Introduction
Osteosarcoma (OS) is the most common malignant tumor
of the bone, with a peak incidence in young children and
adolescents. The incidence of osteosarcoma is 0.20–0.35/100,000
individuals (1). OS is associated
with a high rate of mortality: The five year overall survival is
75–77% for the primary non-metastatic osteosarcoma, and no more
than 20% for metastatic osteosarcoma (2,3). Despite
the use of surgical excision combined with chemotherapy and
radiotherapy, the median survival rate of OS remains poor (4). It has been demonstrated that
dysregulation of oncogenes or tumor suppressor genes is involved in
the development and progression of OS (5). Establishing novel therapeutic targets is
urgently required for the diagnosis and treatment of OS.
Receptor tyrosine kinase (RTK)-like orphan receptor
2 (ROR2) belongs to the RTK family, which is important in the
regulation of numerous cellular biological processes, including
proliferation, apoptosis, differentiation, adhesion and migration
(6–8).
ROR2 is expressed in heart, brain and lung tissue, and is also
involved in the development of the nervous system and bones
(9–11). It has been hypothesized that ROR2 is
involved in the early formation of chondrocytes as well as the
development of cartilage and growth plates (12). In addition, mutations in the
ROR2 gene can cause the autosomal recessive form of Robinow
syndrome, a rare disorder that is characterized by skeletal
dysplasia, limb bone shortening, segmental defects of the spine,
brachydactyly and facial abnormalities (13).
It has been demonstrated that ROR2 is frequently
downregulated in a number of common types of malignancy, including
esophageal, nasopharyngeal, gastric, colorectal, hepatocellular,
lung and breast cancers (14,15). ROR2 acts as a tumor suppressor by
inhibiting the epithelial-mesenchymal transition and tumor cell
stemness through repressing β-catenin and AKT signaling (16). Recently, it has been indicated that
Wnt5a/ROR2 signaling may be associated with OS severity, and plays
a promotive role in the regulation of OS cell migration and
invasion (17–19). However, the precise roles of ROR2 in
the regulation of OS cell proliferation, as well as the underlying
mechanism, have not previously been reported.
The present study aimed to explore the role of ROR2
in the regulation of OS cell proliferation and to investigate the
underlying molecular mechanisms.
Materials and methods
Reagents and materials
RPMI 1640 medium, fetal bovine serum (FBS), Trizol
Reagent and Lipofectamine 2000 were purchased from Life
Technologies (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) and
MTT were purchased from Sigma-Aldrich (St. Louis, MO, USA).
PrimeScript RT Reagent Kit and SYBR Premix Ex Taq II were purchased
from Takara Biotechnology Co., Ltd., (Dalian, China).ROR2-specific
small interfering RNA (siRNA) and non-specific siRNA were generated
from Nlunbio (Changsha, China). A Pierce Enhanced Chemiluminescence
(ECL) kit was purchased from Thermo Fisher Scientific (Rockford,
IL, USA). Transwell inserts were purchased from BD Biosciences (San
Jose, CA, USA). Mouse anti-ROR2 monoclonal antibody, mouse
anti-GAPDH monoclonal antibody and rabbit anti-mouse secondary
antibody were purchased from Abcam (Cambridge, UK).
Tissue specimen collection
All protocols in this study were approved by the
Ethics Committee of Jishou University (Jishou, China). A total of
18 OS tissues as well as their matched adjacent normal tissues were
collected at The Second Department of Orthopedics of the First
Affiliated Hospital of Jishou University between December 2012 and
December 2013. The 18 cases included 7 female and 11 male who
ranged in age between 25 and 64 years, with a mean of 48.5 years.
All patients received neither radiation therapy nor chemotherapy
before surgical resection. Among all OS patients, 2 cases were
classified as grade I, 7 grade II, 6 grade III, and 3 grade IV.
Written informed consent was obtained from all patients. Tissues
were immediately snap-frozen in liquid nitrogen following surgical
removal, and stored at −70°C until use.
Cell culture
The human OS cell lines Saos-2, MG-63 and U-2 OS and
the human osteoblast cell line hFOB 1.19 were obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin (Sigma-Aldrich) in a
humidified atmosphere of 5% CO2 at 37°C.
Reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR) analysis
Trizol Reagent was used to extract total RNA from
tissues or cells, in accordance with the manufacturer's
instructions, and a total of 800 ng RNA was subsequently reverse
transcribed into cDNA using a PrimeScript RT Reagent Kit, according
to the manufacturer's protocol. Reverse transcription was performed
at 16°C for 30 min, followed by an incubation at 42°C for 30 min
and enzyme inactivation at 85°C for 5 min. The mRNA expression
level was determined using SYBR Premix Ex Taq II, on ABI 7500
thermocycler (Thermo Fisher Scientic, Inc.), in accordance with the
manufacturer's instructions. The reaction conditions were as
follows: 95°C for 5 min, followed by 40 cycles of denaturation at
95°C for 15 sec and an annealing/elongation step at 60°C for 30
sec. The specific primer pairs are as follows: ROR2 sense,
5′-GTGCGGTGGCTAAAGAATGAT-3; ROR2 antisense,
5′-ATTCGCAGTCGTGAACCATATT-3′; GAPDH (internal reference) sense,
5′-ACAACTTTGGTATCGTGGAAGG-3′; and GAPDH antisense,
5′-GCCATCACGCCACAGTTTC-3′. Independent experiments were repeated
three times. The relative expression of ROR2 mRNA was analyzed
using the 2-∆∆Ct method.
Transfection
Cells were cultured to 70% confluence and
resuspended in serum-free medium. Lipofectamine 2000 was used to
transfect cells with ROR2 siRNA or with non-specific siRNA as a
negative control (NC). In brief, serum-free medium was used to
dilute Lipofectamine 2000 and siRNA, and the diluted Lipofectamine
2000 was then added into the diluted siRNA. Following incubation
for 20 min at room temperature, the mixture was added to the cell
suspension. The cells and transfection reagents were incubated at
37°C in an atmosphere of 5% CO2 for 6 h, before the
medium was replaced by normal serum-containing medium. After
transfection for 48 h, the following assays were performed.
Proliferation assay
An MTT assay was performed to determine cell
proliferation. In brief, 104 cells per well were plated in a
96-well plate, and incubated for 6, 12, 24 or 48 h at 37°C in an
atmosphere of 5% CO2. Subsequently, 10 µl of MTT
solution (5 mg/ml) was added to each well and incubated for 4 h at
37°C in 5% CO2. The supernatant was then removed, and
100 µl of DMSO was added to dissolve the precipitate. The
absorbance [optical density (OD)] was detected at 492 nm with a 680
microplate reader (Bio-Rad Laboratories, Inc.).
Colony formation assay
For all three groups, 150 cells in 3 ml complete
medium were added to each well of a 6-well plate, which were then
incubated at 37°C in 5% CO2 for 14 days. At the end of
this period, the cells were washed and stained with Giemsa
(Sigma-Aldrich). Colonies composed of ≥50 cells were then
counted.
Cell cycle analysis
Cells were collected in 1X phosphate-buffered saline
(PBS) and fixed in 70% ethanol overnight at −20°C. The cells were
subsequently pelleted at 200 × g for 5 min, washed in 1X PBS, and
pelleted again at 200 × g for 5 min. Cells were resuspended in 300
µl propidium iodide (PI; Sigma-Aldrich) and incubated at room
temperature for 30 min. DNA content analyses were conducted using a
C6 flow cytometer (Beckman Coulter, Brea, CA, USA).
Western blotting
Western blotting was performed to determine relative
protein expression. In brief, tissues or cells were solubilized in
cold radioimmunoprecipitation assay lysis buffer (Sbjbio, Nanjing,
China). Proteins were separated using 10% SDS-PAGE (Sbjbio), and
transferred onto a polyvinylidene fluoride (PVDF) membrane (Thermo
Fisher Scientific), which was then incubated with mouse monoclonal
anti-human ROR2 (ab201962, 1:100), monoclonal mouse anti-human
cyclin D1 (ab187892, 1:200), monoclonal mouse anti-human cyclin E
(ab3927, 1:50), monoclonal mouse anti-human CDK4 (ab75511, 1:200),
monoclonal mouse anti-human c-myc (ab56, 1:200) and
monoclonal mouse anti-human GAPDH (ab8245, 1:200, used as internal
control) at room temperature for 3 h. Following three washes in
PBS-Tween 20, the PVDF membrane was incubated with the rabbit anti
mouse secondary antibody (ab175743, 1:10,000) at room temperature
for 1 h. Chemiluminescence detection was performed using an ECL kit
and the relative protein expression was analyzed using Image-Pro
Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA), represented as the ROR2 density ratio relative to that of
GAPDH.
