Introduction
Cervical cancer is the fourth most common cancer in
women, and the seventh overall, with an estimated 528,000 new cases
in 2012. There were an estimated 266,000 mortalities from cervical
cancer worldwide in 2012, accounting for 7.5% of all female cancer
mortalities. Around 87% of mortalities from cervical cancer occur
in less developed regions (1).
Guidelines for the treatment of uterine cervical
cancer, based on the results of randomized controlled trials, have
been published by the National Comprehensive Cancer Network
(2) and National Cancer Institute
(3). For early-stage cervical cancer,
the first-line treatment consists of radical surgery alone or in
combination with adjuvant radiotherapy, while concurrent
chemoradiotherapy with cisplatin may also be used as an adjuvant
therapy in cases of high-risk early-stage cervical cancer,
particularly those positive for lymph node metastasis.
Chemoradiotherapy is also recommended in cases of locally advanced
cancer (3). However, with the
exclusion of chemoradiotherapy, the role of chemotherapy as a
treatment for uterine cervical cancer has not been clearly
established.
In cases involving distant metastasis or recurrence,
chemotherapy consisting of cisplatin combined with paclitaxel is
currently considered to be among the most effective regimens, based
on findings from a randomized controlled study (4). In addition, for cases of intermediate-
and high-risk, early-stage disease, the postoperative use of
cisplatin-based chemotherapy has demonstrated some degree of
clinical efficacy (5–8). However, chemotherapeutic protocols for
cervical cancer have are poorly defined at present; in order to
establish the effect of chemotherapy on clinical outcome in cases
of cervical cancer, large-scale clinical trials are necessary.
Furthermore, it is important to establish which factors may be used
to predict and alter chemosensitivity to cisplatin to facilitate
the individualization of treatment and inform clinical
decisions.
We have previously studied the chemosensitivity of
the platinum analog nedaplatin (NDP) using the histoculture drug
response assay (HDRA; see methods) (9). The sensitivity of cervical cancers to
NDP was predicted by the HDRA; the true positive rate was
determined to be 100%, whilst the true negative rate was 55.6% and
the accurate prediction rate was 77.8%. Furthermore, the
disease-free survival rate for the high-sensitivity group was
generally higher compared with that of the low-sensitivity group in
patients who received postoperative adjuvant chemotherapy with NDP
(9). Thus, NDP is associated with a
high response rate in cervical cancers that is comparable to the
response rate achieved with cisplatin. In addition, NDP has fewer
gastrointestinal side effects, is less nephrotoxic and requires
less additional fluid during infusion (10,11). For
this reason, NDP has been widely used in Japan for the treatment of
cervical cancer and was the agent selected for the present HDRA
chemosensitivity analysis.
With the current trend of individualizing
chemotherapy treatment protocols, studies must consider the various
factors that predict and affect chemosensitivity in a given tumor.
Establishing more information with regard to these factors may
allow prediction of the chemosensitivity of cervical cancer in
routine clinicopathological tests without using HDRA, and
individualized chemotherapy may become clinically feasible in the
treatment of cervical cancer.
The current study investigated the association
between NDP sensitivity and the expression of biological factors
affecting chemosensitivity, including the cell proliferation marker
protein Ki-67, apoptosis-related factors [p53, B-cell lymphoma-2
(Bcl-2), Bcl-2-associated X protein (Bax), cleaved caspase-3, and
cyclooxygenase-2 (COX-2)] and the nucleotide excision
repair-related protein excision repair cross-complementation group
1 (ERCC1), in cervical cancer specimens using
immunohistochemistry.
Materials and methods
Subjects
The current study was performed as an extension of
our previous study, and patient characteristics and the study
design are described elsewhere (9). A
total of 179 patients with invasive cervical squamous cell
carcinoma were treated between April 2002 and August 2009 at Fujita
Health University Hospital (Toyoake, Japan). Among these 179
patients, 45 with International Federation of Gynecology and
Obstetrics (FIGO) stage IB1-IVB disease were enrolled in the study
after providing informed consent. The median age of patients was 46
years (range, 30–67 years). Clinical FIGO stages were as follows:
Stage IB1, 12 patients; stage IB2, 3 patients; stage IIA, 6
patients; stage IIB, 16 patients; stage IIIA, 1 patient; stage
IIIB, 4 patients; and stage IVB, 3 patients. HDRA was performed on
18 pretreatment biopsies and 27 surgical specimens obtained from
these patients to determine the chemosensitivity of cervical cancer
to NDP. The HDRA procedure was described previously (12). Briefly, collagen gel sponge (Gelform,
Pfizer, USA) was cut into ~1 cm3 cubes and placed into
the wells of a 24-well plate. The concentration of NDP in the
medium was set at 6.25, 12.5, 25, 50 and 100 µg/ml. Specimens were
washed and cut into ~1 mm3 pieces in medium (1 ml/well),
and placed on the collagen gel sponge. Specimens were then cultured
for 7 days. Following incubation, cell viability was assessed using
an MTT assay to identify the concentration that produced 50%
inhibition of tumor growth (IC50). The optimal cut-off
value of NDP was set at 48 µg/ml based on the IC50 of
NDP determined in our previous study (9); cases with an IC50 below or
above the cut-off concentrations were defined as having high or low
sensitivity to NDP, respectively.
Immunohistochemistry
Immunohistochemical staining was performed using the
avidin-biotin-peroxidase complex method for 18 pretreatment
biopsies and 27 surgical specimens. Briefly, formalin-fixed,
paraffin-embedded sections were deparaffinized, rehydrated, and
treated with 3% H2O2 in methanol to block
endogenous peroxidase activity. Following antigen retrieval in a
microwave oven with 10 mM citrate buffer (pH 6.0; Cosmo Bio Co.,
Ltd., Tokyo, Japan) at 90°C, the sections were incubated overnight
with primary antibodies at 4°C, followed by incubation with
biotinylated goat anti-mouse IgG (cat. no. BA-9200) or goat
anti-rabbit IgG (cat. no. BA-1000) secondary antibodies (Vector
Laboratories, Inc., Burlingame, CA, USA). Staining was visualized
using avidin-biotin-peroxidase complex solution (Vectastain ABC
kit, Vector Laboratories, Inc.) and
3,3′-diaminobenzidine-H2O2 solution, and
counterstaining with hematoxylin was conducted.
