Introduction
The cancer stem-like cell (CSC) theory hypothesizes
that tumors contain a small subpopulation of cancer cells that
share numerous properties with normal stem cells, including
proliferative potential and self-renewal (1). These rare stem-like tumor initiators are
considered to be associated with initiating and maintaining the
growth of tumors, and may be responsible for the local recurrence
and distant metastasis of tumors (1).
Previous studies have indicated that CSCs exist in numerous human
tumors, including hematopoietic cancer (2), brain tumors (3), breast cancer (4), melanoma (5) and bone sarcoma (6,7). Advanced
methods and techniques have contributed to the identification of
CSCs (2). These methods include the
detection of specific surface markers that are selectively
expressed on CSCs, but not on the majority of tumor cells,
serum-free suspension culture medium for colony formation in
vitro and a unique pattern of staining with certain dyes,
including Hoechst 33342, for detecting side population (SP) cells
(2–5):
The characteristics of side population cells include proliferative
potential and self-renewal.
Previous studies have revealed that specific surface
molecules, including cluster of differentiation (CD)133 and CD44,
may be markers for certain CSC populations (8–11). Several
studies have indicated that CSCs may demonstrate the ability of
increased resistance to chemotherapy, due to the high expression of
specific drug transporters, including multidrug resistance protein
1 and adenosine triphosphate (ATP)-binding cassette (ABC)
sub-family G member 2 (ABCG2) (12,13).
Certain cell enzymes have also been demonstrated to be useful
molecules for the selection and detection of CSCs, and aldehyde
dehydrogenase (ALDH) 1 is one of the possible candidates for a stem
cell marker that may be used for the isolation of CSCs from tumors
in cancers that include leukemia, breast cancer and sarcoma
(14–16).
Although the mechanisms of drug resistance in CSCs
are poorly understood, previous studies have revealed that these
may be associated with the ABC drug transporters (17,18).
CSC markers remain limited, but the sphere culture
system is particularly useful as a functional approach to enrich
the potential CSC subpopulations which including proliferative
potential and self-renewal. (6). The
sphere culture system, which comprises stressful growth conditions
of serum starvation and anchorage independence, is frequently used
to identify and enrich stem and progenitor cells by eliminating the
differentiated cells that are unable to survive (6). Reynolds and Weiss (19) first employed this system to
demonstrate that the adult mammalian brain contained cells that
gave rise to neurosphere clones. This method is also termed the
neurosphere/sarcomaspere culture system. Previous studies have
demonstrated that spherical forming colonies derived from various
tumors demonstrated stem-like properties with the ability of
self-renewal, increased expression of certain embryonic stem (ES)
genes, and tumorigenicity in mouse models (6,7,20–23). The
present study aimed to detect SP and ALDH+ cell
populations in human fibrosarcoma HT1080 and malignant fibrous
histiocytoma (MFH) NMFH-1 cells.
Materials and methods
Cell lines and culture
The human synovial sarcoma SW982 cells and
fibrosarcoma HT1080 cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA). The novel myxofibrosarcoma
NMFH-1 cell line, which was considered to be a myxoid variant of
MFH, was provided by Dr. Akira Ogose (Division of Orthopedic
Surgery, Niigata University Graduate School of Medical and Dental
Sciences, Niigata, Japan) (24). The
SW982 cells were maintained in Leibovitz's L-15 medium (Gibco Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; HyClone, Logan, UT, USA), the HT1080 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco Life
Technologies) supplemented with 10% FBS, and the NMFH-1 cells were
maintained in RPMI-1640 medium (Gibco Life Technologies)
supplemented with 10% FBS. All cells were maintained at 37°C in a
5.0% CO2 atmosphere.
Identification of SP cells
The cell suspensions were labeled with Hoechst 33342
dye (Sigma-Aldrich, St. Louis, MO, USA), using the method described
by Goodell et al (25).
Briefly, the cells were trypsinized and re-suspended in pre-warmed
Leibovitz's L-15 medium, DMEM or RPMI-1640 medium supplemented with
5% FBS at a concentration of 1×106 cells/ml. Hoechst
33342 dye was added at a final concentration of 5.0 µg/ml in the
presence or absence of 50 µM verapamil (Sigma-Aldrich), which acts
as an inhibitor of the ABC transporter. The cells were incubated at
37°C for 90 min with continuous agitation. At the end of the
incubation, the cells were washed with ice-cold PBS supplemented
with 5% FBS, centrifuged at 4°C and resuspended in ice-cold PBS
containing 5% FBS. Flow cytometry was performed using BD FACSAria
II (BD Biosciences, Franklin Lakes, NJ, USA). The Hoechst 33342 dye
was excited at 357 nm and the fluorescence was analyzed using a
dual wavelength (blue, 402–446 nm; red, 650–670 nm).
Spherical colony formation assay
Monolayer cells at ~70% confluence in RPMI medium
supplemented with 10% FBS were dissociated into single-cell
suspensions using 0.25% trypsin and 0.05% EDTA (Sigma-Aldrich). The
cells were then inoculated into B27-supplemented RPMI-1640 medium
and 1% methylcellulose medium mixed with 10 ng/ml human recombinant
epidermal growth factor (EGF) and 10 ng/ml basic fibroblast growth
factor (βFGF) at a cell density of 6×104 cells per well
in ultra low attachment 6-well plates (Corning Inc., Corning, NY,
USA). EGF and βFGF were purchased from PeproTech, Inc. (Rocky Hill,
NJ, USA).
Fresh aliquots of EGF and βFGF were added every
other day. Subsequent to 7–12 days of culture, the colonies that
contained >40 cells were quantitated by inverted phase contrast
microscopy (Olympus CKX41; Olympus, Tokyo, Japan). Spherical
colonies were dissociated and re-introduced into 96-well ultra low
attachment plates at least 5 times, in normal medium and in
anchorage-independent methylcellulose medium, to investigate the
self-renewal ability of the cells through secondary sphere
formation.
Aldefluor assay and detection of the
ALDH+ subpopulations by FACS
The Aldefluor (StemCell Technologies, Inc.,
Vancouver, BC, Canada) was used to detect cell populations with
high ALDH enzymatic activity. The cells were labeled with Aldefluor
reagent, according to the manufacturer's instructions. Briefly,
cultures of NMFH-1 and SP cells were harvested by trypsin-EDTA and
resuspended in Aldefluor assay buffer, containing 1 µmol/l of the
ALDH substrate BODIPY aminoacetaldehyde (StemCell Technologies,
Inc.) per 1×106 cells. The cells were then incubated for
30 min at 37°C. As a negative control for each sample of cells, an
aliquot was treated with 50 mmol/l 4-diethylaminobenzaldehyde
(DEAB), an ALDH-specific inhibitor (StemCell Technologies, Inc.).
Flow cytometry was performed using BD FACSAria.
