Introduction
Tumor suppressor p53 plays a central role in
protecting cells against carcinogenesis, mainly functioning as a
transcription factor. In response to stress signals, such as DNA
damage, oncogenic stimuli and hypoxia, the p53 protein regulates
the transcription of numerous different genes, leading to cell
cycle arrest, apoptosis, DNA repair or senescence (1). The inactivation of p53 by mutation is a
frequent event in carcinogenesis. Mutations in the TP53 gene, which
encodes the p53 protein, occur in around half of all tumor
specimens, but the overall frequency of p53 mutations in breast
cancer is only 20–30% (2,3). It is believed that, in breast cancer
harboring the wild-type p53 gene, p53 function is compromised by
other genetic or epigenetic alterations (4,5). A number
of studies have demonstrated that changes in interactome components
or the target genes of p53 could contribute to reduce the roles of
p53 during stress [reviewed in (4,5)].
Recently, a number of microRNAs (miRNAs) have been
found to be involved in the p53 signaling pathway and breast
carcinogenesis (6). Certain miRNAs
directly target the mRNA of p53 and negatively regulate p53
expression, such as miR-125b (7),
miR-375 (8) and miR-504 (9). A study in murine models of
postmenopausal breast cancer suggested that miR-504 expression
induced by obesity contributes to the reduced p53 protein
expression and mammary tumor progression (9). Another class of miRNAs indirectly
affected p53 signaling through regulating genes associated with
p53. For example, miR-21 antagonizes the p53 pathway in breast
cancer by inhibiting the expression of p53-regulated genes
(10); oncomiRs miR-221/222 promoted
proliferation in breast cancer by inhibiting p53 upregulated
modulator of apoptosis expression (10). Conversely, p53 can regulate miRNA
transcription, for example, that of miR-10b (11), miR-22 (12), miR-26a (13), miR-34a (14), miR-148a (15), miR-200b (16), miR-200c (16) and miR-205 (17), or miRNA processing, such as that of
miR-16 (13,18), miR-145 (18,19) and
miR-203 (20).
Given the rapidly increasing amount of literature
regarding the interaction between p53 and miRNAs, and as
complexities in the association between p53 and miRNAs exist, the
present study systematically analyzed p53-related miRNAs and their
targets in breast cancer using a literature-based discovery
approach, natural language processing (NLP).
Materials and methods
NLP analysis of miRNAs associated with
p53 and breast cancer
NLP analysis was performed as described by Gao et
al (21) and Tang et al
(22). Briefly, a PubMed search was
conducted with the following combination of query terms: (‘mammary
cancer’ OR ‘mammary tumour’ OR ‘mammary tumor’ OR ‘mammary
neoplasm’ OR ‘mammary carcinoma’ OR ‘breast cancer’ OR ‘breast
tumour’ OR ‘breast tumor’ OR ‘breast neoplasm’ OR ‘breast
carcinoma’) AND (‘p53’ OR ‘TP53’ OR ‘TRP53’). All the miRNAs
reported in each of the studies were compiled in a list, and then
subjected to gene mention tagging using A Biomedical Named Entity
Recognizer, an open source tool for automatically tagging genes,
proteins and other entity names in text (23). For conjugated terms, conjunction
resolution was performed to obtain individual terms, for example,
‘miR-200b/c’ was resolved into ‘miR-200b’ and ‘miR-200c’. In the
present study, all the genes and miRNAs were named using the
official symbol in the Entrez and miRBase databases, respectively.
Finally, the co-citation frequency of each miRNA with p53 and
breast cancer in the PubMed abstracts was calculated as described
by Gao et al (21). The higher
the co-citation frequency of a miRNA with p53 and breast cancer,
the closer it is associated with p53 and breast cancer.
Prediction of miRNA targets
The targets of the miRNAs were predicted using the
following computational programs: PicTar 2005 (24) (http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi),
miRanda v5 (25) (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5)
and TargetScan 5.1 (26) (http://www.targetscan.org).
Analysis of gene ontology (GO),
pathways and networks
Go analysis was performed with GSEABase package from
R statistical platform (http://www.r-project.org/). Genes were categorized
based on biological process (BP), molecular function (MF) and
cellular component (CC). GenMAPP v2.1 was used to map genes to the
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database,
and calculate the enrichment P-value for each pathway (27).
Network analysis of miRNA targets
To construct gene interaction networks, the
following three different interaction associations were integrated:
i) Protein interaction, gene regulation and protein modification in
the KEGG database; ii) high-throughout protein interaction
experiments such as yeast two-hybrid experiments; and iii) gene
interaction associations that have previously been reported.
Pathway data were downloaded from the KEGG database and used to
analyze the interaction associations of genes [including
enzyme-enzyme associations, protein-protein interactions (PPIs) and
gene expression interactions] with the KEGGSOAP package (http://www.bioconductor.org/packages/2.4/bioc/html/KEGGSOAP.html).
