Introduction
Colorectal cancer (CRC) is one of the most common
causes of cancer-related mortality in the world, and its prevalence
and mortality rates have been increasing (1). Currently, chemoprevention, a strategy
which attempts to prevent cancer using non-toxic chemical entities,
has attracted much attention (2,3). Although
the possible effects of >50 agents, including non-steroidal
anti-inflammatory drugs (NSAIDs), aspirin, metformin, fiber,
statins, eicosapentaenoic acid (EPA), peroxisome
proliferator-activated receptor γ (PPARγ) agonists and folate, have
been evaluated for the protection against CRC development in
epidemiological studies (4–6), the use of the majority of these agents
is only in the early experimental stage. Although the occurrence of
CRC and/or colorectal adenoma is regarded as a reliable endpoint in
chemoprevention trials (7),
evaluation of such an endpoint would require inclusion of hundreds
of subjects in the studies and extremely long observation periods.
To maximize the efficiency of early-phase chemoprevention trials,
more easily modifiable and easy-to-measure surrogate biomarkers are
therefore required.
Colorectal carcinogenesis is based on the
adenoma-carcinoma sequence; CRC is believed to develop through the
accumulation of multiple acquired genetic and epigenetic
alterations that cause the malignant transformation of the normal
colonic epithelium to adenocarcinoma (8,9). Over the
last decade, the emergence of aberrant crypt foci (ACF) as putative
precursors to colorectal adenoma has been witnessed, and these ACF
have come to be regarded as potential biomarkers for CRC (10–18). In
the rodent model, the formation of ACF has been demonstrated to be
enhanced by cancer promoters and suppressed by chemopreventive
agents (19–23). These results indicate that
chemopreventive agents against CRC may also prevent the formation
of ACF, not only in carcinogen-treated rodent models, but also in
humans. However, few studies have investigated the correlations
between the presence of ACF and the use of candidate
chemopreventive agents of CRC in humans. Moreover, the associations
between the presence of ACF and lifestyle factors, including
obesity, smoking and alcohol intake)(24,25), or
comorbid medical conditions, such as diabetes mellitus (DM)
(26), considered to be associated
with CRC development remain unknown.
In the present study, the association between the
presence of ACF and lifestyle factors or comorbid medical
conditions was investigated. Furthermore, the correlation between
the presence of ACF and the use of medications that are believed to
be associated with colorectal carcinogenesis are also investigated.
If ACF were indeed a reliable biomarker of CRC in humans, their
formation may also be expected to be closely associated with the
aforementioned factors. The present results may pave the way for
further evaluations of the potential usefulness of ACF as a
surrogate biomarker of CRC, and also for further evaluation of
candidate chemopreventive agents.
Materials and methods
Patients
The medical records of 1,024 patients who underwent
total colonoscopy and rectal ACF counting at the Yokohama City
University Hospital (Yokohama, Kanagawa, Japan) between May 2004
and October 2013 were reviewed retrospectively. The collected
patient data included the age, gender, body mass index, smoking
history, alcohol intake history, history of medication use (NSAIDs,
aspirin, statins, metformin, EPA, PPARγ agonists and insulin), and
history of comorbid medical conditions (hypertension,
hyperlipidemia and DM). Subjects were excluded if they had a
history of familial adenomatous polyposis, hereditary non-polyposis
CRC, inflammatory bowel disease or radiation colitis, or had
undergone a prior large-bowel resection. Finally, a total of 902
subjects were enrolled in this study. To confirm the close
association between the presence of ACF and colonic tumors, the
subjects were divided into three categories (normal subjects,
adenoma cases and CRC cases), as previously described (15). This study was conducted with the
approval of the Yokohama City University Hospital Ethics Committee.
Written informed consent was obtained from all the subjects prior
to their participation in the study.
Endoscopic identification of ACF
ACF are usually identified as clusters of crypts
that stain darker than the surrounding normal mucosa. Crypts with a
larger size, a raised appearance, a thicker epithelial lining,
dilated or slit-like crypt lumina, and an increased pericryptal
area as compared to the surrounding normal mucosa are the most
frequently used criteria to identify ACF (18). A Fujinon EC-490ZW5/M magnifying
colonoscope (Fujifilm Medical Co., Ltd., Tokyo, Japan) was used for
identifying the ACF. All the subjects underwent bowel preparation
using a polyethylene glycol-based solution and total colonoscopy
prior to the rectal ACF imaging. Subsequently, 0.25% methylene blue
was applied to the mucosa using a spray catheter and allowed to
stand for 2 min. ACF counting was performed in the lower rectal
region, from the middle Houston valve to the dentate line.