Assay to determine sensitivity to
chemotherapy drugs, with or without verapamil
To assess the sensitivity of NMFH-1 cells to
cisplatin (CDDP) and doxorubicin (DXR), which are frequently used
for chemotherapy in patients with sarcoma, the NMFH-1 cells were
dissociated and inoculated into 96-well microtiter plates (Corning
Inc.) at a concentration of 2,000 cells/90 µl/well. The cells were
allowed to attach to the plates in RPMI-1640 supplemented with 10%
FBS at 37°C. Subsequent to 12 h incubation, the cells were then
exposed to various concentrations of CDDP or DXR (1, 5, 10µM) with
or without 50 µM verapamil. Following 48 h incubation, with or
without chemotherapy drugs, the cell viability was measured by MTS
assay using CellTiter 96 Aqueous One Solution Cell Proliferation
Assay reagent (Promega, Madison, WI, USA), according to the
manufacturer's instructions. This was compared with the control
cells, which were incubated without drugs. NMFH-1 cells were then
inoculated into 96-well ultra low attachment microplates (Corning,
Inc.) in RPMI-1640 supplemented with B27 with 1% methylcellulose
medium at a concentration of 5,000 cells/90 µl/well for 10 days to
allow sphere formation. The cells were then treated with CDDP or
DXR at a final concentration of 1, 5 or 10 µM, with or without 50
µM verapamil, and subsequently cell viability was measured by MTS
assay following 48 h of treatment.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from frozen packed cells
using the RNeasy Total RNA system (Qiagen GmbH, Hilden, Germany)
and first-strand cDNA was synthesized from 500 ng samples using the
Superscript II RNase H Reverse Transcriptase system (Invitrogen
Life Technologies). PCR was performed using 0.5 µl reaction mixture
as templates. The primer sequences used for the amplification of
the Nanog, Oct3/4, signal transducer and activator of transcription
3 (STAT3), sex determining region Y-box 10 (SOX10) and ABCG2 genes
are listed in Table I. The GAPDH gene
was used as an internal control to adjust the quantities of the
template. Aliquots of amplification products (10 µl) were separated
by electrophoresis in 1.5% agarose gels and visualized by ethidium
bromide staining (Qiagen GmbH, Hilden, Germany).
 | Table I.Primer sets for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sets for reverse
transcription-polymerase chain reaction.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| Nanog |
GCTGAGATGCCTCACACGGAG |
TCTGTTTCTTGACTGGGACCTTGTC |
| Oct3/4 |
TGGAGAAGGAGAAGCTGGAGCAAAA |
GGCAGATGGTCGTTTGGCTGAATA |
| STAT3 |
GGGTGGAGAAGGACATCAGCGGTAA |
GCCGACAATACTTTCCGAATGC |
| SOX10 |
TATATACGACACTGTCCCGGC |
AGTGTGGGTGCAACAGTCAAC |
| ABCG2 |
ACCTGAAGGCATTTACTGAA |
TCTTTCCTTGCAGCTAAGAC |
| GAPDH |
CAGCCGAGCCACATCG |
TGAGGCTGTTGTCATACTTCT |
Western blotting
The cells were lysed in 50 mM Tris-HCl (pH 7.4), 150
mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 1% Na-deoxycholate, 1 mM
Na-vanadate, and protease inhibitors consisting of 5 mg/ml
pepstatin, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml leupeptin