The PPI data were downloaded from the MIPS database (http://mips.helmholtz-muenchen.de/proj/ppi/) (28). For the interactions that have been
reported, the co-citation frequency of each gene pair in the PubMed
abstracts was calculated as described by Gao et al (21). Finally, all three types of data were
integrated and mapped in a network structure using Medusa (29).
Construction of miRNA target
expression plasmid
Each pair of complementary oligonucleotides
containing the predicted miRNA target region were synthesized,
annealed and ligated into pmirGLO Dual-Luciferase miRNA target
expression vectors (Promega, Madison, WI, USA) at
NheI/SalI sites. To ensure that the overhangs created
by oligonucleotide annealing were complementary to the two ends of
linearized vector, CTAG (protruding sequence of NheI
digestion) and TCGA (protruding sequence of SalI digestion)
were added to the 5 ends of the forward and reverse
oligonucleotides, respectively. For clone confirmation, a
KpnI restriction site was added to each pair of
oligonucleotides. When digested with KpnI, the correct
construct releases an ~500-bp insert due to a KpnI site at
position 478 in the vector. All the plasmids were further confirmed
by DNA sequencing.
Cell culture, transfection and
luciferase assay
Cell culture, transfection and luciferase assays
were performed as previously described (30). Human embryonic kidney 293 (HEK293)
cells (American Type Culture Collection, Manassas, VA, USA) were
cultured on 24-well plates, and co-transfected with 10 pmol miRNA
mimics (GenePharma, Shanghai, China) and 0.4 µg miRNA target
expression plasmids. At 24-h post-transfection, the cells were
harvested and assayed for luciferase activity using the Dual-Glo
luciferase assay system (Promega). The Firefly luciferase
activities were normalized to Renilla luciferase activity.
The relative Firefly luciferase activity of the cells transfected
with miRNA mimics was represented as the percentage of activity
relative to that of the cells transfected with negative control
miRNA mimics. For each transfection, the luciferase activity was
averaged from three replicates.
Statistical analysis
The Student's t-test was used to evaluate
statistical significance and all statistical analyses were
performed using R project statistical software (http://www.r-project.org/). P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification of miRNAs associated
with p53 and breast cancer by NLP analysis
NLP has been successfully used to identify molecular
interactions. To find the miRNA interacting with p53 in breast
cancer, the present study searched PubMed with the following
combination of query terms: (‘mammary cancer’ OR ‘mammary tumour’
OR ‘mammary tumor’ OR ‘mammary neoplasm’ OR ‘mammary carcinoma’ OR
‘breast cancer’ OR ‘breast tumour’ OR ‘breast tumor’ OR ‘breast
neoplasm’ OR ‘breast carcinoma’) AND (‘p53’ OR ‘TP53’ OR ‘TRP53’),
and obtained 5,525 studies reporting on p53 and breast cancer.
Further analysis, as described in the Materials and methods
section, identified 22 miRNAs that are reported to interact with
p53 in breast cancer (Table I). Among
these miRNAs, the three most frequently cited were miR-34a, miR-21
and miR-200c, which were cited by 8, 6 and 5 studies,
respectively.
 | Table I.p53- and breast cancer-related miRNAs
and their predicted targets. |
Table I.
p53- and breast cancer-related miRNAs
and their predicted targets.
| miRNA | PubMed count | Predicted
targets |
|---|
| miR-34a | 8 | ZNF281,
RPS6KA4, PNOC, SYVN1, MYRIP, CRHR1, TAF5, MPP2, CACNB3,
DPYSL4, EVI5L, STRN3, UHRF2, AXL, COPS7B,
ACSL4, ASB1, SNX15, ALDOA |