Cell proliferative activity of the
rectal colonic epithelial mucosa
To evaluate the association between the colonic
epithelial mucosal proliferative activity and the presence of ACF,
the Ki-67 labeling index was calculated. In certain cases,
normal-appearing rectal mucosa was biopsied, fixed with formalin
and embedded in paraffin, and 4-µm thick sections were prepared.
Immunohistochemical staining for Ki-67 with anti-Ki-67 rabbit
monoclonal antibody (Nichirei Bioscience Inc., Tokyo, Japan) was
performed as previously described (27).
Statistical analysis
The differences in the prevalence and number of ACF
among the three groups (normal subjects, adenoma cases and CRC
cases) were compared using the χ2 (or Fisher's exact)
test or the Kruskal-Wallis test. The associations between the
presence of ACF and lifestyle factors, history of use of oral
medications or comorbid medical conditions were evaluated by the
χ2 (or Fisher's exact) test and/or Student's t-test. To
identify the risk factors for the increased prevalence of ACF,
univariate and multivariate logistic regression analyses were
performed. The differences in the Ki-67 labeling indices were
evaluated by the Mann-Whitney U test. Unless otherwise specified,
P<0.05 was considered to denote a statistically significant
difference. All analyses were performed using the SPSS statistical
software package (version 11.0; SPSS, Inc., Chicago, IL, USA).
Results
Association between the presence of
ACF and colorectal tumors
The prevalence and number of ACF in each of the
three study groups (normal subjects, adenoma cases and CRC cases)
are shown in Table I. The
prevalence/number of ACF were 66.1%/5.3±4.8, 84.0%/5.3±4.8 and
88.9%/7.3±5.7, in the normal subjects, adenoma cases and CRC cases,
respectively. Consistent with previous studies (15,18), the
prevalence and number of ACF increased significantly from the
normal subjects to the CRC cases (P<0.01 and P<0.01,
respectively).
 | Table I.Prevalence and number of ACF in the
study groups. |
Table I.
Prevalence and number of ACF in the
study groups.
| Factor | Normal | Adenoma cases | CRC cases | P-value |
|---|
| Number of
patients | 391 | 412 | 99 |
|
| ACF prevalence
(%)a | 66.1 | 84.0 | 88.9 | <0.001 |
| ACF number (mean ±
SD)b | 5.3±4.8 | 5.6±5.3 | 7.3±5.7 | <0.001 |
Associations between the presence of
ACF and clinical factors that are believed to be associated with
colorectal carcinogenesis
The associations between the presence of ACF and
lifestyle factors, use of oral medications or comorbid medical
conditions are shown in Table II.
The prevalence of ACF was significantly higher in patients with an
older age (69.3 vs. 95.6%; P<0.01), a male gender (70.9 vs.
79.9%; P<0.01), a positive smoking habit (69.3 vs. 85.4%;
P<0.01), positive alcohol intake (72.3 vs. 81.0%; P<0.01), DM
(74.3 vs. 84.8%; P<0.01) and in those receiving insulin therapy
(75.5 vs. 97.8%; P<0.01) than in those without these factors.
ACF were present in higher numbers in patients with an older age
(2.7±3.9 vs. 8.6±5.5; P<0.01), a male gender (3.9±5.1 vs.
4.7±5.2; P=0.03), a positive smoking habit (3.7±5.0 vs. 5.2±5.3;
P<0.01), DM (4.1±5.0 vs. 5.3±5.7; P=0.04) and in those receiving
insulin therapy (4.3±5.1 vs. 6.4±6.2; P<0.01) than in those
without these factors. However, a lower prevalence and number of
ACF were not significantly associated with any of the examined
factors.
 | Table II.Association between the presence of
ACF and clinical factors believed to be associated with colorectal
carcinogenesis. |
Table II.