Statistical analysis
All data is represented as the mean ± standard
deviation of at least triplicate samples. A Student's t-test or
χ2 test was used to statistically analyze data with SPSS
software version 17 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate statistically significant differences.
Results
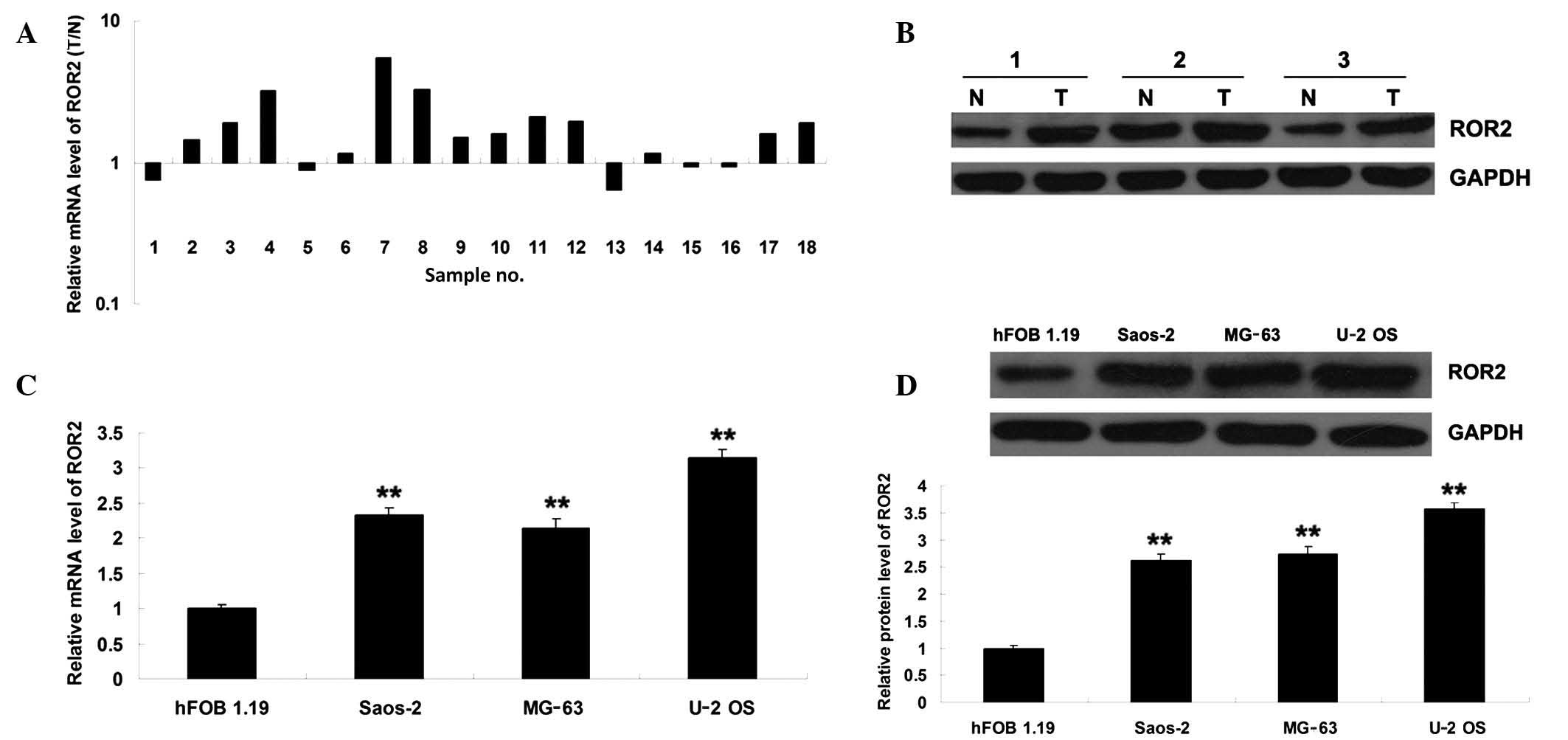
ROR2 expression was significantly
upregulated in OS tissues and cell lines
To investigate the role of ROR2 in OS, its
expression levels were examined in OS tissue specimens as well as
their matched adjacent normal tissues by RT-qPCR. As shown in
Fig. 1A, mRNA expression was
significantly upregulated in OS tissues compared with that of
matched adjacent normal tissue samples. Similarly, the results of
western blotting revealed that ROR2 protein expression was also
upregulated in OS tissues (Fig. 1B).
