Introduction
A unique characteristic in the epidemiology of
esophageal carcinoma is the significant differences in incidence
rate which exist between regions and ethnic groups (1,2). The
worldwide incidence and morbidity of esophageal carcinoma is
greatest in China compared with other countries (3). Xinjiang is a residential province of
China, populated by multiple ethnic groups, and is one of the areas
associated with high incidence of esophageal carcinoma (4). Current treatments available for
esophageal carcinoma result in poor prognosis (1). The majority of patients with esophageal
carcinoma are diagnosed in the progressive or resection-resistant
stage and novel treatment strategies are urgently required.
Research has focused on understanding the characteristics of the
progression and transformation of esophageal carcinoma at the
genetic and molecular level.
Research has indicated that abnormal activation of
the kinase activity of epidermal growth factor receptor (EGFR)
(5,6)
is significant in the occurrence and development of esophageal
carcinoma. Domestic (Chinese) and foreign studies have identified
an overexpression of EGFR protein in esophageal carcinoma (7,8). This
phenomenon is correlated with tumor cell proliferation, invasion,
metastasis, vascular growth and inhibition of cell apoptosis, and
is associated with poor prognosis (7,9). Reports
of the expression rate of EGFR in esophageal cancer tissues are
vary significantly, ranging from 29 to 99% (10,11).
In the process of tumor growth, overexpression of
vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR)
disrupt the balance between angiogenesis inducers and angiogenesis
inhibiting factors, promoting tumor angiogenesis (12,13).
VEGFR-2 distribution in the endothelial cells has a critical role
in the process of tumor angiogenesis. The main function of VEGFR-2
is to mediate VEGF-dependent proliferation of vascular endothelial
cells and chemotaxis of endothelial cells, as well as enhance
vascular permeability (12,13). VEGFR-2 is therefore the main
functional receptor of VEGF (12).
VEGFR is a protein similar to EGFR in terms of their
roles in cancer occurrence and development, therefore evaluating
the expression of VEGFR and tumor angiogenesis-associated VEGFR
tyrosine kinase activity may identify a potential target for
anti-angiogenesis in tumor therapy. Previous studies have found
that the overexpression of EGFR and VEGFR-2 is closely associated
with the invasion and metastasis of a variety of solid tumors
(8,13). To date, comprehensive domestic
research on these two proteins in the invasion and metastasis of
esophageal cancer has been rarely reported. The present study
detected the expression levels of esophageal carcinoma-associated
proteins EGFR and VEGFR-2 in three ethnic populations (Uygur, Han
and Kazak) in Xinjiang, in order to elucidate the differences and
correlations between the expression levels of these proteins and
the occurrence and development of esophageal cancer. The results
may reveal potential novel anti-tumor treatments for esophageal
carcinoma at the molecular level, and may aid the elucidation of
differences in esophageal cancer between the ethnic groups
evaluated.
Materials and methods
Clinical characteristics
A total of 119 pairs of esophageal carcinoma and
corresponding pericarcinoma tissue were collected between February
2011 and December 2012 from patients with esophageal carcinoma
following surgical treatment at the First Affiliated Hospital of
Xinjiang Medical University (Urumqi, China). All samples were
dewaxed twice with xylene (Tianjin Yong Sheng Chemical Co., Ltd.,
Tianjin, China) for 15 min after collection and stored at the
Xinjiang Esophageal Cancer Research Institute/Medical Research
Center of Xinjiang Medical University (Urumqi, China). Of these 119
samples, 41 cases were Uygur, 38 cases were Han and 40 cases were
Kazak. A total of 80 cases were male, and 39 cases were female. The
age of patients ranged from 38 to 79 years, with a median age of 60
years. An additional five carcinoma in situ samples obtained
via biopsy during preoperative endoscopy (n=3) or postoperatively
(n=2) between February 2011 and August 2013 were acquired from the
Xinjiang Esophageal Cancer Research Institute/Medical Research
Center of Xinjiang Medical University for the analysis of the
expression levels of EGFR and VEGFR-2 in the early stages of
esophageal carcinoma. The samples were staged according to the
seventh edition of the TNM staging criteria (14), classified based on the 2010 World
Health Organization histological tumor classification standard
(14) and divided by differentiation
degree. Pathological type distribution included 117 cases of
squamous cell carcinoma and 2 cases of adenocarcinoma. The degree
of cellular differentiation was high in 38 cases, medium in 43
cases medium and low in 38 cases. Tumor T stage classification
identified 12 cases as T1, 34 cases as T2, 59 cases as T3 and 14
cases as T4. N stage classification revealed 59 cases of N0, 49
cases of N1 and 11 cases of N2. Postoperative pathological pTNM
staging indicated 9 cases of Ia, 21 cases of Ib, 30 cases of IIa,
13 cases of IIb, 28 cases of IIIa, 8 cases of IIIb and 10 cases of
IIIc, plus an additional 5 cases of carcinoma in situ. Specimen
collection was approved by the Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University. All the
patients and their families provided informed consent following an
explanation of the significance of the study.
