poor prognosis. There has been no unified optimal strategy put
forward for skeletal muscle metastases. Although chemotherapy,
radiotherapy and even surgical excision may be applied for local
control or palliation, it is unknown whether these therapeutic
methods can achieve a survival benefit (32). In fact, the majority of patients with
skeletal muscle metastasis from lung cancer succumb within a few
months, despite various forms of treatment being administered
(33). On the other hand, lung cancer
at stage IIIB should receive chemotherapy and definitive
radiotherapy. In the present study, the anticancer treatment was
not affected by the coexistence of IMM. The dominating treatment
for IMM is wide local excision for its absolutely benign nature. A
recent retrospective study of IMM over 10 years found no recurrence
after resection (34). The prognosis
of IMM is excellent if the tumor is completely excised with a
pathological safe margin.
In conclusion, this report represents the first case
of lung cancer coexisted with IMM, which was initially suspected to
be intramuscular metastasis. The two lesions are quite rare in
daily clinical work, so there is a lack of experience for forming a
differential diagnosis. When a soft-tissue lesion is concomitant
with malignant carcinoma, a primary soft-tissue tumor and muscle
metastasis should be considered. In the present case, the nature of
the lesion in the psoas muscle determined the lung cancer stage and
significantly affected the following treatment strategy. Although
imaging studies can offer certain characteristic information,
histopathological examination remains the gold standard and its
importance should be highlighted. This case indicates that extreme
caution is essential in forming a diagnosis when a soft-tissue
lesion is concomitant with malignancy. IMM should be considered in
the differential diagnosis.
Acknowledgements
The authors would like to acknowledge Shandong
Cancer Hospital and Institute for providing the details of this
rare case.
References
|
1
|
Stout AP: Myxoma, the tumor of primitive
mesenchyme. Ann Surg. 127:706–719. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allen PW: Myxoma is not a single entity: A
review of the concept of myxoma. Ann Diagn Pathol. 4:99–123. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heymans O, Gebhart M, Alexiou J, de Saint
Aubain N and Larsimont D: Intramuscular myxoma. Acta Chir Belg.
98:120–122. 1998.PubMed/NCBI
|
|
4
|
Enzinger FM: Intramuscular myxoma; A
review and follow-up study of 34 cases. Am J Clin Pathol.
43:104–113. 1965.PubMed/NCBI
|
|
5
|
Charron P and Smith J: Intramuscular
myxomas: A clinicopathologic study with emphasis on surgical
management. Am Surg. 70:1073–1077. 2004.PubMed/NCBI
|
|
6
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwas HH, Zendah I and Ghedira H: Skeletal
muscle metastases from lung cancer. Asian Cardiovasc Thorac Ann.
21:741–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pop D, Nadeemy AS, Venissac N, Guiraudet
P, Otto J, Poudenx M and Mouroux J: Skeletal muscle metastasis from
non-small cell lung cancer. J Thorac Oncol. 4:1236–1241. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falavigna A, Righesso O, Volquind D and
Teles AR: Intramuscular myxoma of the cervical paraspinal muscle.
Eur Spine J. 18:245–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ripalda E, Beni R, Reguero ME, Nistal J
and Carda P: Recurrent Intramuscular Psoas Myxoma. Am Surg.