To confirm this, mRNA and protein expression of ROR2 was also
evaluated in three OS cell lines, with the human osteoblast cell
line hFOB 1.19 used as a control. As shown in Fig. 1C and D, the expression level of ROR2
was markedly increased in OS cell lines compared with that in hFOB
1.19 cells. These findings indicate that ROR2 may be involved in
the development of OS.
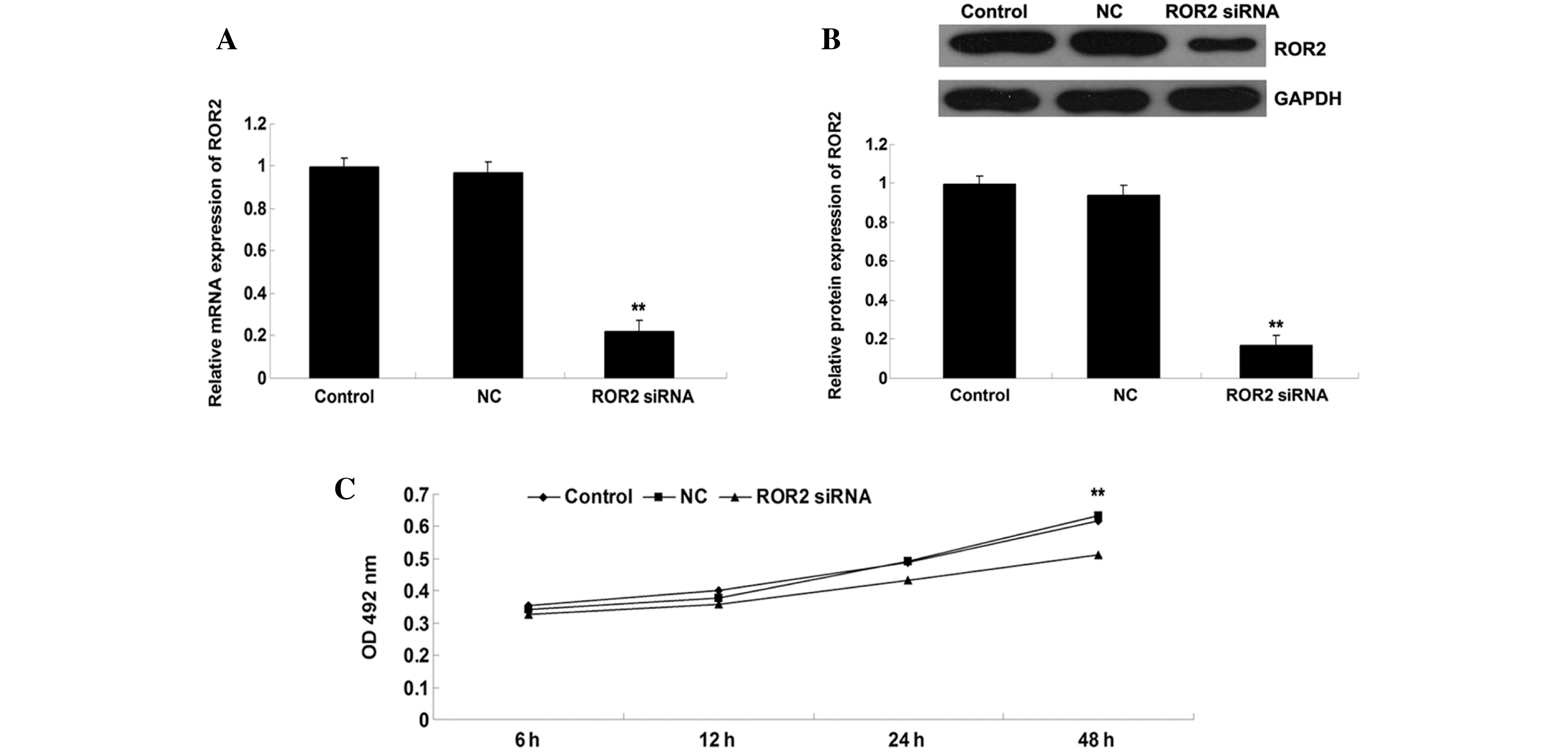
siRNA-induced ROR2 downregulation
inhibits OS cell proliferation
As U-2 OS cells demonstrated the highest ROR2 mRNA
and protein expression, this cell line was used in the subsequent
in vitro experiments. ROR2-specific siRNA was transfected
into U-2 OS cells to knockdown ROR2 expression. As shown in
Fig. 2A and B, the relative mRNA and
protein expression of ROR2 in the ROR2 siRNA group was markedly
decreased when compared with that of the Control (untransfected)
group, while the difference between the NC and Control groups was
not statistically significant. These data indicated that the
expression of ROR2 in U-2 OS cells was successfully downregulated.