Immunohistochemistry
The streptavidin peroxidase immunohistochemical
method was used for immunostaining. Rabbit polyclonal anti-EGFR and
anti-VEGFR-2 antibodies were purchased from Abcam (Cambridge, MA,
USA). Immunostaining was performed as follows: Samples were first
conventionally dewaxed and hydrated using Triton X-100 (Shanghai
Solarbio Co., Shanghai, China). Subsequently, to eliminate
endogenous peroxidase activity, 3% hydrogen peroxide was added and
the mixture was incubated for 10 min at room temperature. Antigens
were retrieved by high pressure heating in a microwave (NN-GF352M;
Panasonic Corporation, Osaka, Japan) at 96°C for 10 min. Samples
were blocked in 10% normal goat serum (Shanghai Solarbio Co.) at
37°C for 30 min, and then incubated with polyclonal rabbit
anti-mouse EGFR (1:100 dilution; cat. no. ab2430; Abcam) or
polyclonal rabbit anti-mouse VEGFR-2 (1:50 dilution; cat. no.
ab2349; Abcam) antibody at 4°C overnight, followed by 3 washes with
phosphate-buffered saline (PBS; Abcam) for 5 min. The biotinylated
goat anti-rabbit IgG secondary antibody (cat. no. SP-9000;
ZSGB-BIO, Beijing, China) was added and the mixture was incubated
at 37°C for 30 min. Subsequently, the samples were washed 3 times
with PBS, colorized with diaminobenzidine (Shanghai Solarbio Co.),
rinsed with distilled water, stained with hematoxylin (Shanghai
Solarbio Co.) and finally sealed using the Histostain-Plus Kit
(ZSGB-BIO). PBS (0.01 mol/l) was used as a negative control.
Assessment of results
Two pathologists observed the sections and performed
double-blinded diagnoses. The dyeing area intensity score and
positive cell area ratio scoring methods (10,12) were
adopted to compare cellular differences in EGFR and VEGFR-2 protein
expression. Positive EGFR staining was localized in the cell
membrane, and cell membrane staining with yellow, brown or deeper
brown particles were regarded as positive cells. According to the
degree of cell positive staining (antigen content), the samples
were divided into: 0 (no coloring), 1 point (yellow), 2 points
(yellow-brown) or 3 points (brown). Each section was observed under
a light microscope (DM3000; Leica Microsystems Ltd., Wetzlar,
Germany) in five randomized high-power fields (magnification, ×20),
and the percentage of positive cells was counted and scored as
follows: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (>75%).
The product of the cell coloring intensity score and positive area
ratio score provided the final score points: 0 (−), 1–2 (+), 3–4
(++), >4 points (+++). Low expression was indicated by (−) and
(+), while (++) and (+++) indicated high (positive) expression.
Identical scoring methods were used for VEGFR-2 and EGFR.
Statistical analysis
Data were analyzed using SPSS 17 software (SPSS,
Inc., Chicago, IL, USA). The expression levels of EGFR and VEGFR-2
protein are presented as percentages. Protein expression levels
between groups were compared using the χ2 test. Multiple
independent samples were compared using multiple independent
samples non-parametric tests. P<0.05 was considered to indicate
a statistically significant difference.
Results
Comparison of clinicopathological
characteristics of patients with esophageal carcinoma of varying
ethnicity
According to the ethnic composition of the Xinjiang
area, the 119 cases of esophageal cancer were divided into 3
groups: Uygur, Han and Kazak. The percentage of patients <60
years old in the three groups was 58% in Uygur, 26% in Han and 47%