Primary antibodies against human Ki-67 (MIB-1; mouse
monoclonal; cat. no. N1653; dilution, 1:100; Dako, Glostrup,
Denmark), p53 (DO-7; mouse monoclonal; cat. no. N1581; dilution,
1:100; Dako), Bcl-2 (124; mouse monoclonal; cat. no. 713141;
dilution, 1:1; Nichirei Biosciences, Inc., Tokyo, Japan), Bax (B-9;
mouse monoclonal; cat. no. sc-7480; dilution, 1:100; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), cleaved caspase-3
(Asp175; mouse monoclonal; cat. no. 9661S; dilution, 1:400; Cell
Signaling Technology, Danvers, MA, USA), COX-2 (rabbit monoclonal;
cat. no. 18516; dilution, 1:50; Immuno-Biological Laboratories Co.,
Ltd., Fujioka, Japan) and ERCC1 (8F1; mouse monoclonal; cat. no.
MA5-13912; dilution, 1:100; Thermo Fisher Scientific, Waltham, MA,
USA) were used. The primary antibody was omitted for the negative
control. For positive controls, sections known to overexpress each
protein from the initial study were always run.
Immunostaining for Bcl-2, Bax and COX-2 was graded
according to immunohistochemical score; scores from 0–18 were
determined by the multiplication of scores for frequency (scored
from 1–6) and intensity (scored from 0–3) of staining (12). Staining frequency was scored based on
the proportion of positively staining cells as follows: 1, 0–4%; 2,
5–19%; 3, 20–39%; 4, 40–59%; 5, 60–79%; or 6, 80–100%. Staining
intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3
(strong). Cases having a score >2 were defined as positive in
the current study. Evaluation of immunostaining for ERCC1 was
graded according to immunohistochemical score as described by Kim
et al (13); scores ranged
from 0–9 and were determined by multiplying the scores for staining
frequency (0, 0%; 1, 0–9%; 2, 10–49%; 3, 50–100%) and intensity
(scored from 0–3, as above). Based on immunoreactivity, samples
with scores of ≥4 were defined as positive in this study. For Ki-67
and p53, the percentage of immunostained tumor cells from 10 fields
that showed relatively higher expression levels throughout the
tumor was employed as the labeling index (LI) to evaluate the
expression level. For cleaved caspase-3, the number of positively
stained cells per 1000 tumor cells exhibiting the highest level of
expression on the section was designated as the apoptotic index
(AI) and was used to evaluate apoptotic cells.
Evaluations were performed by two gynecological
oncologists, neither of whom had knowledge of any clinical
information related to the cases.
Statistical analysis
All measured values were represented as the mean ±
standard deviation and were analyzed by Student's t-test and
Fisher's exact test. SPSS software version 12.0 (SPSS, Inc.,
Chicago, IL, USA) was used to conduct the analyses. P<0.05 was
considered to indicate statistical significance.
Results
NDP chemosensitivity of 45 patients
with cervical cancer
Using IC50 values below or above the
cut-off concentration to define high or low sensitivities to NDP,
respectively (9), 17 cases were
classified into the high-sensitivity group and the remaining 28
cases were classified into the low-sensitivity group.
Association between chemosensitivity
and the expression of various proteins
The typical protein expression of seven biological
factors evaluated in this study is presented in Fig. 1. The associations between
chemosensitivity to NDP and expression of these seven factors are
described in Tables I and II. The analysis revealed that the relative
expression levels of p53 (LI), Bcl-2 (score), cleaved caspase-3
(AI) and COX-2 (score) in the high-sensitivity group versus the
low-sensitivity group were as follows: p53, 14.1±10.5 vs.
28.2±18.6; Bcl-2, 1.1±1.5 vs. 3.9±3.3; cleaved caspase-3, 29.4±18.6
vs. 17.1±10.6; and COX-2, 2.6±2.2 vs. 4.7±3.2 (Table I). The expression levels of these four
factors differed significantly between the high- and
low-sensitivity groups (P=0.002, 0.002, 0.021, and 0.041,
respectively). However, no significant differences in the
expression levels of Ki-67 (LI), Bax (score) or ERCC1 (score) were
observed between the high- and low-sensitivity groups (Table I).