| miR-21 | 6 | WWP1, NFIB, CCL1, C17ORF39, NTF3,
ASPN, CNTFR, PELI1, SOX2,
JAG1, RECK, TGFBI, MATN2, SPRY2 |
| miR-200c | 5 | DGKA, BAP1, NDST1 |
| miR-200b | 4 | HS3ST1 |
| miR-200a | 3 | SPAG9, DIXDC1,
GATA6, TCERG1, HMG20A, TP53INP1, SOX5, PCDH9 |
| miR-203 | 3 | COPS7B,
PHLDA3, CCNG1,
DLG5, DGKZ, CITED2, GLI3, DUSP5, DLX5, ACO2, ARNTL, FOXK2,
CUL1, C18ORF34,
CSN2, OVOL1,
ZNF281, GABRB2 |
| miR-205 | 3 | DLG2, E2F1, C21ORF63, LRP1,
HS3ST1, ERBB3, PHC2, FRK, ADAMTS9, INHBA, INPPL1,
IPO7 |
| miR-145 | 2 | GLIS1, SEMA3A,
FKBP3, NEDD9,
ATXN2, C11ORF9, SLITRK4, CCNL1, ZBTB10, PLCL2, RGS7, RTKN,
CTNNBIP1, LENG8, SEMA6A, ZNF423, ACTG1, ARPC5, SRGAP1 |
| miR-155 | 2 | SALL1,
IKBKE, SDCBP, HIVEP2, BOC, H3F3A, FBXO11, ACTA1, BRD1, LRP1B,
CARHSP1, SOCS1, TP53INP1, WEE1, RNF123, MYO10, DNAJB7,
AICDA, ASTN2, CSNK1G2, CHD7, MAP3K10, CSF1R, HBP1,
CEBPB |
| miR-10b | 1 | DOCK11, HS6ST2,
CECR6, NCOR2, FXR2, ARIH2,
DAZAP1 |
| miR-133a | 1 | SOLH, PTHR1, CTBP2,
ATP6AP2, RAPH1, CSNK1G3, RCE1, CLTA, EVI1, ELF2, TFAP2D, MLLT3,
VPS54, NRIP3, PTPRD, LRRC7, NDRG1, ABCA2, GDI2 |
| miR-148a | 1 | ATP6AP2,
ITSN2, ROBO1, GTF2H1, YPEL3, USP47, KLF4, AKAP1, ABCB7,
RAB34, CNTN4, WNT10B, ALS2CR2, SFRS11, NOG, PRKAG2, MTF1,
GAP43, CCKBR, SYNJ1, MAF1, GPR116, C1GALT1, GADD45A, DYRK1B, TRPS1,
DNMT1, PLAA,
SFRS2IP, UCP3, MLLT10, USP48, LBR, CHD7, COL2A1, RNF38 |
| miR-16 | 1 | CHRNE, CCNE1, WBP11, LPHN2, SH3GL2,
ZSWIM3, RSBN1, CCNT2, KCTD8, DLL4, ATXN2, ADRB2, OMG,
COPS2, SCOC,
ADAMTS18, YWHAQ,
TGFBR3, SEMA6D,
TAF15, EPHA1, KIF21A, CHEK1, STXBP3, G0S2 |
| miR-191 | 1 | RNF139,
GAP43, PLCD1, NDST1 |
| miR-210 | 1 | EFNA3 |
| miR-22 | 1 | JMJD1A,
PURB, ERBB3,
ODF1, CSF1R, MAX, EMILIN3, SATB2, IPO7, PRR6, RFXANK,
SV2A, EPC1, STAG2,
TRUB1, FAM49B, MTHFD2, IL13RA1, DNAJB5, TAGLN, CAV3, CDKN1A, MECP2, ZFYVE9, NFYA, BCL9L,
MAT2A |
| miR-222 | 1 | RBM24, CDKN1C, GNAI2, IRX5, KHDRBS2, RSBN1L,
CDKN1B, MESDC1 |
| miR-26a | 1 | RCN2, NFE2L3,
USP15, ABHD2, ZDHHC18, ADM, DEPDC1B, EPHA2, PITPNC1,
ULK1, CDK2AP1,
HOXA5, COX5A, RLF, PRKCD, ASPN, MTX2,
SALL1, HAO1, DLG5, SMAD1, PTPN13, HIPK1, PRKAG2, ZNF238,
CAMSAP1, PTER, ZDHHC6, PDHX, NAP1L5, PAPD4, COL1A2, KCNQ4,
ALS2CR2, SENP5, RPS6KA6, EPC2, PAN3,
SACS, MGAT4A |
| miR-9 | 1 | CCNE2, ENTPD5, FOXP4,
NOX4, ONECUT1,
RNF111, RBM9, RPS6KA4, DYRK1B, ITPKC, CNTFR, PYGO2, GAD1,
RAB34, ARPC1A, SLC35B3, ODZ1, PARG, SACS, FBXW2,
FBN1, AUH, ARMCX2, LEPRE1, PLSCR3, LRCH4, MUM1L1, KIF21A,
ERBB2IP, CALB2, TNFRSF21, CTHRC1, TBPL1, EVI5L, NCOR2, TESK2, SLC30A3,
HDAC5, ARID1A, SLC31A2, RANBP2, SLC27A4, DHX40, AP2M1, PCSK6,
LAMP1, PALMD, NID2, CSDA, DBNL, DIAPH1, SLC10A3, SNX7, LMNA,
TGOLN2, P4HA2, TRIM2, AP3B1, LHFP |
| miR-342 | 1 | – |
| miR-497 | 1 | – |
| miR-504 | 1 | – |
Computational prediction and
experimental investigation of miRNA targets
To make a reliable prediction, three popular online
tools (PicTar, miRanda and TargetScan) were used to predict the
targets of each p53- and breast cancer-related miRNA. These tools
make predictions based on different features of miRNA-mRNA
interactions (31). Therefore, for a
certain miRNA, the three tools provide different lists of predicted
targets. The common targets predicted by these three prediction
tools were chosen for further analysis in the present study. With
the exception of miR-342, miR-497 and miR-504, each miRNA exhibited
a different number of predicted targets. The miRNA with the most
targets was miR-9, with 59 targets, while miR-200b and miR-210 only
had one target. A total of 320 genes were predicted to be targeted
by these 19 miRNAs (Table I). Among
these, 25 genes were able to be targeted by two miRNAs.