Association between the presence of
ACF and clinical factors believed to be associated with colorectal
carcinogenesis.
| Factor | Variable (−) | Variable (+) | P-value |
|---|
| Demographic and
lifestyle factors |
|
|
|
| Older age >70
years |
|
|
|
| ACF
prevalence, n (%) | 451 (69.3) | 240 (95.6) | <0.01 |
| ACF
number (mean ± SD) | 2.7±3.9 | 8.6±5.6 | <0.01 |
| Male gender |
|
|
|
| ACF
prevalence, n (%) | 234 (70.9) | 457 (79.9) | <0.01 |
| ACF
number (mean ± SD) | 3.9±5.1 | 4.7±5.2 |
0.03 |
| BMI ≥25 |
|
|
|
| ACF
prevalence, n (%) | 512 (75.7) | 179 (79.2) |
0.27 |
| ACF
number (mean ± SD) | 5.3±6.8 | 6.2±6.6 |
0.08 |
| Smoking habit |
|
|
|
| ACF
prevalence, n (%) | 341 (69.3) | 350 (85.4) | <0.01 |
| ACF
number (mean ± SD) | 3.7±5.0 | 5.2±5.3 | <0.01 |
| Alcohol intake |
|
|
|
| ACF
prevalence, n (%) | 329 (72.3) | 362 (81.0) | <0.01 |
| ACF
number (mean ± SD) | 4.3±5.6 | 4.4±4.6 |
0.84 |
| Comorbid medical
conditions |
|
|
|
| Hypertension |
|
|
|
| ACF
prevalence, n (%) | 443 (75.3) | 248 (79.0) |
0.25 |
| ACF
number (mean ± SD) | 4.4±4.9 | 4.8±5.5 |
0.08 |
| Hyperlipidemia |
|
|
|
| ACF
prevalence, n (%) | 555 (76.3) | 136 (77.7) |
0.77 |
| ACF
number (mean ± SD) | 4.5±4.9 | 4.9±5.9 |
0.06 |
| DM |
|
|
|
| ACF
prevalence, n (%) | 524 (74.3) | 167 (84.8) | <0.01 |
| ACF
number (mean ± SD) | 4.1±5.0 | 5.3±5.7 |
0.04 |
| Medication use |
|
|
|
| NSAIDs |
|
|
|
| ACF
prevalence, n (%) | 661 (76.6) | 30
(76.9) | >0.99 |
| ACF
number (mean ± SD) | 4.4±5.1 | 4.4±6.2 |
0.94 |
| Aspirin |
|
|
|
| ACF
prevalence, n (%) | 650 (76.9) | 41
(71.9) |
0.42 |
| ACF
number (mean ± SD) | 4.4±5.1 | 4.7±5.4 |
0.65 |
| Statins |
|
|
|
| ACF
prevalence, n (%) | 610 (77.3) | 81
(71.7) |
0.19 |
| ACF
number (mean ± SD) | 4.3±5.0 | 4.9±6.1 |
0.27 |
| Metformin |
|
|
|
| ACF
prevalence, n (%) | 673 (76.5) | 18
(81.8) |
0.80 |
| ACF
number (mean ± SD) | 4.4±5.2 | 4.3±3.3 |
0.93 |
| EPA |
|
|
|
| ACF
prevalence, n (%) | 685 (76.5) | 6
(85.7) | >0.99 |
| ACF
number (mean ± SD) | 4.4±5.1 | 3.6±3.0 |
0.69 |
| PPARγ agonists |
|
|
|
| ACF
prevalence, n (%) | 677 (76.4) | 14
(87.5) |
0.39 |
| ACF
number (mean ± SD) | 4.3±5.1 | 3.6±2.4 |
0.58 |
| Insulin |
|
|
|
| ACF
prevalence, n (%) | 646 (75.5%) | 45
(97.8) | <0.01 |
| ACF
number (mean ± SD) | 4.3±5.1 | 6.4±6.2 | <0.01 |
Risk factors for the increased
prevalence of ACF
To gain insight into the contribution of the
increased prevalence of ACF, univariate and multivariate logistic
regression analyses were performed, as shown in Table III. Univariate analysis identified
an older age [odds ratio (OR), 9.68; 95% confidence interval (CI),
5.17–18.1; P<0.01], the male gender (OR, 1.63; 95% CI,
1.19–2.23; P<0.01), the presence of adenoma (OR, 2.65; 95% CI,
1.90–3.71; P<0.01), the presence of CRC (OR, 4.12; 95% CI,
2.13–7.99; P<0.01), a history of smoking (OR, 2.56; 95% CI,
1.83–3.58; P<0.01), a history of alcohol intake (OR, 1.63; 95%
CI, 1.19–2.23; P<0.01), the presence of DM (OR, 1.92; 95% CI,
1.26–2.94; P<0.01), a history of insulin therapy (OR, 14.6; 95%
CI, 2.00–106.8; P<0.01) as significant factors associated with
the increased prevalence of ACF. Furthermore, age- and
gender-adjusted multivariate logistic regression analysis
identified a history of smoking (OR, 1.78; 95% CI, 1.20–2.63;
P<0.01) and a history of insulin therapy (OR, 9.97; 95% CI
1.28–77.5; P=0.03) as showing a significant independent association
with an increased prevalence of ACF, regardless of the
presence/absence of colonic tumors.
 | Table III.Univariate and multivariate logistic
regression analyses to identify the factors associated with the
increased prevalence of ACF. |
Table III.