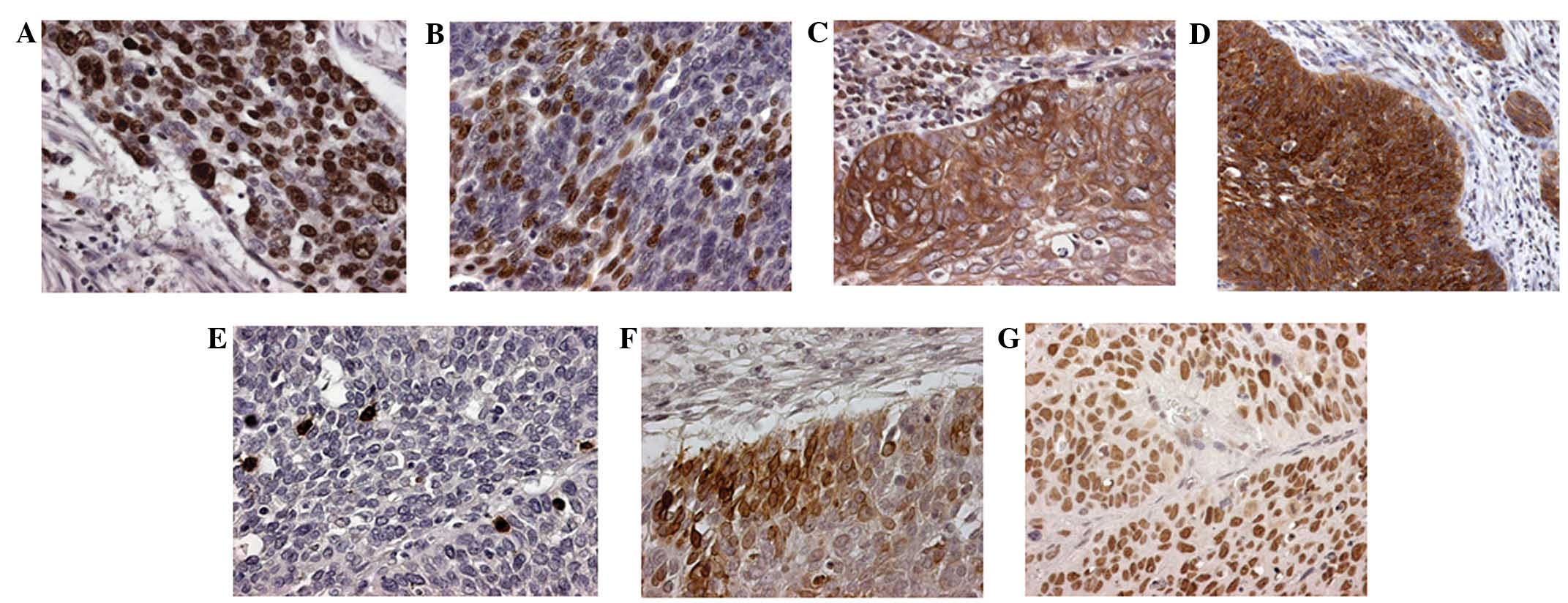 | Figure 1.Typical protein expression of seven
biological factors evaluated in the present study, observed by
immunohistochemistry (original magnification, ×200). (A) Ki-67 (LI,
75%); (B) p53 (LI, 39%); (C) B-cell lymphoma-2 (score, 8); (D)
Bcl-2-associated X protein (score, 6); (E) cleaved caspase-3 (AI,
38.6); (F) cyclooxygenase-2 (score, 15); (G) excision repair
cross-complementation group 1 (score, 9). LI, labeling index; AI,
apoptotic index (per 1000). |
 | Table I.Expression levels of various proteins
in high and low nedaplatin sensitivity groups. |
Table I.
Expression levels of various proteins
in high and low nedaplatin sensitivity groups.
|
| Nedaplatin
sensitivity |
|
|---|
|
|
|
|
|---|
| Factor | High (n=17) | Low (n=28) | P-value |
|---|
| Ki-67 LI, % | 43.9±20.0 | 47.4±18.7 | 0.428 |
| p53 LI, % | 14.1±10.5 | 28.2±18.6 | 0.002 |
| Bcl-2 score | 1.1±1.5 | 3.9±3.3 | 0.002 |
| Bax score | 1.8±2.3 | 1.2±1.5 | 0.632 |
| Cleaved caspase-3
AI | 29.4±18.6 | 17.1±10.6 | 0.021 |
| COX-2 score | 2.6±2.2 | 4.7±3.2 | 0.041 |
| ERCC1 score | 3.4±1.9 | 4.7±3.2 | 0.399 |
 | Table II.Association between expression of
various proteins and nedaplatin sensitivity. |
Table II.
Association between expression of
various proteins and nedaplatin sensitivity.
|
|
| Nedaplatin
sensitivity, n |
|
|---|
|
|
|
|
|
|---|
| Factor | Patients, n | High (n=17) | Low (n=28) | P-value |
|---|
| Ki-67 LI, % |
|
|
| 0.608 |
|
<40 | 19 | 8 | 11 |
|
|
≥40 | 26 | 9 | 17 |
|
| p53 LI, % |
|
|
| 0.009 |
|
<30 | 29 | 15 | 14 |
|
|
≥30 | 16 | 2 | 14 |
|
| Bcl-2 |
|
|
| 0.0008 |
|
Negative | 20 | 13 | 7 |
|
|
Positive | 25 | 4 | 21 |
|
| Bax |
|
|
| 0.608 |
|
Negative | 26 | 9 | 17 |
|
|
Positive | 19 | 8 | 11 |
|
| Cleaved caspase-3
AI |
|
|
| 0.016 |
|
<40 | 41 | 13 | 28 |
|
|
≥40 | 4 | 4 | 0 |
|
| COX-2 |
|
|
| 0.023 |
|
Negative | 6 | 5 | 1 |
|
|
Positive | 39 | 12 | 27 |
|
| ERCC1 |
|
|
| 0.079 |
|
Negative | 19 | 10 | 9 |
|
|
Positive | 26 | 7 | 19 |
|
Furthermore, using cut-off values able to
significantly distinguish between high and low expression groups, a
p53 LI of <30 (n=29) was significantly associated with high NDP
sensitivity (P=0.009; Table II), as
was a cleaved caspase-3 AI ≥40 (n=4; P=0.016). Patients with
negative immunostaining of Bcl-2 (n=20) and COX-2 (n=6) were also
significantly more sensitive to NDP (P=0.0008 and 0.023,
respectively). For ERCC1, patients with negative immunostaining
(n=19) tended to be more sensitive to NDP (P=0.079); however, this
difference was not significant (Table
II).
Discussion
HDRA, developed by Hoffman et al (14,15) in
1991, and improved by Ohie et al (16) in 2000, resembles an in vivo
assay as it supports three-dimensional proliferation on a collagen
gel matrix. The advantages of HDRA are that it has a high
evaluation rate, permits the prompt acquisition of results,
correlates with clinical responses, allows for the testing of
multiple anticancer drugs and is relatively inexpensive.