To validate the above prediction, the pmirGLO
Dual-Luciferase miRNA target expression vector was used to
investigate whether miR-21 could bind to its targets as predicted.
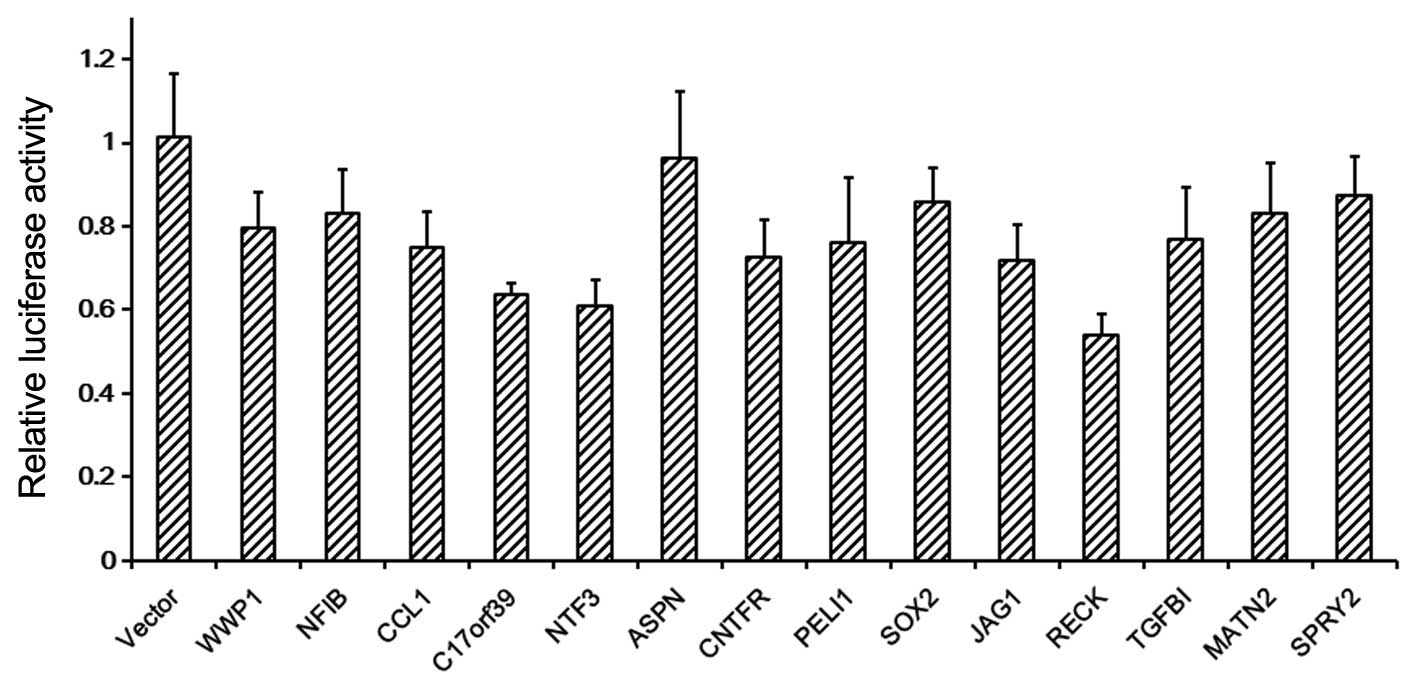
Each of the 14 predicted miR-21 binding sites was cloned downstream
of the Firefly luciferase of the pmirGLO vector, and co-transfected
with miR-21 or scramble mimics into HEK293 cells. Luciferase assay
showed that 13/14 of the predicted targets of miR-21 (not the ASPN
gene) could be regulated by miR-21 (Fig.
1). Moreover, 8/14 of the predicted miR-21 targets have been
validated by other studies [WWP1 (32), NFIB (33), PELI1 (34), SOX2 (35), JAG1 (36), RECK (37), TGFBI (36) and SPRY2 (38)]. These results suggested that the
present miRNA target prediction is reliable.
GO annotation analysis of miRNA
targets
These 320 miRNA target genes were subjected to GO
enrichment analysis. All these genes were categorized based on BP,
MF and CC (Table II). In the CC
category, the nucleus term was the most significant term (with the
lowest P-value) and contained the largest number of genes. In the
MF category, the term with the lowest P-value was transcription
regulatory activity. In the BP category, the most significantly
enriched genes belonged to the cell cycle and proliferation
process, and a total of 42 genes were categorized to this process.