in Kazak; however, these differences in age were not determined to
be statistically significant (P>0.05).
EGFR and VEGFR-2 are not
differentially expressed between ethnic groups
Only base levels of staining were observed in the
epithelial basal cells of normal esophageal mucosa epithelium
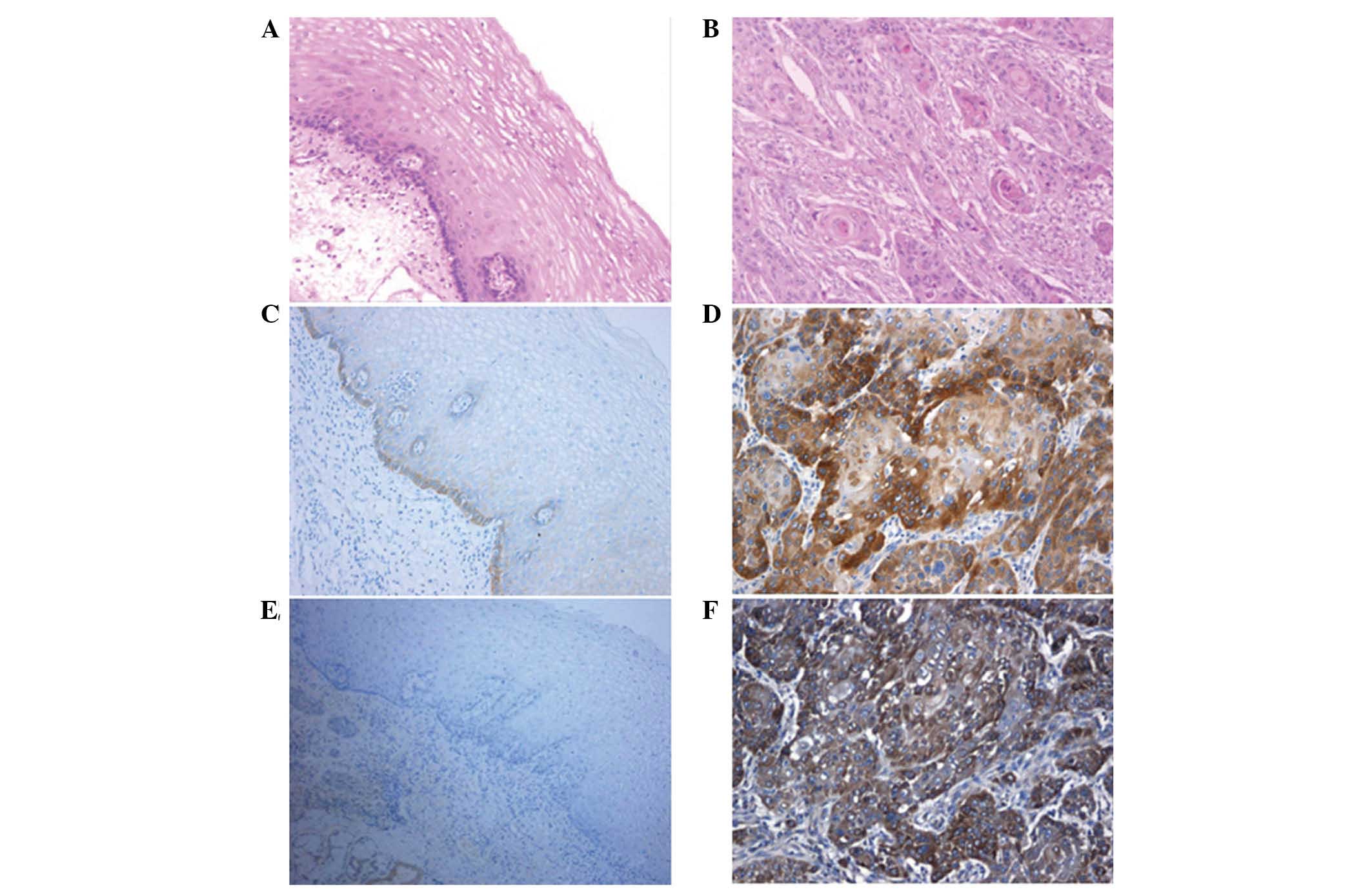
(distance from the tumor margin, >5 cm) (Fig. 1A and B), and therefore basal cells
were considered negative for EGFR expression (Fig. 1C). Hematoxylin-eosin staining
indicated that EGFR localized at the cell membrane and cytoplasm of
esophageal cancer cells (Fig. 1D).
EGFR expression in esophageal carcinoma of the three groups was as
follows: Uygur, 3 cases negative, 7 cases weakly positive, 15 cases
positive and 16 cases strongly positive; Han, 4 cases negative, 7
cases weakly positive, 9 cases positive and 18 cases strongly
positive; and Kazak, 3 cases negative, 7 cases weakly positive, 14
cases positive, and 16 cases strongly positive. The normal
esophageal mucosa epithelium was negative for VEGFR-2 (Fig. 1E), while VEGFR-2-positive staining was
identified to be at the cytoplasm and vascular endothelial cells of
esophageal carcinoma samples (Fig.
1F). VEGFR-2 expression in esophageal carcinoma of the three
ethnic groups were as follows: Uygur, 6 cases negative, 4 cases
weakly positive, 22 cases positive and 9 cases strongly positive;
Han, 6 cases negative, 5 cases weakly positive, 19 cases positive
and 8 cases strongly positive; and Kazak, 6 cases negative, 7 cases
weakly positive, 21 cases positive and 6 cases strongly
positive.
Statistical analysis indicated no significant
differences in the protein expression of EGFR and VEGFR-2 among the
three ethnic groups of patients (Tables
I and II; P>0.05).
 | Table I.Protein expression levels of EGFR in
Uygur, Han and Kazak esophageal carcinoma tissues. |
Table I.
Protein expression levels of EGFR in
Uygur, Han and Kazak esophageal carcinoma tissues.
|
|
| EGFR expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Ethnicity | n | − | + | ++ | +++ | Positive rate, % | P-value |
|---|
| Uygur | 41 | 3 | 9 | 13 | 16 | 92.68 | P>0.05 |
| Han | 38 | 4 | 8 | 9 | 17 | 89.47 |
|
| Kazak | 40 | 2 | 11 | 15 | 12 | 95.00 |
|
 | Table II.Protein expression levels of VEGFR-2
in Uygur, Han and Kazak esophageal carcinoma tissues. |
Table II.
Protein expression levels of VEGFR-2
in Uygur, Han and Kazak esophageal carcinoma tissues.
|
|
| VEGFR-2
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Ethnicity | n | − | + | ++ | +++ | Positive rate, % | P-value |
|---|
| Uygur | 41 | 8 | 3 | 21 | 9 | 80.49 | P>0.05 |
| Han | 38 | 8 | 4 | 17 | 9 | 78.95 |
|
| Kazak | 40 | 7 | 6 | 22 | 5 | 82.50 |
|
Association between expression of EGFR
and VEGFR-2 and clinicopathological factors
No significant differences were detected in the
EGFR- or VEGFR-2-positive rate of esophageal carcinoma tissues
according to age, gender, tumor size or tumor differentiation of
the Uygur, Han and Kazak groups (Tables
III–V; P>0.05). However, a
significant difference was identified in lymph node metastasis
between the three groups (P<0.05). In addition, although
statistically insignificant, EGFR expression was strongly positive
(+++) in low and medially differentiated esophageal carcinoma, and
moderate (++) or weakly positive (+) in highly differentiated
cancer tissues.
 | Table III.Correlation between expression levels
of EGFR and VEGFR-2 and various clinicopathological factors in
Uygur patients with esophageal carcinoma. |
Table III.