A number of clinical studies using HDRA have
demonstrated that it has a high evaluation rate or accuracy in a
number of tumor types, including non-small cell lung cancer
(17), esophageal cancer (18), stomach and colorectal cancer (19), breast cancer (20,21),
urothelial cancer (22), head and
neck cancer (23) and soft tissue
sarcoma (24). In gynecological
cancers, the accurate prediction rates of sensitivity to platinum
were 83% and 87.5% in ovarian and endometrial cancers, respectively
(25,26). Additionally, a correlation between
chemosensitivity to platinum and prognosis in endometrial cancer
has been observed (26). Furthermore,
in our previous study, the evaluation rate of HDRA was 94.0%, with
a true positive rate of 100%, true negative rate of 55.6% and
accurate prediction rate of 77.8% in cervical cancer (squamous cell
carcinoma). Additionally, disease-free survival in the
high-sensitivity group tended to be higher compared with that of
the low-sensitivity group in patients who received postoperative
adjuvant chemotherapy with NDP (9).
In the current study, we investigated whether the
expression levels of Ki-67, p53, Bcl-2, Bax, cleaved caspase-3,
COX-2, and ERCC1 were associated with chemosensitivity to NDP, as
evaluated by HDRA, in cervical cancer. Identification of factors
associated with NDP sensitivity may potentially facilitate the
prediction of platinum sensitivity by immunohistochemistry in
pretreatment biopsies or surgical specimens, which would be easier
than using HDRA.
In general, tumors that have high proliferative
activity are more sensitive to chemotherapeutic drugs or radiation
compared with tumors that have low proliferative activity. This
association between tumor proliferative activity and response to
chemotherapy has been reported in breast cancer (27,28).
Additionally, in cervical cancer, Sultana et al (29) reported that high expression of Ki-67
in preoperative biopsies was associated with the clinical response
to platinum-containing neoadjuvant chemotherapy and improved
prognosis. On the other hand, Costa et al (30) reported that there was no correlation
between Ki-67 expression and clinical response to neoadjuvant
chemotherapy or prognosis. In the current study, no association was
identified between expression of Ki-67 and sensitivity to NDP, as
evaluated by HDRA, consistent with the findings of Costa et
al. However, the number of patients recruited in these studies,
including the present study, was relatively small; therefore,
further studies must be conducted with larger numbers of patients
to confirm this association.
Platinum-induced apoptosis in tumor cells is
generally dependent on the p53 pathway. However, when tumors
possess P53 mutations, platinum does not induce apoptosis as
effectively, and the clinical effects of platinum are restricted
accordingly. Studies have demonstrated that patients with advanced
ovarian cancer harboring P53 mutations or P53
overexpression exhibit low sensitivity to cisplatin-containing
chemotherapy (31,32). The rate of P53 mutation in
cervical cancer has been reported to be <10%, which is lower
than that in certain other types of cancers (33). However, the relationship between
P53 mutations and human papillomavirus (HPV) has not been
clearly defined (34). In the current
study, high-risk type HPV infections were also evaluated in 22 of
45 specimens; these specimens were frozen and adequately stored,
allowing them to be used for polymerase chain reaction
(PCR)-restriction fragment length polymorphism analysis. From this
analysis, high-risk type HPV infections were identified in 19 of 22
specimens (86.4%; data not shown). Furthermore, in the same 22
specimens, only 2 (9.1%) were found to possess P53 mutations
(exons 5 and 6) by PCR-single strand conformation polymorphism
(data not shown). This indicates that P53 mutations are
relatively infrequent in cervical cancer compared with other types
of cancer. Additionally, the rate of p53 protein overexpression has
been reported to be 20–85% (33). p53
protein overexpression is not fully explained by P53
mutations, and several mechanisms have been proposed for this
discrepancy (35–37); however, the precise reasons for p53
overexpression, including HPV integration, have not been
proven.
In addition, the association between p53
overexpression and radiation sensitivity or sensitivity to
platinum-containing chemotherapy have not been clearly defined in
cervical cancer. Sultana et al (29) reported that there was no correlation
between p53 expression in tumors and the clinical effects of
platinum-containing chemotherapy. In the current study, a
significant difference in p53 expression (LI) was observed between
the high- and low-NDP sensitivity groups. Furthermore, patients
with p53 LIs of <30% were significantly more sensitive to NDP;
therefore, these data suggest that the p53 LI may be utilized as a
parameter for the easy prediction of platinum sensitivity using
pretreatment biopsies or surgical specimens without requiring
HDRA.
Bcl-2 is an oncogene that inhibits radiation- or
chemotherapy-induced apoptosis (38).
Cervical cancers with Bcl-2 expression have been reported to
exhibit radiation resistance and poor prognoses (39–41).
However, contradictory results have also been found in terms of
Bcl-2 expression and patient prognosis (42–49).
Furthermore, in one study, no significant association was observed
between Bcl-2 expression and the clinical effects of neoadjuvant
chemotherapy (29). Thus, the
association between Bcl-2 expression and
chemosensitivity/radiosensitivity or prognosis has not been
precisely defined. In the current study, a significant difference
in Bcl-2 expression was observed between the high- and
low-sensitivity groups. Additionally, tumors that were positive for
Bcl-2 expression exhibited low sensitivity to platinum. However,
because few studies have examined the relationship between Bcl-2
expression and chemosensitivity, further studies are necessary to
clarify this relationship with larger numbers of patients.
Bax is a member of the Bcl-2 family and plays a role
in the p53-Bax pathway, which enhances apoptosis. In cervical
cancer patients treated by radiotherapy alone, patients with
Bax-positive tumors have been demonstrated to have a higher
sensitivity to radiotherapy compared with those with Bax-negative
tumors (50). Additionally, Bax
expression in tumors may be used to predict radiosensitivity in
these tumors (50). On the other
hand, few studies have reported the association between Bax
expression and the effects of chemotherapy. In one such study,
Sultana et al (29) observed
an association between Bax expression in preoperative biopsies of
cervical cancer and the response rate to neoadjuvant
chemotherapy.
In the current study, no association was found
between Bax expression and NDP sensitivity in cervical cancer
specimens. However, in cervical cancer, p53 function may be
inactivated by HPV integration; therefore, various aspects of the
p53-Bax pathway may be maintained in cervical tumors, and these
mechanisms require additional investigation.