These 42 genes belonged to the targets of 16 miRNAs (Table I). KEGG pathway analysis also showed
similar results, with the number of genes involved in the cell
cycle being the largest (Table
III). This suggested that the targets of p53-related miRNAs
mainly play roles in the cell cycle and proliferation process.
 | Table II.Top 5 significantly enriched Gene
Ontology terms in the microRNA targets. |
Table II.
Top 5 significantly enriched Gene
Ontology terms in the microRNA targets.
| Category | Term | n | P-value |
|---|
| CC | Nucleus | 108 |
1.67×10−7 |
|
| Extracellular
matrix | 14 |
2.98×10−4 |
|
| ER/golgi | 23 | 0.347901 |
|
| Plasma
membrane | 47 | 0.351261 |
|
| Cytosol | 6 | 0.530201 |
| MF | Transcription
regulatory activity | 40 |
9.53×10−7 |
|
| Kinase
activity | 30 |
3.15×10−5 |
|
| Extracellular
structural activity | 3 | 0.007524 |
|
| Enzyme regulator
activity | 19 | 0.009502 |
|
| Nucleic acid
binding activity | 56 | 0.010738 |
| BP | Cell cycle and
proliferation | 42 |
5.36×10−6 |
|
| RNA metabolism | 70 |
3.13×10−5 |
|
| Cell organization
and biogenesis | 50 |
4.74×10−4 |
|
| Protein
metabolism | 57 | 0.027053 |
|
| Cell death | 21 | 0.031232 |
 | Table III.Kyoto Encyclopedia of Genes and
Genomes pathways overrepresented in the lists of microRNA
targets. |
Table III.
Kyoto Encyclopedia of Genes and
Genomes pathways overrepresented in the lists of microRNA
targets.
| Pathway | n | P-value |
|---|
| Cell cycle | 11 |
3.83×10−5 |
| Axon guidance | 9 | 0.002147584 |
| p53 signaling
pathway | 6 | 0.003804563 |
| Notch signaling
pathway | 4 | 0.019950756 |
|
Phosphatidylinositol signaling system | 5 | 0.026477865 |
| Hedgehog signaling
pathway | 4 | 0.037347722 |
| TGF-β signaling
pathway | 5 | 0.041980514 |
| Basal transcription
factors | 3 | 0.042039395 |
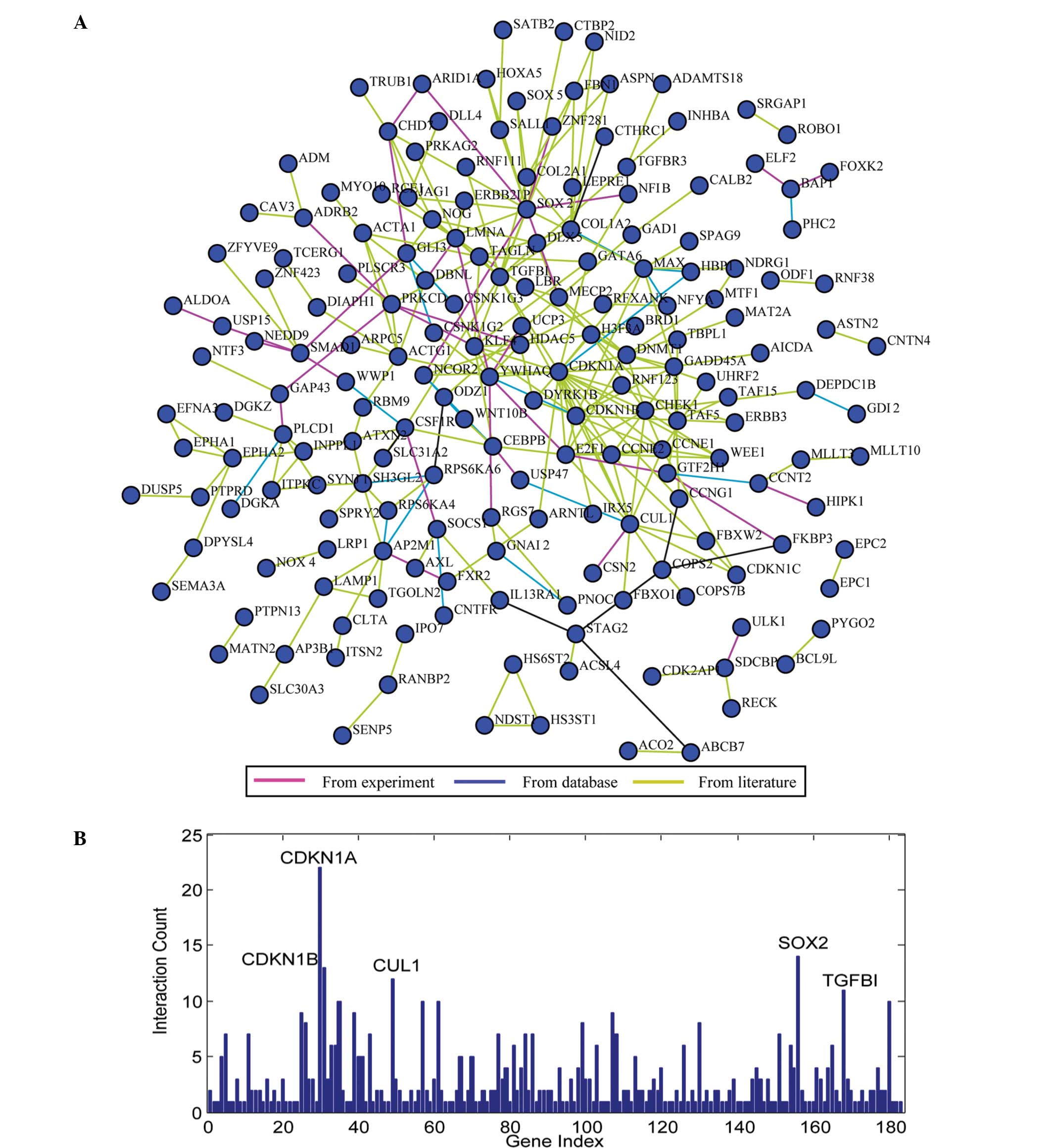
Network analysis of miRNA targets
To understand the association between these miRNA
targets, the KEGG dataset, PPI and Pubmed datasets were integrated
to construct a network of miRNA targets (Fig. 2A). The resulting network was composed
of nodes (genes) and edges (interactions). Fig. 2B shows the degree (i.e., the number of
edges emanating from a node) of each node. In the present network,
the nodes with degree >10 were defined as hubs, including
CDKN1A, SOX2, CDKN1B, CUL1 and TGFBI. According to the
aforementioned GO annotation, these hub genes, with the exception
of TGFBI, were annotated to the term ‘cell cycle and proliferation’