Univariate and multivariate logistic
regression analyses to identify the factors associated with the
increased prevalence of ACF.
|
| OR (95% CI) |
|---|
|
|
|
|---|
| Variable | Univariate | P-value | Multivariate | P-value |
|---|
| Older age >70
years | 9.68
(5.17–18.1) | <0.01 | 9.19
(4.78–17.7) | <0.01 |
| Male gender | 1.63
(1.19–2.23) | <0.01 | 0.71
(0.54–1.03) |
0.08 |
| BMI ≥25 | 1.09
(0.75–1.57) |
0.66 |
|
|
| Smoking habit | 2.58
(1.85–3.61) | <0.01 | 1.80
(1.21–2.67) | <0.01 |
| Alcohol intake | 1.63
(1.19–2.23) | <0.01 | 1.18
(0.80–1.74) |
0.40 |
| Presence of colonic
tumors |
|
|
|
|
| Normal
subject | Reference |
|
|
|
| Adenoma
cases | 2.65
(1.90–3.71) | <0.01 | 2.40
(1.67–3.45) | <0.01 |
| CRC
cases | 4.12
(2.13–7.99) | <0.01 | 3.15
(1.58–6.28) | <0.01 |
| Comorbid medical
conditions |
|
|
|
|
|
Hypertension | 1.23
(0.88–1.71) |
0.22 |
|
|
|
Hyperlipidemia | 1.08
(0.73–1.60) |
0.70 |
|
|
| DM | 1.92
(1.26–2.94) | <0.01 | 1.21
(0.75–1.96) |
0.43 |
| Medication use |
|
|
|
|
|
NSAIDs | 1.02
(0.48–2.18) |
0.96 |
|
|
|
Aspirin | 0.77
(0.42–1.40) |
0.39 |
|
|
|
Statins | 0.74
(0.46–1.16) |
0.19 |
|
|
|
Metformin | 1.38
(0.46–4.14) |
0.56 |
|
|
|
EPA | 1.84
(0.22–15.4) |
0.57 |
|
|
| PPARγ
agonists | 2.16
(0.49–9.59) |
0.31 |
|
|
|
Insulin | 14.6
(2.00–106.8) | <0.01 | 10.2
(1.31–79.0) |
0.03 |
Comparison of the presence of ACF
between diabetic patients receiving and not receiving insulin
therapy
To investigate the difference in the prevalence of
ACF between diabetic patients receiving and not receiving insulin
therapy, the prevalence and number of ACF were compared between 46
diabetic patients receiving insulin therapy and 151 diabetic
patients not receiving insulin therapy (Table IV). The prevalence of ACF in the
diabetic patients receiving insulin therapy was significantly
higher than that in those not receiving insulin therapy (97.8 vs.
80.1%; P<0.01). The median number of ACF in the diabetic
patients receiving insulin therapy was significantly higher than
that in those not receiving insulin therapy (4.5±2.5 vs. 3.0±3.0;
P=0.03).
 | Table IV.Comparison of the presence of ACF
between diabetic patients receiving/not receiving therapy. |
Table IV.
Comparison of the presence of ACF
between diabetic patients receiving/not receiving therapy.