Caspase-3, a member of the caspase family, is
expressed in its active form in apoptotic cells and acts at the
final step of apoptosis. Therefore, immunohistochemistry using
anti-cleaved caspase-3 antibodies may be a relatively convenient
method to detect apoptotic cells (51). In cervical cancer, a number of studies
have reported that a high AI in pretreatment specimens (so-called
spontaneous AI) is correlated with reduced efficacy of radiotherapy
(52–54). In general, high AI in tumors is
associated with intratumoral hypoxia, which in turn may decrease
radiosensitivity. However, there has been no consensus on the
association between intratumoral spontaneous AI and
radiosensitivity due to differences in tumor histology,
intratumoral distribution of hypoxia and cellular proliferative
activity. On the other hand, Sultana et al (29) reported that spontaneous AI in
pretreatment specimens was not correlated with the clinical effect
of neoadjuvant chemotherapy. In the current study, higher
expression of cleaved caspase-3 (AI) was significantly correlated
with chemosensitivity to NDP. Therefore, the expression of cleaved
caspase-3 may also permit detection of NDP chemosensitivity in
cancer. Future studies should investigate the association between
spontaneous AI and chemosensitivity or radiosensitivity.
The role of COX-2 in the development and progression
of various types of cancer has been previously described. COX-2
overexpression is associated with the proliferation of tumor cells,
inhibition of apoptosis, production of growth factors, increased
activity in neoangiogenesis and invasion, and impairment of host
immune responses (55–59). In particular, numerous studies have
revealed that tumors exhibiting high expression levels of COX-2
protein have reduced responses to chemotherapy and poor prognoses;
this is thought to occur because COX-2 inhibits apoptosis by
inducing Bcl-2 expression or reducing intratumoral ceramide
concentrations, which play important roles in apoptosis (60). In cervical cancer, Ferrandina et
al (61) reported that
COX-2-positive cases were resistant to cisplatin and had
unfavorable prognoses compared to that in COX-2-negative cases. In
the current study, patients with negative COX-2 immunostaining were
significantly more sensitive to NDP. These data suggested that
COX-2 expression affected sensitivity to platinum in cervical
cancer. Additionally, as COX-2 induces Bcl-2 expression (60), the association between COX-2 and Bcl-2
expression was also investigated. However, a significant
correlation between these factors was not observed (data not
shown).
ERCC1 is involved in nucleotide excision repair and
has been demonstrated to form a heterodimer with xeroderma
pigmentosum-F (XPF). ERCC1/XPF complexes are responsible for an
incision that cleaves the damaged nucleotide strand at the 5′ end
of the lesion (62). Therefore, ERCC1
has a key role in mediating the response to a range of DNA-damaging
chemotherapeutic agents. Evidence has suggested that increased
expression of ERCC1 protein and mRNA are associated with resistance
to platinum, and may be prognostic indicators of poor patient
survival following treatment with platinum-based chemotherapy or
chemoradiotherapy in various types of malignant neoplasms (63–71).
Park et al (71) reported that evaluating expression
levels of ERCC1 protein in pretreatment specimens of FIGO stage IIB
uterine cervical cancer may allow the prediction of response to
cisplatin-based neoadjuvant chemotherapy; low ERCC1 expression was
also reported to be a significant favorable prognostic indicator of
disease-free survival. Our previous retrospective study indicated
that immunostaining for ERCC1 expression may be useful for
predicting survival in patients with uterine cervical
adenocarcinoma being treated with platinum-based chemotherapy or
chemoradiotherapy with cisplatin (72). Unexpectedly, in the current study, no
significant association was identified between ERCC1 expression and
NDP sensitivity in cervical cancer (squamous cell carcinoma).
However, ERCC1-positive cases tended to exhibit low sensitivity to
NDP. Thus, the association between ERCC1 expression and
chemosensitivity to platinum will likely be clarified when further
investigations with large numbers of patients are conducted.
In conclusion, the results of the present study
indicated that p53, Bcl-2, cleaved caspase-3, and COX-2 were
biological factors affecting NDP sensitivity in cervical cancer.
Low or negative expression of p53, Bcl-2, and COX-2, and high or
positive expression of cleaved caspase-3 were significantly
associated with high sensitivity to NDP. Furthermore, negative
ERCC1 expression may also be correlated with high sensitivity to
NDP. Therefore, sensitivity to platinum can be easily predicted by
immunostaining for these factors using pretreatment biopsies or
surgical specimens. The expression profiles of these proteins may
provide additional information for planning individualized
chemotherapy in patients with uterine cervical cancer. In addition,
a number of other biological factors affecting platinum sensitivity
have been reported (73–75), and further evaluations should be
conducted in the future. As more factors are tested and
incorporated into screening panels, individualized chemotherapy,
designed to maximize treatment response, may become clinically
feasible in cervical cancer.
References
|
1
|
World Health Organization; International
Agency for Research on Cancer: Cervical Cancer - Estimated
Incidence. Mortality and Prevalence Worldwide in. 2012.http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=cervix
|
|
2
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
Cervical Cancer. Version 1. 2013.http://www.alabmed.com/uploadfile/2014/0221/20140221105002791.pdfAccessed.
September 11–2015
|
|
3
|
National Cancer Institute: Cervical cancer
treatment. http://www.cancer.gov/types/cervical/patient/cervical-treatment-pdqAccessed.