Correlation between expression levels
of EGFR and VEGFR-2 and various clinicopathological factors in
Uygur patients with esophageal carcinoma.
| A, EGFR
expression |
|
|
|
|
|
|
|---|
|
|---|
|
|
| EGFR expression |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | n | High | Low | Positive, % | P-value | χ2 |
|---|
| Gender |
|
|
|
| 0.7851 |
|
| Male | 28 | 20 | 8 | 71.43 |
|
|
|
Female | 13 | 8 | 5 | 61.54 |
|
|
| Age, years |
|
|
|
| 0.7404 |
|
|
<60 | 24 | 17 | 7 | 70.83 |
|
|
|
≥60 | 17 | 12 | 5 | 70.59 |
|
|
| Tumor size, cm |
|
|
|
| 0.7625 |
|
|
<4 | 22 | 16 | 6 | 72.73 |
|
|
| ≥4 | 19 | 13 | 6 | 68.62 |
|
|
| Tumor
differentiation |
|
|
|
| >0.05 | 1.29 |
|
High | 12 | 8 | 4 | 66.66 |
|
|
|
Medium | 16 | 11 | 5 | 68.75 |
|
|
|
Low | 13 | 11 | 2 | 84.61 |
|
|
| Lymph node
metastasis |
|
|
|
| 0.0004 |
|
|
Yes | 23 | 20 | 3 | 86.96 |
|
|
| No | 18 | 6 | 12 | 33.33 |
|
|
|
| B, VEGFR-2
expression |
|
|
|
| VEGFR-2
expression |
|
|
|
|
|
|
|
|
|
|
| Clinicopathological
factor | n | High | Low | Positive, % | P-value | χ2 |
|
| Gender |
|
|
|
| 0.7969 |
|
|
Male | 28 | 22 | 6 | 78.57 |
|
|
|
Female | 13 | 9 | 4 | 69.23 |
|
|
| Age, years |
|
|
|
| 0.9652 |
|
|
<60 | 24 | 18 | 6 | 75.00 |
|
|
|
≥60 | 17 | 12 | 5 | 70.58 |
|
|
| Tumor size, cm |
|
|
|
| 0.945 |
|
|
<4 | 22 | 16 | 6 | 72.72 |
|
|
| ≥4 | 19 | 14 | 5 | 73.68 |
|
|
| Tumor
differentiation |
|
|
|
| >0.05 | 0.37 |
|
High | 12 | 8 | 4 | 66.66 |
|
|
|
Medium | 16 | 11 | 5 | 68.75 |
|
|
|
Low | 13 | 10 | 3 | 76.92 |
|
|
| Lymph node
metastasis |
|
|
|
| 0.0003 |
|
|
Yes | 23 | 21 | 2 | 91.30 |
|
|
| No | 18 | 7 | 11 | 38.88 |
|
|
 | Table V.Correlation between expression levels
of EGFR and VEGFR-2 with various clinicopathological factors in
Kazak patients with esophageal carcinoma. |
Table V.
Correlation between expression levels
of EGFR and VEGFR-2 with various clinicopathological factors in
Kazak patients with esophageal carcinoma.
| A, EGFR
expression |
|
|
|
|
|
|
|---|
|
|---|
|
|
| EGFR