and 1 mM NaF (Sigma-Aldrich) for 1 h in ice. Following
centrifugation at 13,000 × g for 10 min at 4°C, the protein
concentration of the supernatants was measured using a
bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA).
The lysates were mixed with Laemmli buffer (dilution, 1:1;
Sigma-Aldrich). In total, 50 µg of protein per lane was
electrophoresed in 10% SDS polyacrylamide gels and transferred onto
polyvinylidene difluoride membranes (Sigma-Aldrich). The membranes
were blocked with non-fat milk for 1 h at room temperature and
incubated overnight at 4°C with rabbit monoclonal anti-CD44
(dilution, 1:2,000; ab51037, Abcam, Cambridge, MA, USA), rabbit
polyclonal anti-CD133 (dilution, 1:1,000; SAB2107606,
Sigma-Aldrich) and rabbit polyclonal IgG anti-GAPDH (dilution,
1:5,000; sc-25778, Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
in 5% bovine albumin (Sigma-Aldrich), Tris-buffered saline (TBS)
and 0.1% Tween-20 (Bio-Rad Laboratories, Hercules, CA, USA).
Subsequent to being washed three times in TBS with 0.1% Tween-20,
the blots were incubated with Goat anti-rabbit IgG (5366S; Cell
Signaling Technology, Danvers, MA, USA; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). Immunoreactive bands were
detected by ECL Plus SuperSignal West Pico (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 60 sec.
Statistical analysis
The data are expressed as the mean ± standard
deviation. The χ2 test and Fisher's exact test were used
where appropriate (n>40 and n<40, respectively). All
statistical analyses were performed using SPSS 13.0 statistical
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
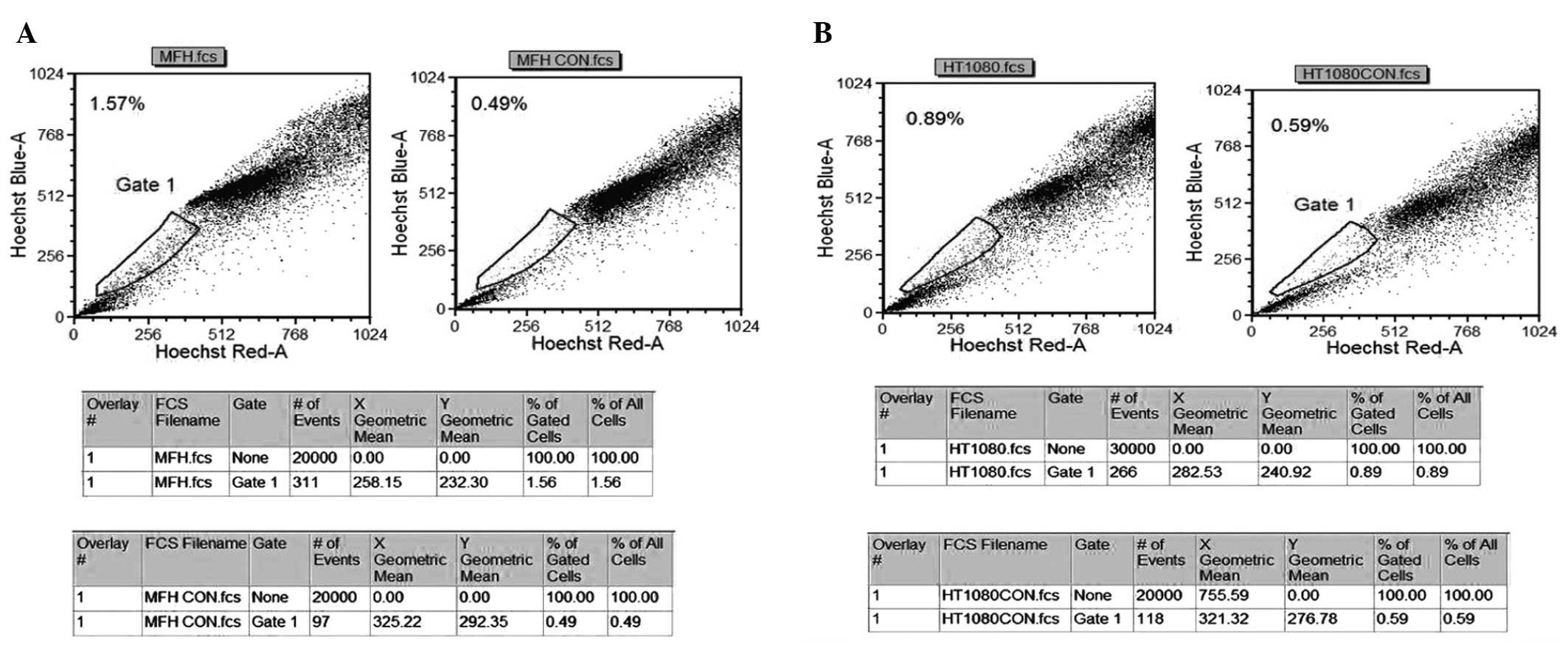
Detection of SP cells in human sarcoma
cell lines
The present study aimed to detect the proportion of
the SP cells in bone and soft tissue sarcoma cell lines. The NMFH-1
(Fig. 1A) and HT1080 (Fig. 1B) cell lines consisted of 0.3 and
1.08% SP cells, respectively. In each cell line, the percentage of
SP cells was markedly diminished by treatment with verapamil, which
is an inhibitor of the protein pumps, such as ABCG2, responsible
for the exclusion of Hoechst 33342 dye, indicating that this
population accurately indicated the proportion of SP cells.
However, staining of the synovial sarcoma SW982 cells did not
reveal the presence of SP cells, which was either due to
inappropriate culture conditions or the cells lacking a stem cell
population that was able to be defined. Therefore, the NMFH-1
cells, which contained the highest proportion of SP cells, was
selected and underwent additional analysis.
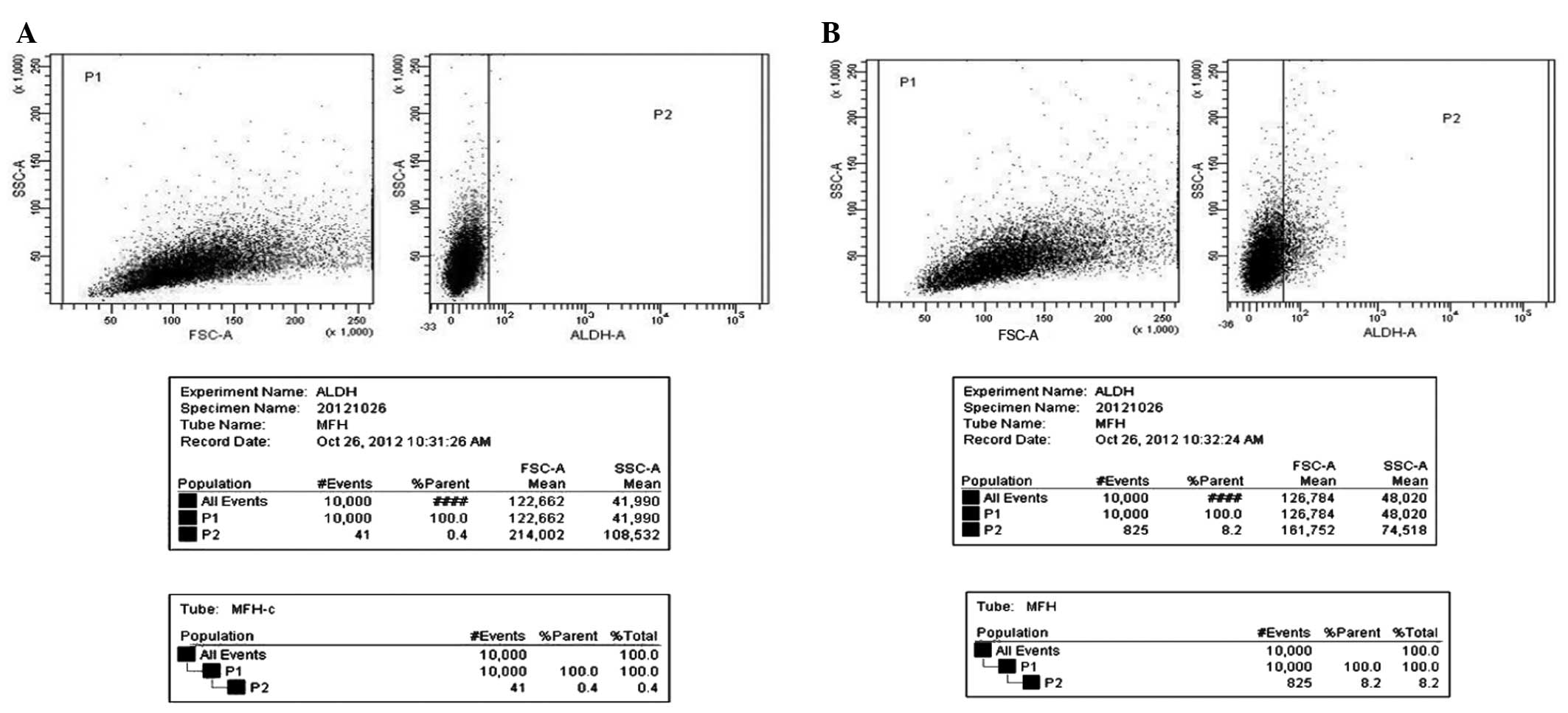
ALDH+ populations of NMFH-1
cells
The presence and size of the cell populations that
demonstrated ALDH enzymatic activity were assessed in NMFH-1 cells
using an Aldefluor assay. The results revealed that NMFH-1 cells
contained populations of cells exhibiting ALDH activity, with a
frequency of 8.2% in NMFH-1 cells (Fig.
2A), compared to the frequency of 0.4% in the control NMFH-1
cells treated with the ALDH inhibitor DEAB (Fig. 2B).