| Factor | Insulin therapy
(+) | Insulin therapy
(−) | P-value |
|---|
| Number of
patients | 46 | 151 |
|
| Age, years (mean ±
SD)a | 64.1±10.6 | 66.8±10.4 |
0.10 |
| Gender
(M/F)b | 23/23 | 117/34 | <0.01 |
| ACF |
|
|
|
|
Prevalence, n (%)b | 45 (97.8%) | 121 (80.1%) | <0.01 |
| Number,
median (range)c | 4.5 (2.0–7.0) | 3.0 (0–6.0) |
0.03 |
Comparison of the proliferative
activity of the colonic mucosal epithelium in diabetic patients
receiving and not receiving insulin therapy
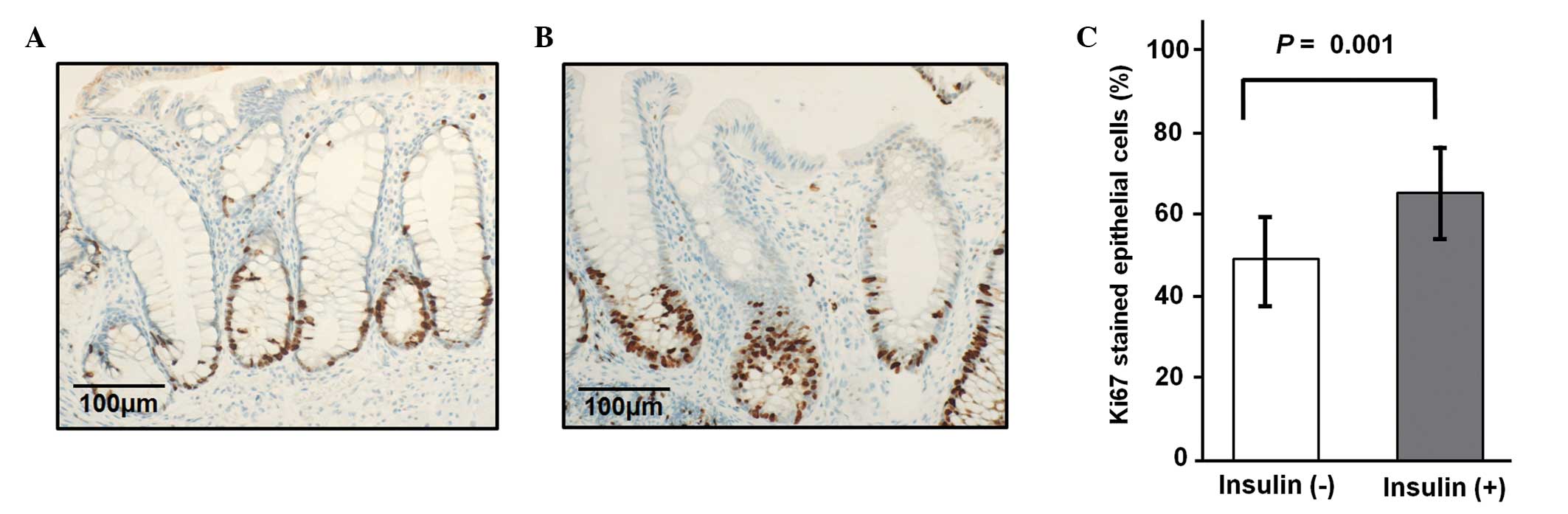
The Ki-67 data from a total of 20 biopsy samples of
normal-appearing rectal mucosa obtained from diabetic patients
(including 10 patients receiving insulin and 10 patients not
receiving insulin) were measured. The differences in the
immunohistochemical staining and labeling index for Ki-67 between
the specimens obtained from the diabetic patients receiving and not
receiving insulin therapy are shown in Fig. 1. The proportion of proliferating cells
in the patients receiving insulin therapy was significantly higher
than that in the patients not receiving insulin therapy (64.1 vs.
48.6%; P=0.01).
Discussion
The current study presents the results of a large,
cross-sectional study conducted to investigate the associations
between factors associated with the development of CRC and the
presence of rectal ACF. The main aim of the study was to evaluate
the potential usefulness of ACF as a surrogate biomarker of CRC,
and to discuss candidate chemopreventive agents.
Consistent with previous studies (15,16,18), the
present study confirmed that the prevalence and number of ACF
increased significantly from normal subjects to CRC cases. In
addition, it was also confirmed that an older age, the male gender
and a positive smoking habit were associated with an increased
prevalence and number of ACF, as found in previous studies
(12,18,23,28). The
close link between tobacco smoking and an increased risk of CRC is
considered to be mediated by the large number of carcinogens that
can cause irreversible genetic damage to the normal colonic mucosa
(29). Moreover, Stevens et al
reported that the number of ACF was higher in patients with a
family history of CRC compared with that in patients without a
family history of CRC (30). In a
recent study, we demonstrated that the number of ACF can be a
useful predictor of the likelihood of colorectal adenoma recurrence
(31). Taken together, the present
results and those of previous studies lend support to the notion
that ACF can serve as a reliable surrogate biomarker of CRC.