expression |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | n | High | Low | Positive, % | P-value | χ2 |
|---|
| Gender |
|
|
|
| 0.498 |
|
|
Male | 28 | 21 | 7 | 75.00 |
|
|
|
Female | 12 | 7 | 5 | 58.33 |
|
|
| Age, years |
|
|
|
| 0.9058 |
|
|
<60 | 19 | 13 | 6 | 68.42 |
|
|
|
≥60 | 21 | 14 | 7 | 66.66 |
|
|
| Tumor size, cm |
|
|
|
| 0.9781 |
|
|
<4 | 26 | 17 | 9 | 65.38 |
|
|
| ≥4 | 14 | 10 | 4 | 71.42 |
|
|
| Tumor
differentiation |
|
|
|
| >0.05 | 1.72 |
|
High | 15 | 10 | 5 | 66.67 |
|
|
|
Medium | 14 | 7 | 6 | 57.14 |
|
|
|
Low | 11 | 9 | 2 | 81.82 |
|
|
| Lymph node
metastasis |
|
|
|
| 0.0131 |
|
|
Yes | 17 | 14 | 3 | 82.35 |
|
|
| No | 23 | 10 | 13 | 43.47 |
|
|
|
| B, VEGFR-2
expression |
|
|
|
|
|
|
|
|
|
| VEGFR-2
expression |
|
|
|
|
|
|
|
|
|
|
| Clinicopathological
factor | n | High | Low | Positive, % | P-value | χ2 |
|
| Gender |
|
|
|
| 0.2385 |
|
|
Male | 28 | 21 | 7 | 75.00 |
|
|
|
Female | 12 | 6 | 6 | 50.00 |
|
|
| Age, years |
|
|
|
| 0.577 |
|
|
<60 | 19 | 12 | 7 | 63.16 |
|
|
|
≥60 | 21 | 15 | 6 | 71.43 |
|
|
| Tumor size, cm |
|
|
|
| 0.5013 |
|
|
<4 | 26 | 19 | 7 | 73.08 |
|
|
| ≥4 | 14 | 8 | 6 | 57.14 |
|
|
| Tumor
differentiation |
|
|
|
| >0.05 | 0.21 |
|
High | 15 | 10 | 5 | 66.67 |
|
|
|
Medium | 14 | 9 | 5 | 64.29 |
|
|
|
Low | 11 | 8 | 3 | 72.73 |
|
|
| Lymph node
metastasis |
|
|
|
| 0.0189 |
|
|
Yes | 17 | 13 | 4 | 76.47 |
|
|
| No | 23 | 9 | 14 | 39.13 |
|
|
Association between EGFR and VEGFR-2
expression
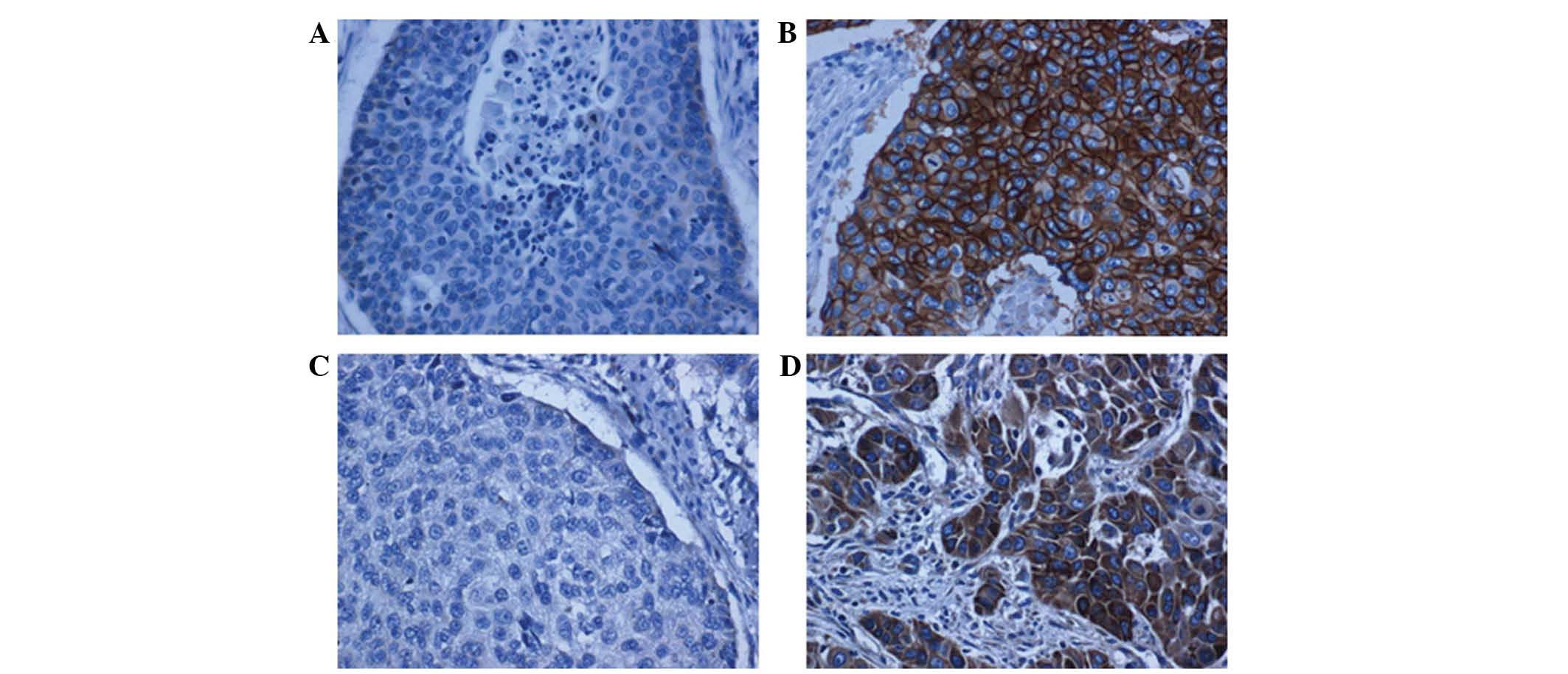
A correlation study was performed on the protein
expression of EGFR and VEGFR-2 in 119 esophageal cancer specimens
from the Uygur, Han and Kazak ethnic groups. Low expression was
defined as (−) and (+), while (++) and (+++) were considered to
indicate positive expression (Fig.
2). Statistical analysis revealed a significant correlation
between the expression of EGFR and VEGFR-2 in all three groups
(Table VI; P<0.05).
 | Table VI.Association between the expression of
EGFR and VEGFR-2. |
Table VI.