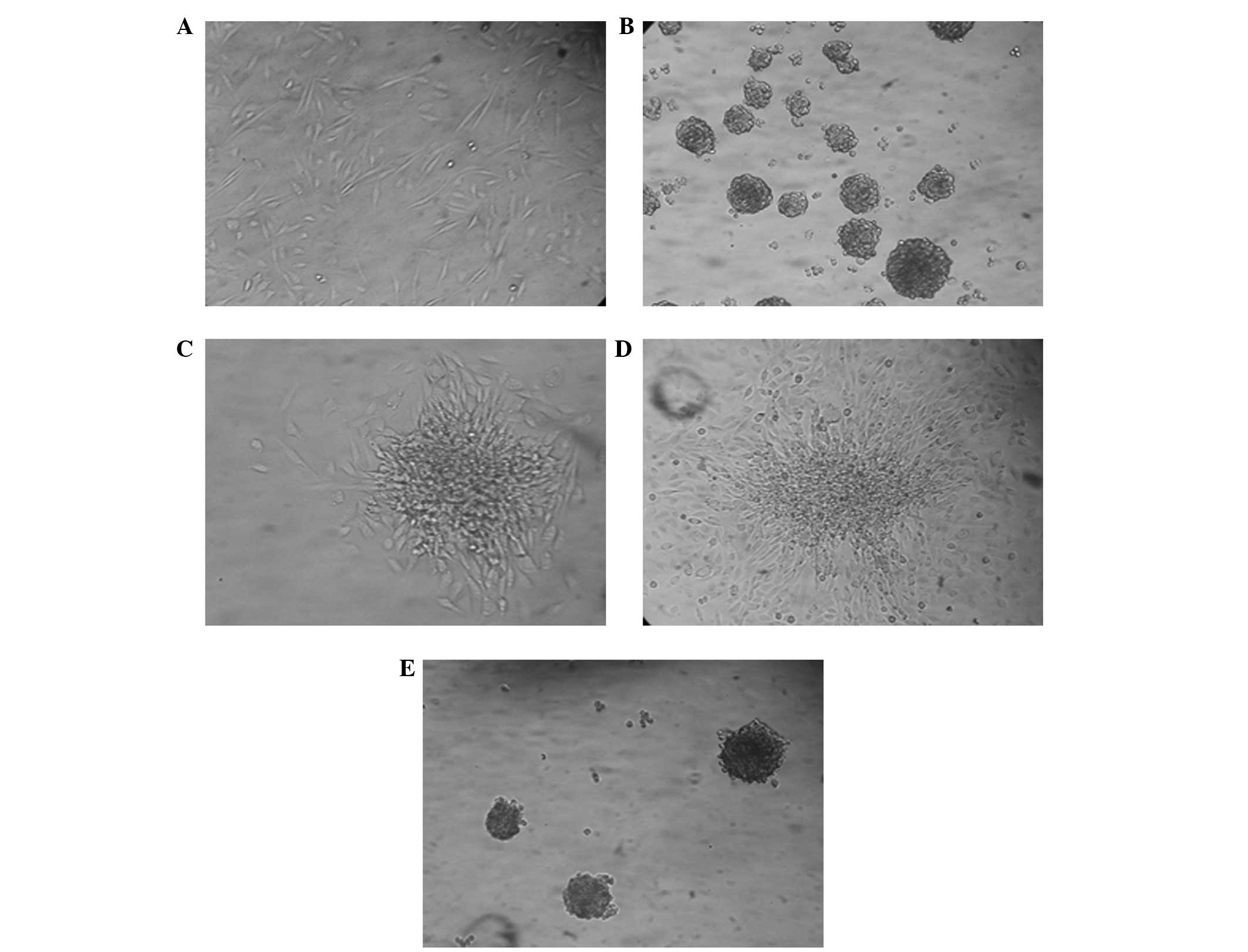
Spherical colony formation in NMFH-1
cells
The ability of NMFH-1 cells to generate spherical
clones and self-renew was evaluated in a serum-starved culture
assay. To investigate cell self-renewal, cultured spheres were
dissociated into single cells and allowed to grow in monolayer
culture and serum-starved anchorage-independent conditions with 1%
methylcellulose medium: This process was repeated twice. The
spherical colonies of NMFH-1 cells revealed expansion in the
monolayer culture, which led to cell differentiation and
self-renewal through the secondary formation of spherical colonies
(Fig. 3).
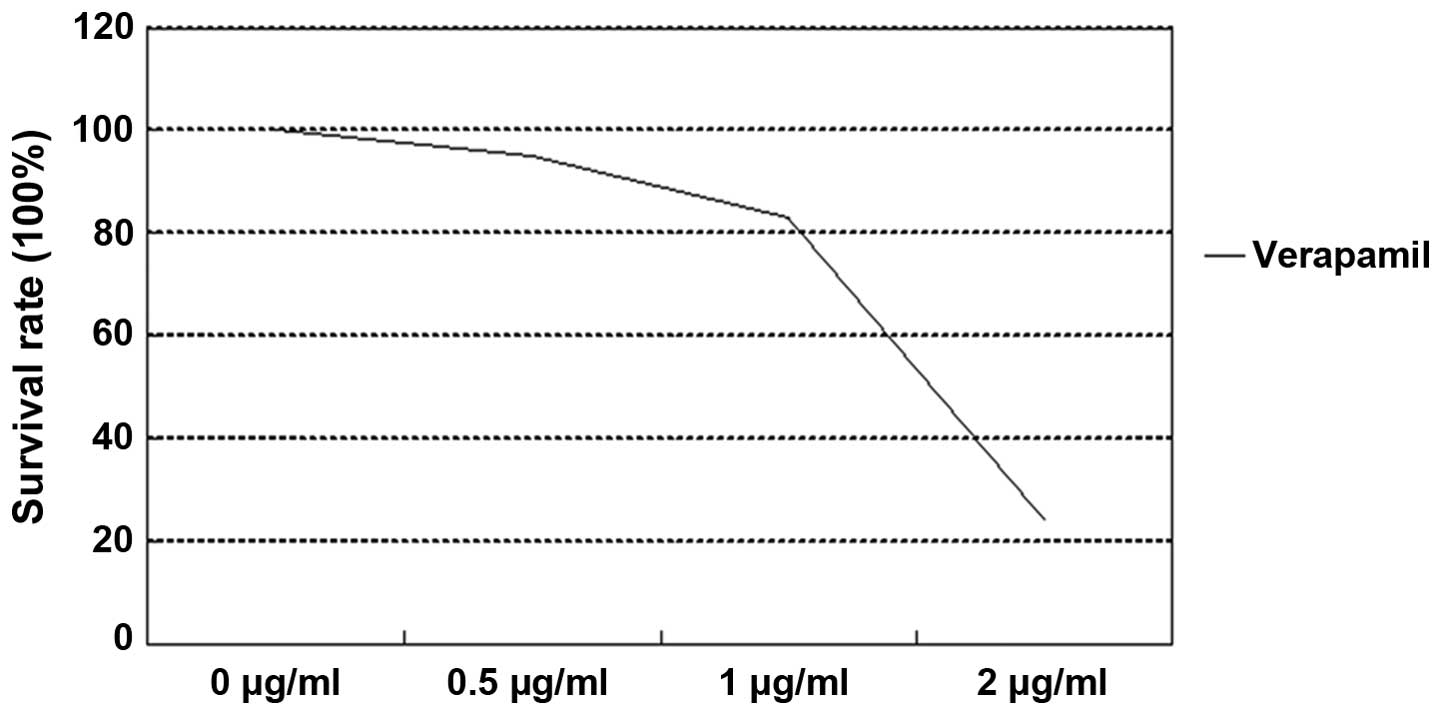
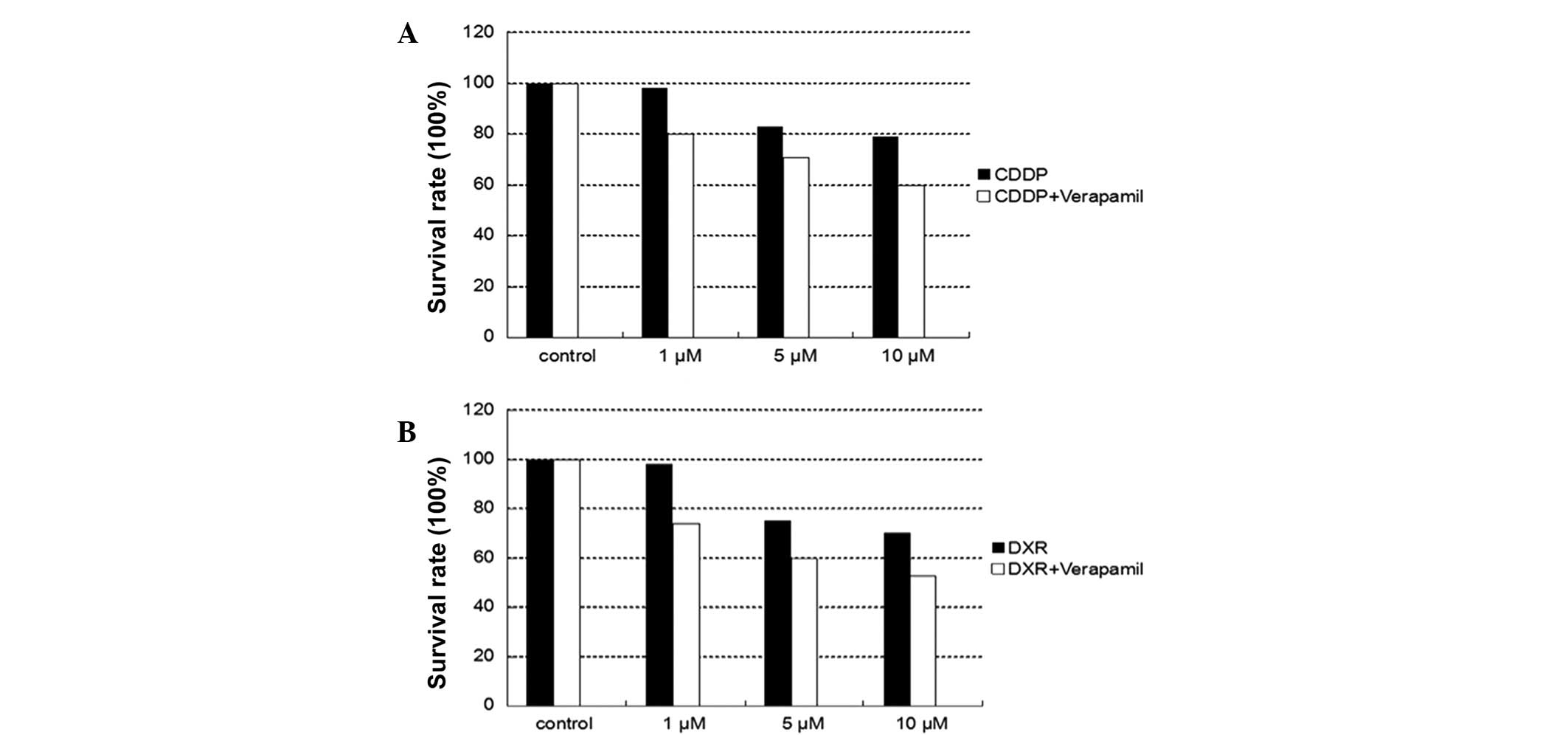
Chemotherapy drug resistance between
NMFH-1 and spherical colonies
CDDP and DXR inhibited the growth of NMFH-1 cells in
a dose-dependent manner. The survival rates of adherent and
spherical colonies subsequent to 48-h drug treatment are shown in
Table II and Figs. 4–6. The
difference in the growth inhibition rate between the adherent and
spherical colonies was statistically significant (P=0.0003), as 10
µM of CDDP inhibited cell growth by 50% in NMFH-1 cells in
monolayer culture and 23% in sphere conditions, respectively. In
addition, 10 µM DRX inhibited cell growth by 58% in adherent
conditions and by 31% in spherical colony conditions. These results
suggest that the spherical colonies are resistant to CDDP and DXR,
which are the most commonly available chemotherapy drugs for
sarcomas. The application of verapamil, an ABCG2 inhibitor, in
combination with either CDDP or DXR resulted in increased cell
growth inhibition compared with non-verapamil treatment. However,
the growth inhibition rates were limited to only 26.8% for CDDP
combined with verapamil and 31.1% for DXR combined with verapamil
in adherent conditions. In spherical conditions, the growth
inhibition rates were 19.4% for CDDP combined with verapamil and
25.5% for DXR combined with verapamil.
 | Table II.Cell survival rates of NMFH-1 cells
subsequent to 48 h treatment with CDDP, with or without 50 µM
verapamil. |
Table II.
Cell survival rates of NMFH-1 cells
subsequent to 48 h treatment with CDDP, with or without 50 µM
verapamil.