September 11–2015
|
|
4
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combination in stage IVB,
recurrent or persistent cervical carcinoma: A gynecologic oncology
group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thigpen JT: The role of chemotherapy in
the management of carcinoma of the cervix. Cancer J. 9:425–432.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tattersall MH, Ramirez C and Coppleson M:
A randomized trial of adjuvant chemotherapy after radical
hysterectomy in stage Ib-IIa cervical cancer patients with pelvic
lymph node metastasis. Gynecol Oncol. 46:176–181. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeshima N, Umayahara K, Fujiwara K,
Hirai Y, Takizawa K and Hasumi K: Treatment results of adjuvant
chemotherapy after radical hysterectomy for intermediate-and
high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 103:618–622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lahousen M, Haas J, Pickel H, Hackl A,
Kurz C, Ogris H, Stummvoll W and Winter R: Chemotherapy versus
radiotherapy versus observation for high-risk cervical carcinoma
after radical hysterectomy: A randomized, prospective, multicenter
trial. Gynecol Oncol. 73:196–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kato R, Hasegawa K, Achiwa Y, Okamoto H,
Torii Y, Oe S and Udagawa Y: Predicting nedaplatin sensitivity of
cervical cancer using the histoculture drug response assay. Eur J
Gynaecol Oncol. 32:381–386. 2011.PubMed/NCBI
|
|
10
|
Yamamoto K, Kokawa K, Nishimura R,
Hasegawa K, Konishi I, Saji F, Nishida M, Noguchi H and Takizawa K:
Phase I study of combination chemotherapy with irinotecan
hydrochloride and nedaplatin for cervical squamous cell carcinoma:
Japanese gynecologic oncology group study. Oncol Rep. 21:1005–1009.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe Y, Nakai H, Etoh T, Kanemura K,
Tsuji I, Ishizu A and Hoshiai H: Feasibility study of docetaxel and
nedaplatin for recurrent squamous cell carcinoma of the uterine
cervix. Anticancer Res. 28:2385–2388. 2008.PubMed/NCBI
|
|
12
|
Detre S, Jotti Saclani G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MK, Cho KJ, Kwon GY, Park SI, Kim YH,
Kim JH, Song HY, Shin JH, Jung HY, Lee GH, et al: ERCC1 predicting
chemoradiation resistance and poor outcome in oesophageal cancer.
Eur J Cancer. 44:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vescio RA, Connors KM, Kubota T and
Hoffman RM: Correlation of histology and drug response of human
tumors grown in negative-state three-dimensional histoculture and
in nude mice. Proc Natl Acad Sci USA. 88:5163–5166. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffman RM: In vitro assay for
chemotherapy sensitivity. Crit Rev Oncol Hematol. 15:99–111. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohie S, Udagawa Y, Kozu A, Komuro Y, Aoki
D, Nozawa S, Moossa AR and Hoffman RM: Cisplatin sensitivity of
ovarian cancer in the histoculture drug response assay correlates
to clinical response to combination chemotherapy with cisplatin,
doxorubicin and cyclophosphamide. Anticancer Res. 20:2049–2054.
2000.PubMed/NCBI
|
|
17
|
Yoshimasu T, Ohta F, Oura S, Tamaki T,
Shimizu Y, Naito K, Kiyoi M, Hirai Y, Kawago M and Okamura Y:
Histoculture drug response assay for gefitinib in non-small-cell
lung cancer. Gen Thorac Cardiovasc Surg. 57:138–143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita Y, Hiramatsu M, Kawai M, Nishimura
H, Miyamoto A and Tanigawa N: Histoculture drug response assay
predicts the postoperative prognosis of patients with esophageal
cancer. Oncol Rep. 21:499–505. 2009.PubMed/NCBI
|
|
19
|
Furukawa T, Kubota T and Hoffman RM:
Clinical application of the histoculture drug response assay. Clin
Cancer Res. 1:305–311. 1995.PubMed/NCBI
|
|
20
|
Furukawa T, Kubota T, Tanino H, et al:
Chemosensitivity of breast cancer lymph node metastasis compared to
the primary tumor from individual patients tested in the
histoculture drug response assay. Anticancer Res. 20:3657–3658.
2000.PubMed/NCBI
|
|
21
|
Tanino H, Oura S, Hoffman RM, et al:
Acquisition of multidrug resistance in recurrent breast cancer
demonstrated by the histoculture drug response assay. Anticancer
Res. 21:4083–4086. 2001.PubMed/NCBI
|
|
22
|
Hirano Y, Kageyama S, Ushiyama T, Suzuki K
and Fujita K: Clinical usefulness of chemotherapy based on an in
vitro chemosensitivity test in urothelial cancer patients.
Anticancer Res. 21:4061–4066. 2001.PubMed/NCBI
|
|
23
|
Singh B, Li R, Xu LI, et al: Prediction of
survival in patients with head and neck cancer using the
histoculture drug response assay. Head Neck. 24:437–442. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morioka H, Yabe H, Morii T, Yamada R, Kato
S, Yuasa S and Yano T: In vitro chemosensitivity of human soft
tissue sarcoma. Anticancer Res. 21:4147–4151. 2001.PubMed/NCBI
|
|
25
|
Nakata S, Aoki D, Ohie S, Horiguchi M,
Suzuki N, Kanasugi M, Susumu N, Udagawa Y and Nozawa S:
Chemosensitivity testing of ovarian cancer using the histoculture
drug response assay: Sensitivity to cisplatin and clinical
response. Int J Gynecol Cancer. 15:445–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanasugi M, Aoki D, Suzuki N, Susumu N,
Nakata S, Horiguchi M, Udagawa Y and Nozawa S: Sensitivity to
cisplatin determined by the histoculture drug response assay and
clinical response of endometrial cancer. Int J Gyenecol Cancer.
16:409–415. 2006. View Article : Google Scholar
|
|
27
|
Bonetti A, Zaninelli M, Rodella S, Molino
A, Sperotto L, Piubello Q, Bonetti F, Nortilli R, Turazza M and
Cetto GL: Tumor proliferative activity and response to first-line
chemotherapy in advanced breast carcinoma. Breast Cancer Res Treat.
38:289–297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Reilly SM, Camplejohn RS, Rubens RD and
Richards MA: DNA flow cytometry and response to preoperative
chemotherapy for primary breast cancer. Eur J Cancer. 28:681–683.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sultana H, Kigawa J, Kanamori Y, Itamochi
H, Oishi T, Sato S, Kamazawa S, Ohwada M, Suzuki M and Terakawa N:
Chemosensitivity and p53-Bax pathway-mediated apoptosis in patients
with uterine cervical cancer. Ann Oncol. 14:214–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Costa S, Terzano P, Santini D, Ceccarelli
C, Martoni A, Angelelli B, Panetta A, Bovicelli A, Cristiani P,
Lipponen P, et al: Neoadjuvant chemotherapy in cervical carcinoma:
Regulators of cell cycle, apoptosis, and proliferation as
determinants of response to therapy and disease outcome. Am J Clin
Pathol. 116:729–737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Righetti SC, Della Torre G, Pilotti S,
Ménard S, Ottone F, Colnaghi MI, Pierotti MA, Lavarino C,
Cornarotti M, Oriana S, et al: A comparative study of p53 gene
mutations, protein accumulation and response to cisplatin-based
chemotherapy in advanced ovarian carcinoma. Cancer Res. 56:689–693.