Association between the expression of
EGFR and VEGFR-2.
|
|
|
| EGFR |
|
|---|
|
|
|
|
|
|
|---|
| Ethnicity | n | VEGFR-2 | High | Low | P-value |
|---|
| Uygur | 41 | High | 27 | 4 | <0.05 |
|
|
| Low | 2 | 8 |
|
| Han | 38 | High | 24 | 2 | <0.05 |
|
|
| Low | 2 | 10 |
|
| Kazak | 40 | High | 25 | 2 | <0.05 |
|
|
| Low | 2 | 11 |
|
EGFR and VEGFR-2 expression in
carcinoma in situ samples
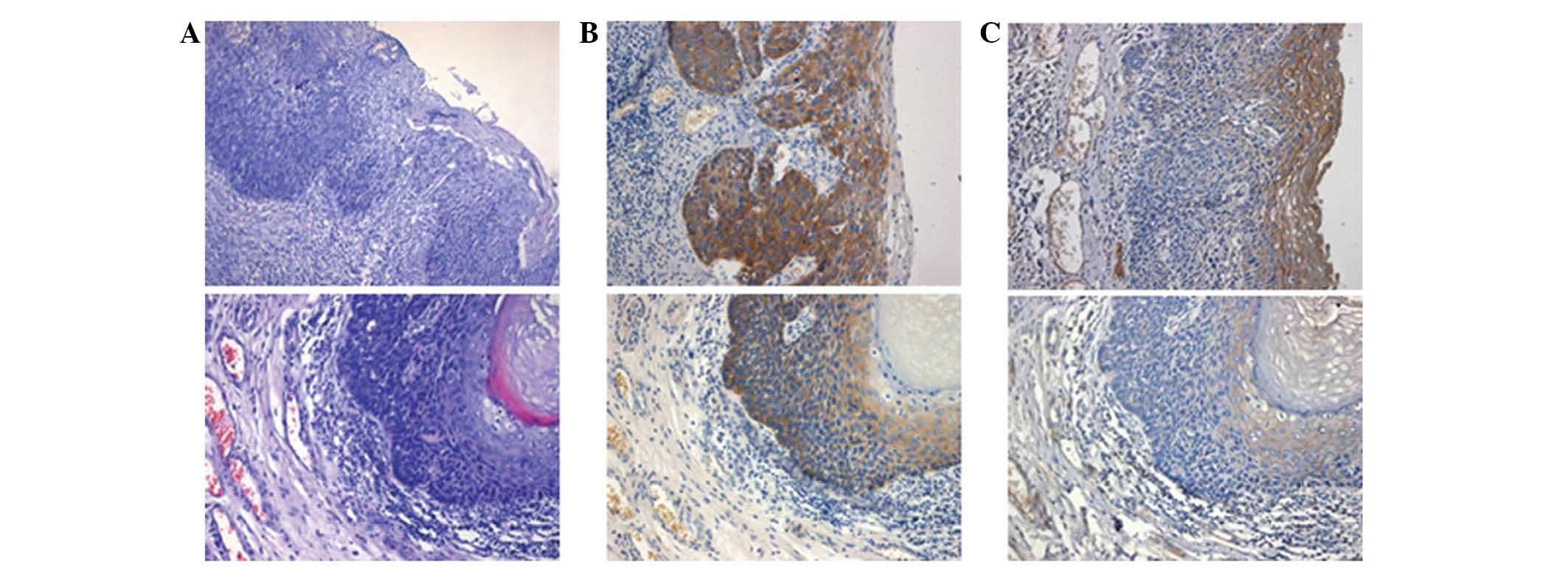
Carcinoma in situ samples were stained with
hematoxylin and eosin (Fig. 3A). EGFR
expression was positive in 5 cases of carcinoma in situ
(Fig. 3B), while VEGFR-2 expression
was negative (Fig. 3C). These results
indicate that EGFR expression may be an early event of esophageal
cancer.
Discussion
To date, surgery remains the primary treatment for
esophageal carcinoma. Unfortunately, due to the atypical early
symptoms of esophageal carcinoma, ~85% patients that seek medical
advice have already progressed to an advanced or metastatic stage,
which cannot be treated by surgery. Since there is currently no
alternative effective treatment available, the prognosis of
esophageal carcinoma is poor, with a 5-year survival rate of 8–11%.
Though comprehensive treatment and radical chemoradiotherapy have
been introduced, esophageal carcinoma survival rates have been
minimally altered (1,2). Thus, improving patient prognosis and
prolonging survival times has become pivotal to the development of
novel treatments for esophageal cancer. Recently, the majority of
research has focused on chemotherapy-based treatments with
combinative targeted therapy, particularly aimed at the epidermal
growth factor and vascular endothelial growth factor receptors.
Proto-oncogene EGFR has a critical role in the development of
esophageal cancer (7,10,13). The
abnormal activation of EGFR promotes tumorigenesis and
proliferation through regulating cell signaling transduction, cell
proliferation, apoptosis and angiogenesis. Gibault et al
(15) demonstrated that EGFR
overexpression was significantly correlated with vascular invasion
(P=0.023), local recurrence (P=0.006) and a lower survival rate
(P=0.003). In addition, a further study found that EGFR inhibitors
were able to inhibit the overexpression of EGFR and human epidermal
growth factor receptor-2, thereby inhibiting the proliferation of
esophageal cancer cell lines (16).
These results suggest that the expression of EGFR is critical for
the determination of targeted therapy for esophageal cancer.