|
| Cell survival rate,
% |
|
|
|---|
|
|
|
|
|
|---|
| Treatment | Adherent
colony | Spherical
colony | χ2 | P-value |
|---|
| CDDP |
|
|
|
|
| 1
µM | 83.91±3.98 | 98.97±0.40 | 446 | 0.0004 |
| 5
µM | 64.38±11.15 | 87.80±12.10 | 337 | 0.0024 |
| 10
µM | 50.17±13.57 | 83.76±14.05 | 279 | 0.0003 |
| CDDP +
verapamil |
|
|
|
|
| 1
µM | 64.18±14.37 | 96.39±6.21 | 370 | 0.0001 |
| 5
µM | 46.29±14.68 | 74.40±16.62 | 259 | 0.0016 |
| 10
µM | 43.39±12.01 | 69.40±16.09 | 241 | 0.0001 |
Expression of Nanog, Oct3/4, STAT3,
SOX10 and ABCG2
The expression of the STAT3, Nanog, Oct3/4, Sox10
and ABCG2 genes, all of which are associated with the marker genes
of pluripotent ES cells, was investigated by semi-quantitative
RT-PCR analysis to determine whether these genes were expressed in
adherent and sphere formation conditions. All five genes were
expressed in spherical NMFH-1 cell colonies, and the spheres
consistently demonstrated increased expression of these genes, with
the exception of Nanog, compared with adherent cells (Fig. 7). ABCG2 encodes a membrane efflux
transporter that is expressed in human hematopoietic stem cells
(2), and the gene is also associated
with chemotherapy resistance (18,25). The
protein encoded by ABCG2 functions as a xenobiotic transporter,
which may play a major role in multi-drug resistance. The ABCG2
protein is likely to act as a cellular defense mechanism in
response to exposure to mitoxantrone and anthracycline (26). ABC transporters have the capacity to
export numerous chemotherapy agents and are upregulated in CSCs
derived from certain cancer cell lines (17,18). In
particular, ABCG2 has been implicated in the high Hoechst 33342 dye
efflux capacity that marks the SP cell phenotype. Therefore, to
investigate abundance of ABC transporters associated with
multi-drug resistance, RT-PCR was used to determine the relative
mRNA expression of ABC transporters in NMFH-1 adherent and
spherical cell colonies.
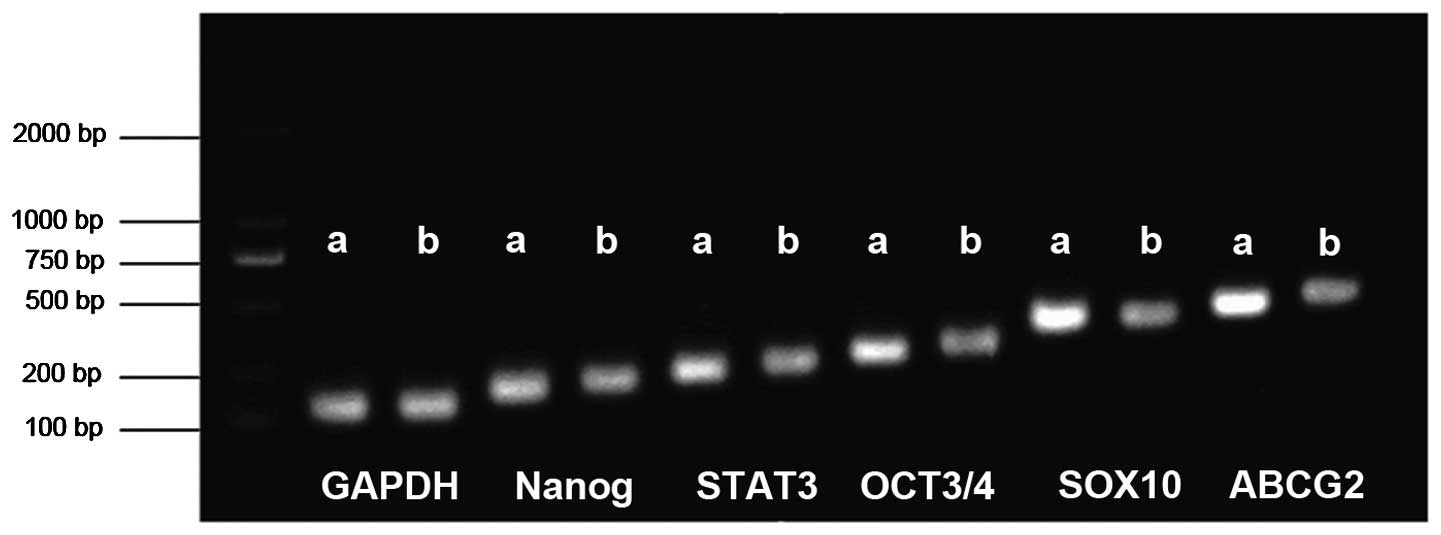 | Figure 7.Expression of Nanog, OCT3/4, STAT3,
SOX10 and ABCG2. Reverse transcription-polymerase chain reaction
revealed strong expression of Nanog, STAT3, OCT3/4, SOX10 and ABCG2
in spherical NMFH-1 cell colonies, compared with the adherent
cells. a, spherical colonies; b, adherent cells; STAT3, signal
transducer and activator of transcription 3; SOX10, sex determining
region Y-box 10; ABCG2, adenosine triphosphate-binding cassette
sub-family G member 2. |
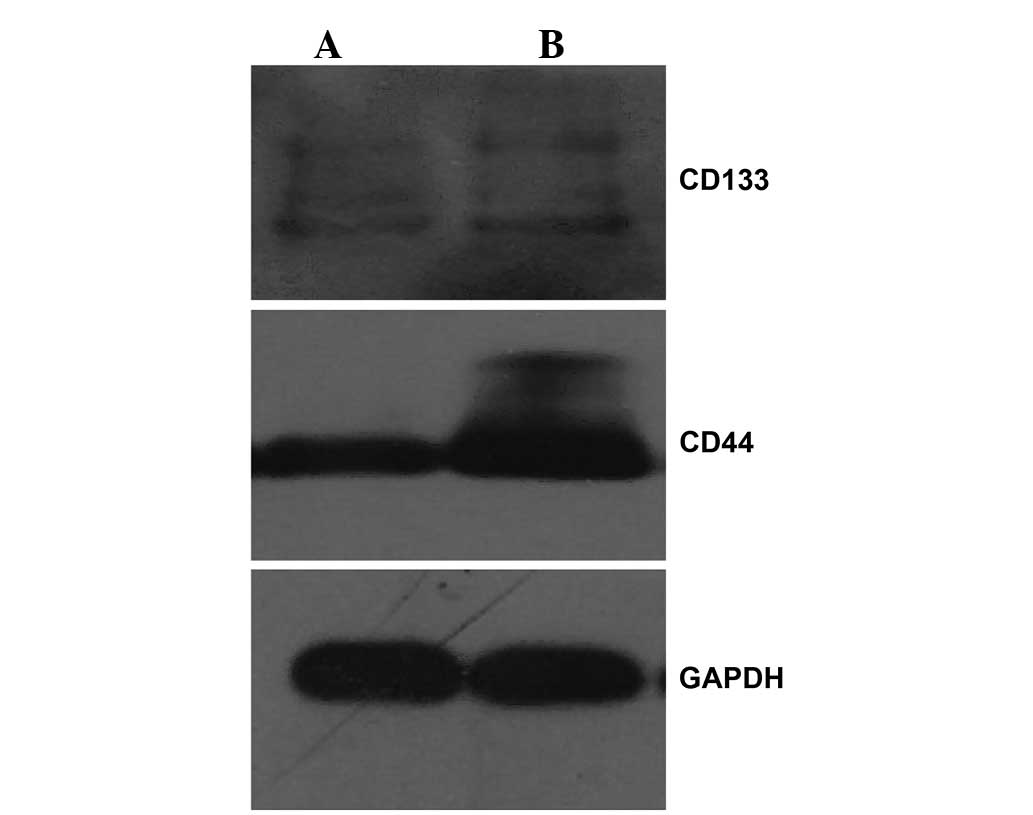
Expression of CD44 and CD133
The expression of the normal stem-associated cell
proteins and candidate CSC markers CD133 and CD44 was examined by
western blotting. The results revealed that the expression of the
two proteins in the spherical colonies was significantly increased
compared with the expression in the adherent NMFH-1 cells (Fig. 8), indicating that the spherical
colonies possess certain stem cell-like properties. However, the
expression of CD44 was considerably increased in the adherent and
spherical colony formation conditions, which suggests that NMFH-1
cells may demonstrate a considerable migratory ability, as CD44
induces a metastatic phenotype in locally growing tumor cells
(27,28). These results indicate that the
spherical colony formation system may increase the expression of
CD44 and CD133 in stem and progenitor cells.