75:862–863. 2009.PubMed/NCBI
|
|
11
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC and
Paulus R: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sudo A, Ogihara Y, Shiokawa Y, Fujinami S
and Sekiguchi S: Intramuscular metastasis of carcinoma. Clin Orthop
Relat Res. 296:213–217. 1993.PubMed/NCBI
|
|
14
|
Ruiz-Tovar J, Ripalda E, Beni R, Reguero
ME, Nistal J and Carda P: Recurrent intramuscular psoas myxoma. Am
Surg. 75:862–863. 2009.PubMed/NCBI
|
|
15
|
Damron TA and Heiner J: Distant soft
tissue metastases: A series of 30 new patients and 91 cases from
the literature. Ann Surg Oncol. 7:526–534. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quint LE, Tummala S, Brisson LJ, Francis
IR, Krupnick AS, Kazerooni EA, Iannettoni MD, Whyte RI and Orringer
MB: Distribution of distant metastases from newly diagnosed
non-small cell lung cancer. Ann Thorac Surg. 62:246–250. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ampil FL, Lall C and Datta R: Palliative
management of metastatic tumors involving the psoas muscle: Case
reports and review of the literature. Am J Clin Oncol. 24:313–314.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strauss JB, Shah AP, Chen SS, Gielda BT
and Kim AW: Psoas muscle metastases in non-small cell lung cancer.
J Thorac Dis. 4:83–87. 2012.PubMed/NCBI
|
|
19
|
Plaza JA, Perez-Montiel D, Mayerson J,
Morrison C and Suster S: Metastases to soft tissue: A review of 118
cases over a 30-year period. Cancer. 112:193–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphey MD, McRae GA, Fanburg-Smith JC,
Temple HT, Levine AM and Aboulafia AJ: Imaging of soft-tissue
myxoma with emphasis on CT and MR and comparison of radiologic and
pathologic findings. Radiology. 225:215–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bancroft LW, Kransdorf MJ, Menke DM,
O'Connor MI and Foster WC: Intramuscular myxoma: Characteristic MR
imaging features. AJR Am J Roentgenol. 178:1255–1259. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tassart M, Roussy M, Boidart F and Tricot
JF: Muscular myxoma. A case of myxoma of the psoas. J Radiol.
74:147–150. 1993.PubMed/NCBI
|
|
23
|
van Roggen JF, McMenamin ME and Fletcher
CD: Cellular myxoma of soft tissue: A clinicopathological study of
38 cases confirming indolent clinical behaviour. Histopathology.
39:287–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dormand EL, Prabhu-Desai A, Rice AJ and
Rosin RD: Not all pain in the left iliac fossa is diverticular
disease: A case study of a psoas myxoma and review. Surgeon.
4:239–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haraguchi N, Yamamoto Y, Sasaki K, Satoh H
and Sekizawa K: Muscle metastases as initial manifestation of lung
cancer. Clin Oncol (R Coll Radiol). 16:586–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pretorius ES and Fishman EK: Helical CT of
skeletal muscle metastases from primary carcinomas. AJR Am J
Roentgenol. 174:401–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams JB, Youngberg RA, Bui-Mansfield
LT and Pitcher JD: MR imaging of skeletal muscle metastases. AJR Am
J Roentgenol. 168:555–557. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang WT, Yeo W and Metreweli C: Imaging of
iliopsoas metastasis. Clin Radiol. 54:85–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart IC, Blaikie KJ and MacLeod HM:
Adenocarcinoma of unknown primary site (ACUPS) presenting as a
psoas abscess. Scott Med J. 34:4701989.PubMed/NCBI
|
|
30
|
Ozawa H, Fujii M, Tomita T and Ogawa K:
Intramuscular myxoma of scalene muscle: A case report. Auris Nasus
Larynx. 31:319–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Molina-Garrido MJ and Guillén-Ponce C:
Muscle metastasis of carcinoma. Clin Transl Oncol. 13:98–101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Alao BS, Westrup J and Shuhaibar MN:
Non-small-cell lung cancer: Unusual presentation in the gluteal
muscle. Gen Thorac Cardiovasc Surg. 59:382–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McKeown PP, Conant P and Auerbach LE:
Squamous cell carcinoma of the lung: An unusual metastasis to
pectoralis muscle. Ann Thorac Surg. 61:1525–1526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Charron P and Smith J: Intramuscular
myxomas: A clinicopathologic study with emphasis on surgical
management. Am Surg. 70:1073–1077. 2004.PubMed/NCBI
|