Similarly, another tyrosine kinase receptor,
VEGFR-2, is closely associated with tumor angiogenesis (8,12,13). In the tumor tissue, tumor cells and
host cells secrete a series of cytokines, generating a favorable
microenvironment for angiogenesis; VEGF is one of these cytokines
(8,13,17).
VEGFR-2 is activated following stimulation with activation signals,
for example binding to its ligand VEGF. A series of cascade
reactions downstream of the signal transduction are initiated,
enhancing gene transcription in the nucleus and promoting the
proliferation and angiogenesis of vascular endothelial cells
(8,13,16). Zhang
et al (18) reported a
xenograft model of human esophageal adenocarcinoma tissue and mouse
tumor through application of neoplastic and non-neoplastic Barrett
epithelial cells, confirming the aforementioned hypothesis.
However, to the best of our knowledge, there has been no report
regarding the correlation between the expression and activation of
EGFR and VEGFR-2 in esophageal cancer tissues. To the best of our
knowledge, the present study is the first study of EGFR and VEGFR-2
in esophageal carcinoma based on samples from various ethnic groups
in Xinjiang.
In the present study, the expression levels of EGFR
and VEGFR-2 in esophageal carcinoma patients of various ethnicities
were detected by immunohistochemistry. The results revealed that
adjacent normal esophageal mucosa tissues were negative for EGFR
and VEGFR-2 staining, while overexpression of EGFR was detected in
70.73% of Uygur, 68.42% of Han and 67.5% of Kazak esophageal cancer
tissue samples and VEGFR-2 was overexpressed in 73.17, 68.42 and
67.5% of the Uygur, Han and Kazak groups, respectively. However,
statistical analysis revealed that the EGFR and VEGFR-2 protein
expression rate was not significantly different between the three
ethnic groups (P>0.05). The expression rate of EGFR in
esophageal carcinoma detected here was comparable to that observed
in the Huizhou Hakka population (19)
and the results of a study by Carneiro et al (20) (69.81%). Therefore, further studies are
required to confirm whether there may be regional differences in
esophageal cancer.
Overexpression of EGFR in tumor cells, leading to
uncontrolled cell growth and malignant transformation, is
associated with the poor prognosis of tumor-associated diseases
(9,18). In addition, the overexpression of EGFR
in esophageal cancer patients is significantly associated with
vascular invasion (21). Based on the
comparison of the expression levels of EGFR and VEGFR-2 in the
three ethnic groups evaluated, the present study further analyzed
the correlation between the expression rate of EGFR, VEGFR-2 and
the clinicopathological parameters of patients with esophageal
cancer. The results identified no significant differences in the
expression rate of EGFR between gender, age or tumor size in
esophageal cancer tissues between the three groups (P>0.05).
However, a significant difference in the expression levels of EGFR
was detected between patients with and without lymph node
metastasis (P<0.05). In addition, although statistically
insignificant, EGFR expression was strongly positive (+++) in low
and medially differentiated esophageal carcinoma, and moderate (++)
or weakly positive (+) in highly differentiated cancer tissues.
Thus, EGFR may have a significant role in the occurrence,
development and lymph node metastasis of esophageal cancer in
various ethnic groups in Xinjiang, and may potentially be used as
an index for the prediction of metastasis and prognosis. Similarly,
VEGFR-2 overexpression is associated with tumor progression, lymph
node metastasis and poor prognosis (12,13,16). A
total of 119 cases of esophageal carcinoma tissue samples from
multi-ethnic patients in Xinjiang were used to evaluate the
correlation between the expression of EGFR and VEGFR-2, and the
results revealed a significant correlation (P<0.05). These
results demonstrate that EGFR and VEGFR-2 may have a synergistic
effect in the development of esophageal carcinoma in various ethnic
groups in Xinjiang.
In addition, EGFR expression was positive and
VEGFR-2 expression was negative in 5 cases of carcinoma in
situ. Therefore, it was hypothesized that overexpression of
EGFR may be an early event of esophageal cancer and may be used as
an early indicator of tumor development. However, further studies
with a larger cohort are required to verify this hypothesis.
In 119 cases of esophageal cancer, the proportion of
Uygur patients ≤60 years was relatively high, compared with that of
the Han and Kazak populations. This may be a result of the dietary
habits typical of the Uygur population, which include large
quantities of barbecue, hot food and low fresh vegetable intake, as
well as the poor sanitary conditions and regional economy in
southern Xinjiang (3,4).
In conclusion, the present study found that
overexpression of EGFR and VEGFR-2 in Xinjiang Uygur, Han and Kazak
patients with esophageal carcinoma was associated with tumor
occurrence, development and lymph node metastases, providing an
experimental basis for tumor therapy targeting EGFR and VEGFR-2.