Discussion
In general, there are two models of heterogeneity in
cancer cells (1,29). The first model is that cancer cells of
numerous phenotypes have the potential to proliferate extensively,
and any one cell may have a low probability of exhibiting this
potential in an assay of clonogenicity or tumorigenicity (1). The second model is that the majority of
cancer cells have only limited proliferative potential, and only a
rare subset of cancer cells consistently proliferates extensively
in clonogenic assays and may form novel tumors on transplantation
(29). This model predicts that a
distinct subset of cells demonstrates an increased ability to form
novel tumors, whereas the majority of cells do not possess this
ability. Previous therapeutic failure to successfully treat the
majority of cancers indicates that the second model of
heterogeneity may be the more accurate model. The CSC hypothesis is
consistent with the latter model that tumors contain a small
subpopulation of cancer cells that share numerous stem cell-like
properties, including proliferative potential and self-renewal,
increased or decreased expression of stem cell-associated genes and
cell surface markers. These rare stem cell-like tumor initiators
are considered to be associated with initiating and maintaining the
growth of tumors, and these cells may be responsible for local
recurrence and distant metastasis.
In the present study, the novel myxofibrosarcoma
cell line NMFH-1 was demonstrated to possess the abilities to form
spherical colonies and self-renewal in anchorage-independent,
serum-starved culture conditions, which were previously developed
to isolate cancer stem cells from hepatoma and certain bone
sarcomas (6,7,20,23). The present study found that NMFH-1
adherent cells and spherical colonies expressed key marker genes of
ES cells, consisting of Nanog, STAT3, Oct3/4 and SOX10. However,
increased expression of STAT3, Oct3/4 and SOX10 was identified in
spherical colonies compared with the adherent cultures. These genes
play important roles in ES cells. Nanog maintains self-renewal in
ES cells (30,31), STAT3 plays important roles in
regulating cell growth, differentiation, apoptosis, angiogenesis
and immune responses (32), SOX10 is
involved in the regulation of embryonic development and the
determination of cell fate (33), and
Oct3/4 is a POU domain, octamer-binding transcription factor that
is expressed in ES cells (34). In
particular, previous studies have revealed that the increased or
decreased expression of these stem cell-associated genes have been
found in numerous primary cancers and CSCs (6,7,21,23,35).
Therefore, certain key marker genes in ES cells may play a role in
sarcoma oncogenesis.
To investigate the CSC properties of spherical
colonies, the sensitivity of spherical colonies to chemotherapy
agents was investigated. The spherical colonies exhibited general
resistance to CDDP and DXR, and demonstrated increased survival
ability compared with the adherent cells. In addition, verapamil,
an ABCG2 inhibitor, in combination with either CDDP or DXR,
demonstrated an increased inhibition of cell growth compared with
non-verapamil treatment. It has been demonstrated that ABC
transporters have the capacity to export numerous chemotherapy
agents and are upregulated in CSCs derived from certain cancer cell
lines (17,18). The ABCG2 protein functions as a
xenobiotic transporter that may play a major role in multi-drug
resistance (2). This protein is
likely to act as a cellular defense mechanism in response to the
exposure of cells to mitoxantrone and anthracycline (26), and is alternatively referred to as a
breast cancer resistance protein (36). The present results indicate that
verapamil may enhance the efficacy of chemotherapy agents by
inhibiting ABCG2 from pumping chemotherapy drugs out of the cells.
However, the ABCG2 inhibitor verapamil may only partially inhibit
the growth adherent cells and spherical colonies. This may be due
to cancer stem cells expressing other drug resistant proteins,
including ABCB1 (37).
Furthermore, the present study demonstrated that the
spherical colony culture system may enrich stem-like cells with the
expression of the ABCG2 gene, according to the present results from
RT-PCR. Previous studies have revealed that the ABCG2 gene is
highly expressed in the placenta, normal stem cells and in certain
tumor stem cells (2,38–40). ABCG2
levels were reduced when stem cells were induced to differentiate.
In particular, ABCG2 has been implicated in the high Hoechst 33342
dye efflux capacity that is characteristic of the SP phenotype, and
was subsequently identified and characterized as a novel stem cell
marker (39). Thus, the spherical
colonies overexpress ABCG2 and are more resistant to CDDP and DXR,
indicating a possible contribution of these cells to cancer
chemoresistance.
Finally, the expression of the candidate CSC markers
CD44 and CD133 was examined in the spherical colonies. The
lymphocyte homing receptor CD44 is expressed in numerous cells and
is considered to be a cell-surface glycoprotein involved in
cell-cell interactions, cell adhesion and migration (41). In particular, CD44 induces a
metastatic phenotype in locally growing tumor cells (27,28). There
is considerable evidence for the contribution of CD44 expression to
the initiation and progression of numerous tumors and CSCs
(42). CD133 is an important
candidate CSC marker that localizes to membrane protrusions and is
often expressed on adult stem cells, where it is hypothesized to
maintaining stem cell properties by suppressing differentiation.
CD133 has been used as a marker for hematopoietic stem cells
(43) and neuronal stem cells
(44). Previously, various studies
have demonstrated that CD133 is associated with the initiation and
progression of cancer stem and progenitor cells, and may be a
valuable CSC marker in a variety of tumors, including epithelial
cancers and solid sarcomas (10,11,45–50).
Therefore, the increased expression of CD44 and CD133 in NMFH-1
spherical colonies may account for the increased survival and
metastatic ability of these cells.
In conclusion, the present study revealed that the
spherical colonies derived from NMFH-1 cells demonstrate stem-like
properties in anchorage-independent conditions, and it was
indicated that spherical colonies may contain CSC subpopulations.