(Table I). In a molecular interaction
network, hubs are more essential for the global network structure
than non-hubs (39). Therefore, it
indicates the roles of targets of p53-related miRNAs in the cell
cycle and proliferation, in accordance with the aforementioned
pathway analysis.
Discussion
In the present study, for the first time, the
interactions of miRNAs and p53 were systematically analyzed in
breast cancer using NLP analysis, and 22 miRNAs associated with p53
in breast cancer were identified. Among these miRNAs, 11 are
transcriptionally or post-transcriptionally upregulated by p53
[miR-10b (11), miR-16 (13,18),
miR-22 (12), miR-26a (13), miR-34a (14), miR-145(18,19),
miR-148a (15), miR-200b (16), miR-200c (16), miR-203 (20) and miR-205 (17)], one directly targets p53 [miR-504
(9)], and others do not directly
interact with p53, but indirectly play roles in the p53 signaling
pathway [e.g., miR-9 (40), miR-21
(41) and miR-222 (10)]. Bioinformatics analysis identified 320
targets of p53-related miRNAs.
Although these 22 p53-related miRNAs have different
sets of targets, GO annotation revealed that the majority of the
miRNA targets were significantly enriched in the cell cycle and
proliferation process. In the network of miRNA targets, the five
hub genes, with the exception of TGFBI, were annotated to cell
cycle processes. TGFBI has been recently reported to affect the
cell cycle via the regulation of p21 and p53 expression (42). Cyclin-dependent kinase inhibitor 1A
(CDKN1A; also known as p21, Cip1 or WAF1) was identified as the
most highly connected hub gene. CDKN1A has been proven to be a
direct target of the p53 tumor suppressor and to play key roles in
mediating p53-dependent cell cycle arrest in response to DNA
damage. In a molecular interaction network, hubs are more essential
for the global network structure than non-hubs (39). The results suggest that p53-related
miRNAs play roles in the cell cycle. A number of studies have
demonstrated that p53 acts as a key regulator of the cell cycle,
mainly by transcriptional regulation of certain key genes in the
cell cycle, such as CDKN1A. The present study suggested that, in
addition to transcriptionally regulating cell cycle-related genes,
p53 also indirectly regulates them through miRNAs. These results
also suggest a previously unknown mechanism for p53 function, and
thus provide an important contribution to our knowledge of p53.