1996.PubMed/NCBI
|
|
32
|
Sato S, Kigawa J, Minagawa Y, Okada M,
Shimada M, Takahashi M, Kamazawa S and Terakawa N: Chemosensitivity
and p53-dependent apoptosis in epithelial ovarian carcinoma.
Cancer. 86:1307–1313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu X and Feki A: Phenotypic features with
p53 alterations related to human papillomavirus and prognostic
evaluation in cervical cancer. Int J Gynecol Cancer. 16:708–717.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soussi T, Dehouche K and Béroud C: P53
website and analysis of p53 gene mutations in human cancer: forging
a link between epidemiology and carcinogenesis. Hum Mutat.
15:105–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kraiss S, Spiess S, Reihsaus E and
Montenarh M: Correlation of metabolic stability and altered
quaternary structure of oncoprotein p53 with cell transformation.
Exp Cell Res. 192:157–164. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banin S, Moyal L, Shieh S, Taya Y,
Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y
and Ziv Y: Enhanced phosphorylation of p53 by ATM in response to
DNA damage. Science. 281:1674–1677. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirao A, Kong YY, Matsuoka S, et al: DNA
damage-induced activation of p53 by the checkpoint kinase Chk2.
Science. 287:1824–1827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reed JC: Bcl-2 and the regulation of
programmed cell death. J Cell Biol. 124:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pillai MR, Jayaprakash PG and Nair MK:
Bcl-2 immunoreactivity but not p53 accumulation associated with
tumour response to radiotherapy in cervical carcinoma. J Cancer Res
Clin Oncol. 125:55–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rajkumar T, Rajan S, Baruah RK, Majhi U,
Selvaluxmi G and Vasanthan A: Prognostic significance of Bcl-2 and
p53 protein expression in stage IIB and IIIB squamous cell
carcinoma of the cervix. Eur J Gynaecol Oncol. 19:556–560.
1998.PubMed/NCBI
|
|
41
|
Wootipoom V, Lekhyananda N, Phungrassami
T, Boonyaphiphat P and Thongsuksai P: Prognostic significance of
Bax, Bcl-2 and p53 expressions in cervical squamous cell carcinoma
treated by radiotherapy. Gynecol Oncol. 94:636–642. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pezzella F, Turley H, Kuzu I, et al: Bcl-2
protein in non-small cell lung carcinoma. N Engl J Med.
329:690–694. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Joensuu H, Pylkkänen L and Toikkanen S:
Bcl-2 protein expression and long-term survival in breast cancer.
Am J Pathol. 145:1191–1198. 1994.PubMed/NCBI
|
|
44
|
Diebold J, Baretton G, Felchner M, et al:
Bcl-2 expression, p53 accumulation, and apoptosis in ovarian
carcinoma. Am J Clin Pathol. 105:341–349. 1996.PubMed/NCBI
|
|
45
|
Crawford RA, Caldwell C, Iles RK, et al:
Prognostic significance of the bcl-2 apoptotic family of proteins
in primary and recurrent cervical cancer. Br J Cancer. 78:210–214.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Itoi T, Yamana K, Bilim V, Takahashi K and
Tomita F: Impact of frequent bcl-2 expression on better prognosis
in renal cell carcinoma patients. Br J Cancer. 90:200–205. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Saito T, Takehara M, Tanaka R, Lee R,
Horie M, Wataba K, Ito E and Kudo R: Correlation between
responsiveness of neoadjuvant chemotherapy and apoptosis-associated
proteins for cervical adenocarcinoma. Gynecol Oncol. 92:284–292.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Munakata S, Watanabe O, Ohashi K and
Morino H: Expression of Fas ligand and bcl-2 in cervical carcinoma
and their prognostic significance. Am J Clin Pathol. 123:879–885.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Padovan P, Salmaso R, Marchetti M and
Padovan R: Prognostic value of bcl-2, p53 and Ki-67 in invasive
squamous carcinoma of the uterine cervix. Eur J Gynecol Oncol.
21:267–272. 2000.
|
|
50
|
Mukherjee G, Freeman A, Moore R,
Kumaraswamy, Devi KU, Morris LS, Coleman N, Dilworth S, Prabhakaran
PS and Stanley MA: Biologic factors and response to radiotherapy in
carcinoma of the cervix. Int J Gynecol Cancer. 11:187–193. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gown AM and Willingham MC: Improved
detection of apoptotic cells in archival paraffin sections:
Immunohistochemistry using antibodies to cleaved caspase 3. J
Histochem Cytochem. 50:449–454. 2000. View Article : Google Scholar
|
|
52
|
Levine EL, Renehan A, Gossiel R, Davidson
SE, Roberts SA, Chadwick C, Wilks DP, Potten CS, Hendry JH, Hunter
RD, et al: Apoptosis, intrinsic radiosensitivity and prediction of
radiotherapy response in cervical carcinoma. Radiother Oncol.
37:1–9. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Levine EL, Davidson SE, Roberts SA,
Chadwick CA, Potten CS and West CM: Apoptosis as predictor of
response to radiotherapy in cervical carcinoma. Lancet.
344:4721994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim JY, Cho HY, Lee KC, Hwang YJ, Lee MH,
Roberts SA and Kim CH: Tumor apoptosis in cervical cancer: Its role
as a prognostic factor in 42 radiotherapy patients. Int J Cancer.