However, the occurrence and development of esophageal cancer is a
gradual and complex process involving numerous factors. Thus
further study focusing on the specific mechanisms of activation of
the EGFR and VEGFR-2 genes involved in the occurrence, development
and metastasis of esophageal carcinoma are required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 30960383). Tumor tissues
were provide by the Department of Pathology, The First Affiliated
Hospital of Xinjiang Medical University (Urumqi, China).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin DX, Tan W, Lu SX, et al: Molecular
epidemiological study of Chinese esophageal cancer. Chin J
Epidemiology. 10:130–135. 2003.(In Chinese).
|
|
4
|
Lu XM, Zhang YM, Lin RY, Arzi G, Wang X,
Zhang YL, Zhang Y, Wang Y and Wen H: Relationship between genetic
polymorphisms of metabolizing enzymes CYP2E1, GSTM1 and Kazakh's
esophageal squamous cell cancer in Xinjiang, China. World J
Gastroenterol. 11:3651–3654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bazley LA and Gullick WJ: The epidermal
growth factor receptor family. Endocr Relat Cancer. 12(Suppl 1):
S17–S27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andl CD, Mizushima T, Nakagawa H, Oyama K,
Harada H, Chruma K, Herlyn M and Rustgi AK: Epidermal growth factor
receptor mediates increased cell proliferation, migration and
aggregation in esophageal keratinocytes in vitro and in vivo. J
Biol Chem. 278:1824–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanawa M, Suzuki S, Dobashi Y, Yamane T,
Kono K, Enomoto N and Ooi A: EGFR protein overexpression and gene
amplification in squamous cell carcinomas of the esophagus. Int J
Cancer. 118:1173–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang RG, Zang GQ, Feng J, et al:
Expression of c-Myb, VEGFR and EGFR protein in human hepatic
cirrhosis and hepatocellular carcinoma tissues. Chin J
Gastroenterol Hapatol. 16:420–423. 2007.(In Chinese).
|
|
9
|
Brand TM, Iida M and Wheeler DL: Molecular
mechanisms of resistance to the EGFR monoclonal antibody cetuximab.
Cancer Biol Ther. 11:777–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu X, Hedman H, Bergqvist M, Bergström S,
Henriksson R, Gullbo J, Lennartsson J, Hesselius P and Ekman S:
Expression of EGFR and LRIG proteins in oesophageal carcinoma with
emphasis on patient survival and cellular chemosensitivity. Acta
Oncol. 51:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kii T, Takiuchi H, Kawabe S, Gotoh M, Ohta
S, Tanaka T, Kuwakado S, Nishitani H and Katsu K: Evaluation of
prognostic factors of esophageal squamous cell carcinoma (stage
II-III) after concurrent chemoradiotherapy using biopsy specimens.
Jpn J Clin Oncol. 37:583–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Tang X, Riquelme E, Behrens C,
Nilsson MB, Giri U, Varella-Garcia M, Byers LA, Lin HY, Wang J, et
al: Increased VEGFR-2 gene copy is associated with chemoresistance
and shorter survival in patients with non-small-cell lung carcinoma
who receive adjuvant chemotherapy. Cancer Res. 71:5512–5521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodríguez-Antona C, Pallares J,
Montero-Conde C, Inglada-Pérez L, Castelblanco E, Landa I, Leskelä
S, Leandro-García LJ, López-Jiménez E, Letón R, et al:
Overexpression and activation of EGFR and VEGFR2 in medullary
thyroid carcinomas is related to metastasis. Endocr Relat Cancer.
17:7–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB, Byrd DR, Compton CC, et al:
Esophageal and esophagogastric junction. AJCC Cancer Staging Manual
(7th). (New York, NY). Springer. 103–115. 2010.
|
|
15
|
Gibault L, Metges JP, Conan-Charlet V,
Lozac'h P, Robaszkiewicz M, Bessaguet C, Lagarde N and Volant A:
Diffuse EGFR staining is associated with reduced overall survival
in locally advanced oesophageal squamous cell cancer. Br J Cancer.
93:107–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mimura K, Kono K, Maruyama T, Watanabe M,
Izawa S, Shiba S, Mizukami Y, Kawaguchi Y, Inoue M, Kono T, et al:
Lapatinib inhibits receptor phosphorylation and cell growth and
enhances antibody-dependent cellular cytotoxicity of EGFR- and
HER2-overexpressing esophageal cancer cell lines. Int J Cancer.
129:2408–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Besh S, Chao J, Das P, Denlinger C, Fanta P, Fuchs CS, et al:
Esophageal and esophagogastric junction cancers, version 1.2015. J
Natl Compr Canc Netw. 13:194–227. 2015.PubMed/NCBI
|
|
18
|
Zhang Q, Yu C, Peng S, Xu H, Wright E,
Zhang X, Huo X, Cheng E, Pham TH, Asanuma K, et al: Autocrine VEGF
signaling promotes proliferation of neoplastic Barrett's epithelial
cells through a PLC-dependent pathway. Gastroenterology.
146:461–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pei XJ, Yang QX and Zou DM: Preliminary
study in Huizhou Hakka population of esophageal cancer EGFR protein
expression and correlation with MAPK signaling pathway. China
Medical Herald. 9:16–19. 2012.
|
|
20
|
Carneiro A, Isinger A, Karlsson A,
Johansson J, Jönsson G, Bendahl PO, Falkenback D, Halvarsson B and
Nilbert M: Prognostic impact of array-based genomic profiles in
esophageal squamous cell cancer. BMC Cancer. 8:982008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaneko K, Kumekawa Y, Makino R, Nozawa H,
Hirayama Y, Kogo M, Konishi K, Katagiri A, Kubota Y, Muramoto T, et
al: EGFR gene alterations as a prognostic biomarker in advanced
esophageal squamous cell carcinoma. Front Biosci (Landmark Ed).
15:65–72. 2010. View
Article : Google Scholar : PubMed/NCBI
|