Furthermore, the results of the present study were consistent with
those of Otsuka et al (43)
which revealed that p53-induced miRNAs control the cell cycle and
cell survival via the repression of cell-cycle regulators and/or
antiapoptotic proteins. Additionally, Rokavec et al
(44) summarized previously published
data regarding the interaction between p53 and miRNAs in
gastrointestinal cancer, and found that a total of 32 p53-related
miRNAs exhibit differential expression between normal and tumor
tissue and are associated with clinical and pathological parameters
of gastrointestinal cancer. Among the 32 miRNAs, only 9 miRNAs
(miR-34a, miR-200a, miR-200b, miR-200c, miR-205, miR-145, miR-16,
miR-22, miR-504) are common in both gastrointestinal cancer and
breast cancer (Table I). Thus, we
hypothesize that p53 regulates different sets of miRNAs in various
types of cancer.
Acknowledgements
The authors would like to thank Shanghai Sensichip
Infoteck Co., Ltd., (Shanghai, China) for assistance with the
bioinformatics analysis. This study was supported by the National
Natural Science Foundation of China (grant nos. 81071656 and
81272318) and the Cooperative Innovation Center of Engineering and
New Products for Developmental Biology of Hunan (grant no.
20134486).
References
|
1
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gasco M, Shami S and Crook T: The p53
pathway in breast cancer. Breast Cancer Res. 4:70–76. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacroix M, Toillon RA and Leclercq G: p53
and breast cancer, an update. Endocr Relat Cancer. 13:293–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krell J, Frampton AE, Colombo T, Gall TM,
De Giorgio A, Harding V, Stebbing J and Castellano L: The p53 miRNA
interactome and its potential role in the cancer clinic.
Epigenomics. 5:417–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu N, Lin X, Zhao X, Zheng L, Xiao L, Liu
J, Ge L and Cao S: MiR-125b acts as an oncogene in glioblastoma
cells and inhibits cell apoptosis through p53 and
p38MAPK-independent pathways. Br J Cancer. 109:2853–2863. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Xing R, Zhang X, Dong W, Zhang J,
Yan Z, Li W, Cui J and Lu Y: miR-375 targets the p53 gene to
regulate cellular response to ionizing radiation and etoposide in
gastric cancer cells. DNA Repair (Amst). 12:741–750. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ford NA, Dunlap SM, Wheatley KE and
Hursting SD: Obesity, independent of p53 gene dosage, promotes
mammary tumor progression and upregulates the p53 regulator
microRNA-504. PLoS One. 8:e680892013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang C, Zhang J, Zhang A, Wang Y, Han L,
You Y, Pu P and Kang C: PUMA is a novel target of miR-221/222 in
human epithelial cancers. Int J Oncol. 37:1621–1626.
2010.PubMed/NCBI
|
|
11
|
Bisio A, De Sanctis V, Del Vescovo V,
Denti MA, Jegga AG, Inga A and Ciribilli Y: Identification of new
p53 target microRNAs by bioinformatics and functional analysis. BMC
Cancer. 13:5522013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Huo R, Xiao L, Zhu X, Xie J, Sun S,
He Y, Zhang J, Sun Y, Zhou Z, et al: A novel p53/microRNA-22/Cyr61
axis in synovial cells regulates inflammation in rheumatoid
arthritis. Arthritis Rheumatol. 66:49–59. 2013. View Article : Google Scholar
|
|
13
|
Lezina L, Purmessur N, Antonov AV, Ivanova
T, Karpova E, Krishan K, Ivan M, Aksenova V, Tentler D, Garabadgiu
AV, et al: miR-16 and miR-26a target checkpoint kinases Wee1 and
Chk1 in response to p53 activation by genotoxic stress. Cell Death
Dis. 4:e9532013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Léveillé N, Elkon R, Davalos V, Manoharan
V, Hollingworth D, Oude Vrielink J, le Sage C, Melo CA, Horlings
HM, Wesseling J, et al: Selective inhibition of microRNA
accessibility by RBM38 is required for p53 activity. Nat Commun.
2:5132011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng
X, Zhu Z, Jiao H, Lin J, Jiang K, et al: Hepatitis B virus X
protein represses miRNA-148a to enhance tumorigenesis. J Clin
Invest. 123:630–645. 2013.PubMed/NCBI
|
|
16
|
Kim T, Veronese A, Pichiorri F, Lee TJ,
Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al:
p53 regulates epithelial-mesenchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piovan C, Palmieri D, Di Leva G, et al:
Oncosuppressive role of p53-induced miR-205 in triple negative
breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki HI, Yamagata K, Sugimoto K, Iwamoto
T, Kato S and Miyazono K: Modulation of microRNA processing by p53.
Nature. 460:529–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spizzo R, Nicoloso MS, Lupini L, Lu Y,
Fogarty J, Rossi S, Zagatti B, Fabbri M, Veronese A, Liu X, et al:
miR-145 participates with TP53 in a death-promoting regulatory loop
and targets estrogen receptor-alpha in human breast cancer cells.