In the present study, no association could be
identified between candidate chemoprevention agents and a low
prevalence and number of ACF, despite these drugs having been
demonstrated to prevent colorectal tumors in experimental models
and/or epidemiological studies. Although this can be explained in
part by the small number of patients in the subgroups of the study,
particularly with regard to medication use, these results raise
doubt about the validity of using ACF as an intermediate endpoint
in CRC chemoprevention trials. Recently, several early-phase CRC
chemoprevention trials have been conducted using ACF as an
intermediate endpoint (5,6,32,33). Takayama et al conducted an
ACF-based open-label chemoprevention trial and demonstrated the
chemopreventive efficacy of sulindac (6). By contrast, a study by Limburg et
al, in which an ACF-based randomized phase II chemoprevention
trial was conducted, did not confirm the chemopreventive efficacy
of sulindac (32). The differences in
the prevalence of dysplastic ACF, presenting as
histologically-confirmed dysplasia, may have an effect on these
results. In addition, the differences in the criteria for the
endoscopic identification of ACF and/or the counting area may also
affect these results. Nonetheless, further investigations are
required prior to incorporating these lesions into chemoprevention
trials as intermediate endpoints.
Recently, a series of studies and meta-analyses
confirmed that the risk for CRC is elevated in diabetic patients
(26,34,35).
Investigation of the molecular mechanisms for this connection has
led to the so-called hyperinsulinemia hypothesis stating that
insulin may, at high serum concentrations, increase the risk of CRC
by acting as a mitogen and promoting the formation of colonic
tumors (36). The present results
indicated that insulin enhances colonic epithelial proliferation
and initiates the formation of ACF, irrespective of the
presence/absence of DM, emphasizing the importance of insulin in
colorectal carcinogenesis. Shpitz et al reported that the
proliferative activity of ACF was significantly increased compared
with that of normal mucosa (17).
Notably, at least one ACF could be identified in >60% of even
normal subjects. Therefore, we hypothesized that the majority of
ACF only reflect the increased proliferative activity of the
colonic mucosa and that only a small proportion of ACF, which show
dysplastic change, could be regarded as preneoplastic lesions of
CRC. Although several studies have reported a high incidence of
KRAS mutation in human ACF (37,38), the
precise molecular trigger for the development of dysplasia in the
ACF remains unknown. Therefore, additional studies are required to
reveal the molecular basis and role of ACF in colorectal
carcinogenesis.
The present study had certain limitations. Firstly,
it was a retrospective study and patients without sufficient
clinical data, including data on the behavioral characteristics,
medication history and comorbid medical conditions, were excluded,
therefore, a selection bias was inevitable. Secondly, the number of
patients taking specific candidate chemopreventive drugs (e.g.,
metformin, EPA and PPARγ) was not sufficiently large, which could
limit any conclusions. Thirdly, the results lacked follow-up data,
and the study could not evaluate whether these clinical factors
truly affected the presence of ACF. Finally, the presence of
dysplastic ACF was not evaluated, as no association between the
shape of the crypt lumen and the histological presence of dysplasia
has been reported (39). The reported
prevalence of dysplastic ACF in patients with CRC varies widely
from 0.5 to 22% (10,16–18,31,40). This indicates that a consistent,
reproducible set of criteria to identify dysplastic ACF by their
descriptive features or a consistent scheme for their histological
classification is still lacking. The histological criteria for the
identification of dysplastic ACF also require standardization.
In conclusion, data from the present retrospective,
large, cross-sectional study revealed the potential usefulness of
ACF as a surrogate biomarker of CRC, however, useful data on
candidate chemopreventive agents could not be obtained. The results
indicated that insulin can enhance colonic epithelial proliferative
activity and induce the formation of ACF, thereby possibly
triggering CRC development.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
|
EPA
|
eicosapentaenoic acid
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
ACF
|
aberrant crypt foci
|
|
BMI
|
body mass index
|
|
DM
|
diabetes mellitus
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Das D, Arber N and Jankowski JA:
Chemoprevention of colorectal cancer. Digestion. 76:51–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niitsu Y, Takayama T, Miyanishi K, Nobuoka
A, Hayashi T, Kukitsu T, Takanashi K, Ishiwatari H, Abe T, Kogawa
T, et al: Chemoprevention of colorectal cancer. Cancer Chemother
Pharmacol. 54(Suppl 1): S40–S43. 2004.PubMed/NCBI
|
|
4
|
Boghossian S and Hawash A: Chemoprevention
in colorectal cancer-where we stand and what we have learned from
twenty year's experience. Surgeon. 10:43–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hosono K, Endo H, Takahashi H, Sugiyama M,
Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, et al:
Metformin suppresses colorectal aberrant crypt foci in a short-term
clinical trial. Cancer Prev Res (Phila). 3:1077–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayama T, Nagashima H, Maeda M, Nojiri
S, Hirayama M, Nakano Y, Takahashi Y, Sato Y, Sekikawa H, Mori M,
et al: Randomized double-blind trial of sulindac and etodolac to
eradicate aberrant crypt foci and to prevent sporadic colorectal
polyps. Clin Cancer Res. 17:3803–3811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arber N, Eagle CJ, Spicak J, Rácz I, Dite
P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al:
Celecoxib for the prevention of colorectal adenomatous polyps. N
Engl J Med. 355:885–895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grady WM and Carethers JM: Genomic and
epigenetic instability in colorectal cancer pathogenesis.
Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adler DG, Gostout CJ, Sorbi D, Burgart LJ,
Wang L and Harmsen WS: Endoscopic identification and quantification
of aberrant crypt foci in the human colon. Gastrointest Endosc.
56:657–662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bird RP: Observation and quantification of
aberrant crypts in the murine colon treated with a colon
carcinogen: Preliminary findings. Cancer Lett. 37:147–151. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moxon D, Raza M, Kenney R, Ewing R,
Arozullah A, Mason JB and Carroll RE: Relationship of aging and
tobacco use with the development of aberrant crypt foci in a
predominantly African-American population. Clin Gastroenterol
Hepatol. 3:271–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohkubo H, Takahashi H, Yamada E, Sakai E,
Higurashi T, Uchiyama T, Hosono K, Endo H, Taguri M and Nakajima A:
Natural history of human aberrant crypt foci and correlation with
risk factors for colorectal cancer. Oncol Rep. 27:1475–1480.
2012.PubMed/NCBI
|
|
14
|
Pretlow TP, Barrow BJ, Ashton WS,
O'Riordan MA, Pretlow TG, Jurcisek JA and Stellato TA: Aberrant
crypts: Putative preneoplastic foci in human colonic mucosa. Cancer
Res. 51:1564–1567. 1991.PubMed/NCBI
|
|
15
|
Sakai E, Takahashi H, Kato S, Uchiyama T,
Hosono K, Endo H, Maeda S, Yoneda M, Taguri M and Nakajima A:
Investigation of the prevalence and number of aberrant crypt foci
associated with human colorectal neoplasm. Cancer Epidemiol
Biomarkers Prev. 20:1918–1924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seike K, Koda K, Oda K, Kosugi C, Shimizu
K, Nishimura M, Shioiri M, Takano S, Ishikura H and Miyazaki M:
Assessment of rectal aberrant crypt foci by standard chromoscopy
and its predictive value for colonic advanced neoplasms. Am J
Gastroenterol. 101:1362–1369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shpitz B, Bomstein Y, Mekori Y, Cohen R,
Kaufman Z, Neufeld D, Galkin M and Bernheim J: Aberrant crypt foci
in human colons: Distribution and histomorphologic characteristics.
Hum Pathol. 29:469–475. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takayama T, Katsuki S, Takahashi Y, Ohi M,
Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H and Niitsu Y:
Aberrant crypt foci of the colon as precursors of adenoma and
cancer. N Engl J Med. 339:1277–1284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Endo H, Hosono K, Fujisawa T, Takahashi H,
Sugiyama M, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Inamori M, et
al: Involvement of JNK pathway in the promotion of the early stage
of colorectal carcinogenesis under high-fat dietary conditions.
Gut. 58:1637–1643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endo H, Hosono K, Uchiyama T, Sakai E,
Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H
and Nakajima A: Leptin acts as a growth factor for colorectal
tumours at stages subsequent to tumour initiation in murine colon
carcinogenesis. Gut. 60:1363–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McLellan E and Bird RP: Effect of
disulfiram on 1,2-dimethylhydrazine- and azoxymethane-induced
aberrant crypt foci. Carcinogenesis. 12:969–672. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McLellan EA and Bird RP: Specificity study
to evaluate induction of aberrant crypts in murine colons. Cancer
Res. 48:6183–6186. 1988.PubMed/NCBI
|
|
23
|
McLellan EA, Medline A and Bird RP:
Sequential analyses of the growth and morphological characteristics
of aberrant crypt foci: Putative preneoplastic lesions. Cancer Res.
51:5270–5274. 1991.PubMed/NCBI
|
|
24
|
Moghaddam AA, Woodward M and Huxley R:
Obesity and risk of colorectal cancer: A meta-analysis of 31
studies with 70,000 events. Cancer Epidemiol Biomarkers Prev.