The current findings support the hypothesis that human NMFH-1 cells
are heterogeneous, and that rare cells within the bulk of a tumor
are responsible for the initiation and growth of NMFH-1 lesions.
Due to the current inability to successfully treat the majority of
tumors, the modulations of drug resistance in cancer chemotherapy
may be promising. ABC transporter protein inhibitors, such as
verapamil, may be valuable candidates. In addition, as CD44 plays
an important role in tumor growth, progression and metastasis, the
present interest in the use of CDs in tumor therapy should be
increased.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30772205 and 81472512) and
the Research Fund for Science and Technology Innovation Talents,
Harbin Science and Technology Bureau (grant no. 2007RFLXS024). The
authors thank Dr. Akira Ogose (Division of Orthopedic Surgery,
Niigata University Graduate School of Medical and Dental Sciences,
Niigata, Japan) for providing the NMFH-1 cell line.
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scharenberg CW, Harkey MA and Torok-Storb
B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump
and is preferentially expressed by immature human hematopoietic
progenitors. Blood. 99:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast
cancer cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
7
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naka N, Takenaka S, Araki N, Miwa T,
Hashimoto N, Yoshioka K, Joyama S, Hamada K, Tsukamoto Y, Tomita Y,
et al: Synovial sarcoma is a stem cell malignancy. Stem Cells.
28:1119–1131. 2010.PubMed/NCBI
|
|
9
|
Zoller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X, Gwye Y, Russell D, Cao C, Douglas
D, Hung L, Kovar H, Triche TJ and Lawlor ER: CD133 expression in
chemo-resistant Ewing sarcoma cells. BMC Cancer. 10:1162010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Chen ZG, Du CZ, Wang HW, Yan L and
Gu J: Cancer stem cell marker CD133+ tumour cells and clinical
outcome in rectal cancer. Histopathology. 55:284–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Korkaya H and Wicha MS: Selective
targeting of cancer stem cells: A new concept in cancer
therapeutics. BioDrugs. 21:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honoki K, Fujii H, Kubo A, Kido A, Mori T,
Tanaka Y and Tsujiuchi T: Possible involvement of stem-like
populations with elevated ALDH1 in sarcomas for chemotherapeutic
drug resistance. Oncol Rep. 24:501–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang M, Zhang R, Yan M, Ye Z, Liang W and
Luo Z: Detection and characterization of side population in Ewing's
sarcoma SK-ES-1 cells in vitro. Biochem Biophys Res Commun.
391:1062–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilson H, Huelsmeyer M, Chun R, Young KM,
Friedrichs K and Argyle DJ: Isolation and characterisation of
cancer stem cells from canine osteosarcoma. Vet J. 175:69–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma
W, Zhang Y, Li C and Zhang S: Spheres derived from the human
SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem
cells. Cancer Lett. 299:150–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murase M, Kano M, Tsukahara T, Takahashi
A, Torigoe T, Kawaguchi S, Kimura S, Wada T, Uchihashi Y, Kondo T,
et al: Side population cells have the characteristics of cancer
stem-like cells/cancer-initiating cells in bone sarcomas. Br J
Cancer. 101:1425–1432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawashima H, Ogose A, Gu W, Nishio J, Kudo
N, Kondo N, Hotta T, Umezu H, Tohyama T, Nishijima H, et al:
Establishment and characterization of a novel myxofibrosarcoma cell
line. Cancer Genet Cytogenet. 161:28–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharom FJ: ABC multidrug transporters
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gunthert U, Hofmann M, Rudy W, Reber S,
Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H and Herrlich P:
A new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Naor D, Wallach-Dayan SB, Zahalka MA and
Sionov RV: Involvement of CD44, a molecule with a thousand faces,
in cancer dissemination. Semin Cancer Biol. 18:260–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Michelson S and Slate D: Emergence of the
drug-resistant phenotype in tumor subpopulations: A hybrid model. J
Natl Cancer Inst. 81:1392–1401. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Honore SM, Aybar MJ and Mayor R: Sox10 is
required for the early development of the prospective neural crest
in Xenopus embryos. Dev Biol. 260:79–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pesce M and Schöler HR: Oct-4: Gatekeeper
in the beginnings of mammalian development. Stem Cells. 19:271–278.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bourguignon LY, Peyrollier K, Xia W and
Gilad E: Hyaluronan-CD44 interaction activates stem cell marker
Nanog, Stat-3-mediated MDR1 gene expression and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cells. J Biol Chem.
283:17635–17651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shukla S, Wu CP and Ambudkar SV:
Development of inhibitors of ATP-binding cassette drug
transporters: Present status and challenges. Expert Opin Drug Metab
Toxicol. 4:205–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim M, Turnquist H, Jackson J, Sgagias M,
Yan Y, Gong M, Dean M, Sharp JG and Cowan K: The multidrug
resistance transporter ABCG2 (breast cancer resistance protein 1)
effluxes Hoechst 33342 and is overexpressed in hematopoietic stem
cells. Clin Cancer Res. 8:22–28. 2002.PubMed/NCBI
|
|
39
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H and Sorrentino BP: The ABC transporter Bcrp1/ABCG2 is
expressed in a wide variety of stem cells and is a molecular
determinant of the side-population phenotype. Nat Med. 7:1028–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Allikmets R, Schriml LM, Hutchinson A,
Romano-Spica V and Dean M: A human placenta-specific ATP-binding
cassette gene (ABCP) on chromosome 4q22 that is involved in
multidrug resistance. Cancer Res. 58:5337–5339. 1998.PubMed/NCBI
|
|
41
|
Gallatin WM, Weissman IL and Butcher EC: A
cell-surface molecule involved in organ-specific homing of
lymphocytes. Nature. 304:30–34. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ratajczak MZ: Cancer stem cells-normal
stem cells ‘Jedi’ that went over to the ‘dark side’. Folia
Histochem Cytobiol. 43:175–181. 2005.PubMed/NCBI
|
|
43
|
Miraglia S, Godfrey W, Yin AH, Atkins K,
Warnke R, Holden JT, Bray RA, Waller EK and Buck DW: A novel
five-transmembrane hematopoietic stem cell antigen: Isolation,
characterization and molecular cloning. Blood. 90:5013–5021.
1997.PubMed/NCBI
|
|
44
|
Uchida N, Buck DW, He D, Reitsma MJ, Masek
M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct isolation
of human central nervous system stem cells. Proc Natl Acad Sci USA.
97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
47
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
49
|
Ossowski L and Aguirre-Ghiso JA: Dormancy
of metastatic melanoma. Pigment Cell Melanoma Res. 23:41–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suva ML, Riggi N, Stehle JC, Baumer K,
Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L, et
al: Identification of cancer stem cells in Ewing's sarcoma. Cancer
Res. 69:1776–1781. 2009. View Article : Google Scholar : PubMed/NCBI
|