Cell Death Differ. 17:246–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang J, Davis-Dusenbery BN, Kashima R, et
al: Acetylation of p53 stimulates miRNA processing and determines
cell survival following genotoxic stress. EMBO J. 32:3192–3205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao W, Xu J, Liu L, Shen H, Zeng H and Shu
Y: A systematic-analysis of predicted miR-21 targets identifies a
signature for lung cancer. Biomed Pharmacother. 66:21–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang J, Zhang ZH and Liu GL: A systematic
analysis of the predicted human La protein targets identified a
hepatitis B virus infection signature. J Viral Hepat. 20:12–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Settles B: ABNER: An open source tool for
automatically tagging genes, proteins and other entity names in
text. Bioinformatics. 21:3191–3192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, et al:
Combinatorial microRNA target predictions. Nat Genet. 37:495–500.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salomonis N, Hanspers K, Zambon AC,
Vranizan K, Lawlor SC, Dahlquist KD, Doniger SW, Stuart J, Conklin
BR and Pico AR: GenMAPP 2: New features and resources for pathway
analysis. BMC Bioinformatics. 8:2172007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pagel P, Kovac S, Oesterheld M, Brauner B,
Dunger-Kaltenbach I, Frishman G, Montrone C, Mark P, Stümpflen V,
Mewes HW, et al: The MIPS mammalian protein-protein interaction
database. Bioinformatics. 21:832–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pavlopoulos GA, Hooper SD, Sifrim A,
Schneider R and Aerts J: Medusa: A tool for exploring and
clustering biological networks. BMC Res Notes. 4:3842011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Y, Xiao Y, Ding X, Zhuo Y, Ren P, Zhou
C and Zhou J: A miR-200b/200c/429-binding site polymorphism in the
3 untranslated region of the AP-2α gene is associated with
cisplatin resistance. PLoS One. 6:e290432011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Witkos TM, Koscianska E and Krzyzosiak WJ:
Practical Aspects of microRNA Target Prediction. Curr Mol Med.
11:93–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang S, Banerjee S, Freitas A, Cui H, Xie
N, Abraham E and Liu G: miR-21 regulates chronic hypoxia-induced
pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol.
302:L521–L529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dellago H, Preschitz-Kammerhofer B,
Terlecki-Zaniewicz L, Schreiner C, Fortschegger K, Chang MW, Hackl
M, Monteforte R, Kühnel H, Schosserer M, et al: High levels of
oncomiR-21 contribute to the senescence-induced growth arrest in
normal human cells and its knock-down increases the replicative
lifespan. Aging Cell. 12:446–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marquez RT, Wendlandt E, Galle CS, Keck K
and McCaffrey AP: MicroRNA-21 is upregulated during the
proliferative phase of liver regeneration, targets Pellino-1, and
inhibits NF-kappaB signaling. Am J Physiol Gastrointest Liver
Physiol. 298:G535–G541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trohatou O, Zagoura D, Bitsika V, Pappa
KI, Antsaklis A, Anagnou NP and Roubelakis MG: Sox2 suppression by
miR-21 governs human mesenchymal stem cell properties. Stem Cells
Transl Med. 3:54–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen B, Chen X, Wu X, Wang X, Wang Y, Lin
TY, Kurata J, Wu J, Vonderfecht S, Sun G, et al: Disruption of
microRNA-21 by TALEN leads to diminished cell transformation and
increased expression of cell-environment interaction genes. Cancer
Lett. 356:506–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sandhir R, Gregory E and Berman NE:
Differential response of miRNA-21 and its targets after traumatic
brain injury in aging mice. Neurochem Int. 78:117–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao J, Tang N, Wu K, Dai W, Ye C, Shi J,
Zhang J, Ning B, Zeng X and Lin Y: MiR-21 simultaneously regulates
ERK1 signaling in HSC activation and hepatocyte EMT in hepatic
fibrosis. PLoS One. 9:e1080052014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He X and Zhang J: Why do hubs tend to be
essential in protein networks? PLoS Genet. 2:e882006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu PY, Deatherage DE, Rodriguez BA,
Liyanarachchi S, Weng YI, Zuo T, Liu J, Cheng AS and Huang TH:
Xenoestrogen-induced epigenetic repression of microRNA-9-3 in
breast epithelial cells. Cancer Res. 69:5936–5945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li B, Wen G, Zhao Y, Tong J and Hei TK:
The role of TGFBI in mesothelioma and breast cancer: Association
with tumor suppression. BMC Cancer. 12:2392012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Otsuka K and Ochiya T: Genetic networks
lead and follow tumor development: microRNA regulation of cell
cycle and apoptosis in the p53 pathways. Biomed Res Int.
2014:7497242014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rokavec M, Li H, Jiang L and Hermeking H:
The p53/microRNA connection in gastrointestinal cancer. Clin Exp
Gastroenterol. 7:395–413. 2014.PubMed/NCBI
|