16:2533–2547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Acott AA, Theus SA, Marchant-Miros KE and
Mancino AT: Association of tobacco and alcohol use with earlier
development of colorectal cancer: Should we modify screening
guidelines? Am J Surg. 196:915–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuhara H, Steinmaus C, Cohen SE, Corley
DA, Tei Y and Buffler PA: Is diabetes mellitus an independent risk
factor for colon cancer and rectal cancer? Am J Gastroenterol.
106:1911–1921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higurashi T, Hosono K, Endo H, Takahashi
H, Iida H, Uchiyama T, Ezuka A, Uchiyama S, Yamada E, Ohkubo H, et
al: Eicosapentaenoic acid (EPA) efficacy for colorectal aberrant
crypt foci (ACF): A double-blind randomized controlled trial. BMC
Cancer. 12:4132012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mutch MG, Schoen RE, Fleshman JW,
Takahashi H, Iida H, Uchiyama T, Ezuka A, Uchiyama S, Yamada E and
Ohkubo H: A multicenter study of prevalence and risk factors for
aberrant crypt foci. Clin Gastroenterol Hepatol. 7:568–574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luchtenborg M, White KK, Wilkens L,
Kolonel LN and Le Marchand L: Smoking and colorectal cancer:
Different effects by type of cigarettes? Cancer Epidemiol
Biomarkers Prev. 16:1341–1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stevens RG, Swede H, Heinen CD, Jablonski
M, Grupka M, Ross B, Parente M, Tirnauer JS, Giardina C, Rajan TV,
et al: Aberrant crypt foci in patients with a positive family
history of sporadic colorectal cancer. Cancer Lett. 248:262–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uchiyama T, Takahashi H, Endo H, Kato S,
Sakai E, Hosono K, Yoneda M, Inamori M, Hippo Y, Nakagama H and
Nakajima A: Number of aberrant crypt foci in the rectum is a useful
surrogate marker of colorectal adenoma recurrence. Dig Endosc.
24:353–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Limburg PJ, Mahoney MR, Ziegler KL, Sontag
SJ, Schoen RE, Benya R, Lawson MJ, Weinberg DS, Stoffel E, Chiorean
M, et al: Randomized phase II trial of sulindac, atorvastatin and
prebiotic dietary fiber for colorectal cancer chemoprevention.
Cancer Prev Res. 4:259–269. 2011. View Article : Google Scholar
|
|
33
|
Cho NL, Redston M, Zauber AG, Carothers
AM, Hornick J, Wilton A, Sontag S, Nishioka N, Giardiello FM,
Saltzman JR, et al: Aberrant crypt foci in the adenoma prevention
with celecoxib trial. Cancer Prev Res (phila). 1:21–31. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Larsson SC, Orsini N and Wolk A: Diabetes
mellitus and risk of colorectal cancer: A meta-analysis. J Natl
Cancer Inst. 97:1679–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng L, Gui Z, Zhao L, Wang J and Shen L:
Diabetes mellitus and the incidence of colorectal cancer: An
updated systematic review and meta-analysis. Dig Dis Sci.
57:1576–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giovannucci E: Insulin, insulin-like
growth factors and colon cancer: A review of the evidence. J Nutr.
131(Suppl 11): S3109–S3120. 2001.
|
|
37
|
Smith AJ, Stern HS, Penner M, Hay K, Mitri
A, Bapat BV and Gallinger S: Somatic APC and K-ras codon 12
mutations in aberrant crypt foci from human colons. Cancer Res.
54:5527–5530. 1994.PubMed/NCBI
|
|
38
|
Takayama T, Ohi M, Hayashi T, Miyanishi K,
Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S and
Niitsu Y: Analysis of K-ras, APC and beta-catenin in aberrant crypt
foci in sporadic adenoma, cancer and familial adenomatous
polyposis. Gastroenterology. 121:599–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siu IM, Pretlow TG, Amini SB and Pretlow
TP: Identification of dysplasia in human colonic aberrant crypt
foci. Am J Pathol. 150:1805–1813. 1997.PubMed/NCBI
|
|
40
|
Hurlstone DP and Cross SS: Role of
aberrant crypt foci detected using high-magnification-chromoscopic
colonoscopy in human colorectal carcinogenesis. J Gastroenterol
Hepatol. 20:173–181. 2005. View Article : Google Scholar : PubMed/NCBI
|