96:305–312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tomozawa S, Tsuno NH, Sunamio E, Hatano K,
Kitayama J, Osada T, Saito S, Tsuruo T, Shibata Y and Nagawa H:
Cyclooxygenase-2 overexpression correlates with tumor recurrence,
especially haematogenous metastasis of colorectal cancer. Br J
Cancer. 83:324–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Madaan S, Abel PD, Chaudhary KS, Hewitt R,
Stott MA, Stamp GW and Lalani EN: Cytoplasmic induction and
overexpression of cyclooxygenase-2 in human prostate cancer:
Implication for prevention and treatment. BJU Int. 86:736–741.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hwang D, Skollard D, Byerne J and Levine
E: Expression of Cyclooxygenase-1 and cyclooxygenase-2 in human
breast cancer. J Natl Cancer Inst. 90:455–460. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Achiwa H, Yatabe Y, Hida T, Kuroishi T,
Kozaki K, Nakamura S, Ogawa M, Sugiura T, Mitsudomi T and Takahashi
T: Prognostic significance of elevated cyclooxygenase-2 expression
in primary resected lung adenocarcinomas. Clin Cancer Res.
5:1001–1005. 1999.PubMed/NCBI
|
|
59
|
Sheehan KM, Sheahan K, O'Donoghue DP,
MacSweeney F, Conroy RM, Fitzgerald DJ and Murray FE: The
relationship between Cyclooxygenase-2 expression and colorectal
cancer. JAMA. 282:1254–1257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu XH, Yao S, Kirschenbaum A and Levine
AC: NS398, a selective cyclooxygenase-2 inhibitor, induces
apoptosis and down regulation bcl-2 expression in LNCaP cells.
Cancer Res. 58:4254–4249. 1998.
|
|
61
|
Ferrandina G, Lauriola L, Distefana MG,
Zannoni GF, Gessi M, Legge F, Maggiano N, Mancuso S, Capelli A,
Scambia G and Ranelletti FO: Increased cyclooxygenase-2 expression
is associated with chemotherapy resistance and poor survival in
cervical cancer patients. J Clin Oncol. 20:973–981. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
de Laat WL, Appeldoorn E, Jaspers NG and
Hoeijmakers JH: DNA structural elements required for ERCC1-XPF
endonuclease activity. J Biol Chem. 273:7835–7842. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lord RV, Brabender J, Gandara D, Alberola
V, Camps C, Domine M, Cardenal F, Sánchez JM, Gumerlock PH, Tarón
M, et al: Low ERCC1 expression correlates with prolonged survival
after cisplatin plus gemcitabine chemotherapy in non-small cell
lung cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
64
|
Azuma K, Komohara Y, Sasada T, Terazaki Y,
Ikeda J, Hoshino T, Itoh K, Yamada A and Aizawa H: Excision repair
cross-complementation group 1 predicts progression-free and overall
survival in non-small cell lung cancer patients treated with
platinum-based chemotherapy. Cancer Sci. 98:1336–1343. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small-lung cancer and cisplatin-based
adjuvant chemotherapy. N Engl J Med. 355:983–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Metzger R, Leichman CG, Danenberg KD, et
al: ERCC1 mRNA levels complement thymidylate synthase mRNA levels
in predicting response and survival for gastric cancer patients
receiving combination cisplatin and fluorouracil chemotherapy. J
Clin Oncol. 16:309–316. 1998.PubMed/NCBI
|
|
67
|
Kim MK, Cho KJ, Kwan GY, et al: ERCC1
predicting chemoradiation resistance and poor outcome in
oesophageal cancer. Eur J Cancer. 44:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bellmunt J, Paz-Ares L, Cuello M, Cecere
FL, Albiol S, Guillem V, Gallardo E, Carles J, Mendez P, de la Cruz
JJ, et al: Gene expression of ERCC1 as a novel prognostic marker in
advanced bladder cancer patients receiving cisplatin-based
chemotherapy. Ann Oncol. 18:522–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Steffensen KD, Waldstrøm M and Jakobsen A:
The relationship of platinum resistance and ERCC1 protein
expression in epithelial ovarian cancer. Int J Gynecol Cancer.
19:820–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Scheil-Bertram S, Tylus-Schaaf P, du Bois
A, Harter P, Oppitz M, Ewald-Riegler N and Fisseler-Eckhoff A:
Excision repair cross-complementation group 1 protein
overexpression as a predictor of poor survival for high-grade
serous ovarian adenocarcinoma. Gynecol Oncol. 119:325–331. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Park JS, Jeon EK, Chun SH, Won HS, Lee A,
Hur SY, Lee KH, Bae SN, Yoon SC and Hong SH: ERCC1 (excision repair
cross-complementation group 1) expression as a predictor for
response of neoadjuvant chemotherapy for FIGO 2B uterine cervix
cancer. Gynecol Oncol. 120:275–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hasegawa K, Kato R, Torii Y, Ichikawa R,
Oe S and Udagawa Y: The relationship between ERCC1 expression and
clinical outcome in patients with FIGO stage I to stage II uterine
cervical adenocarcinoma. Int J Gynecol Cancer. 21:1479–1485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Costa S, Terzano P, Bovicelli A, Martoni
A, Angelelli B, Santini D, Ceccarelli C, Lipponen P, Erzén M,
Syrjänen S and Syrjänen K: CD44 isoform 6 (CD44v6) is a prognostic
indicatior of the response to neoadjuvant chemotherapy in cervical
cancer. Gynecol Oncol. 80:67–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chung HH, Kim MK, Kim JW, Park NH, Song
YS, Kang SB and Lee HP: XRCC1 R399Q polymorphism is associated with
response to platinum-based neoadjuvant chemotherapy in bulky
cervical cancer. Gynecol Oncol. 103:1031–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Faried LS, Faried A, Kanuma T, Sano T,
Nakazato T, Tamura T, Kuwano H and Minegishi T: Predictive and
prognostic role of activated mammalian target of rapamycin in
cervical cancer treated with cisplatin-based neoadjuvant
chemotherapy. Oncol Rep. 16:57–63. 2006.PubMed/NCBI
|