Subsequently, MTT assays were conducted to investigate the effect
of ROR2 downregulation on U-2 OS cell proliferation. As shown in
Fig. 2C, the OD value of U-2 OS cells
in the ROR2 siRNA group was significantly lower than that of the
Control and NC groups (P<0.01), suggesting that inhibition of
ROR2 expression markedly inhibited the proliferation of U-2 OS
cells.
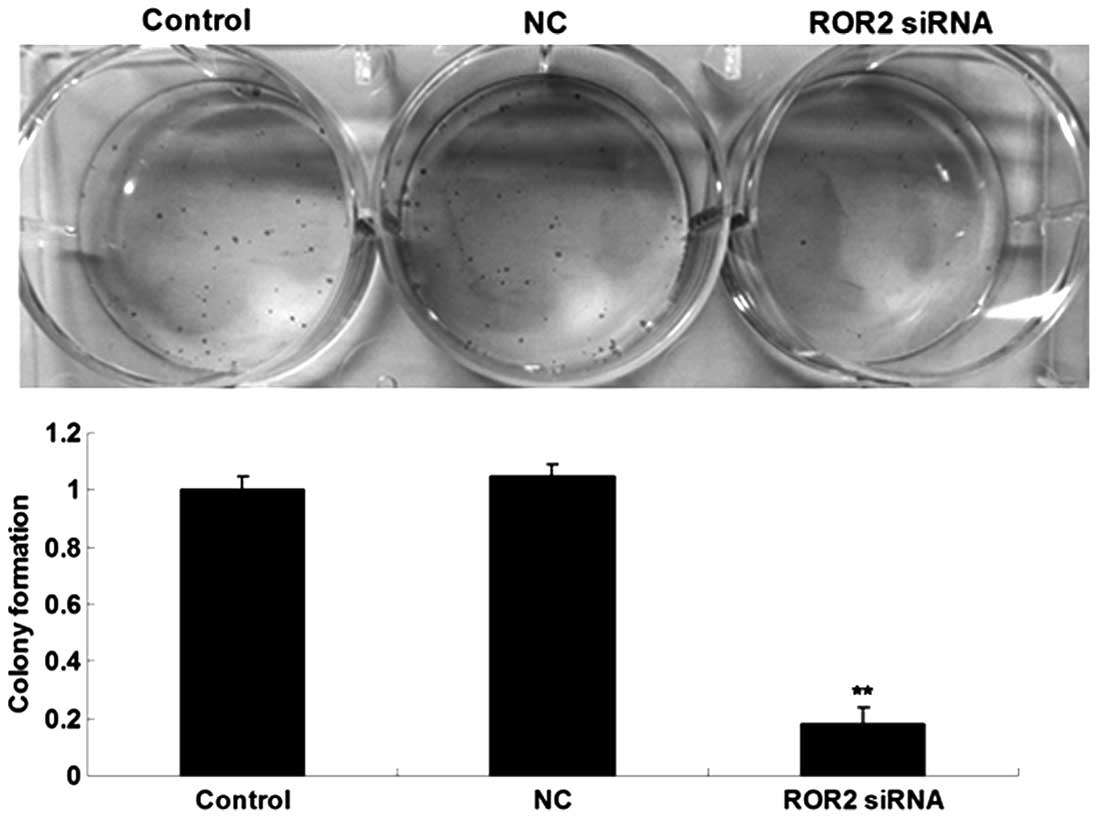
Knockdown of ROR2 expression inhibits
colony formation of OS cells
A colony formation assay was performed to
investigate the effect of ROR2 downregulation on the colony-forming
capacity of U-2 OS cells. As shown in Fig. 3, the colony-forming capacity of U-2 OS
cells transfected with ROR2 siRNA was markedly decreased compared
with that of the Control and NC groups, indicating that
downregulation of ROR2 expression significantly suppressed the
colony-forming capacity of U-2 OS cells.
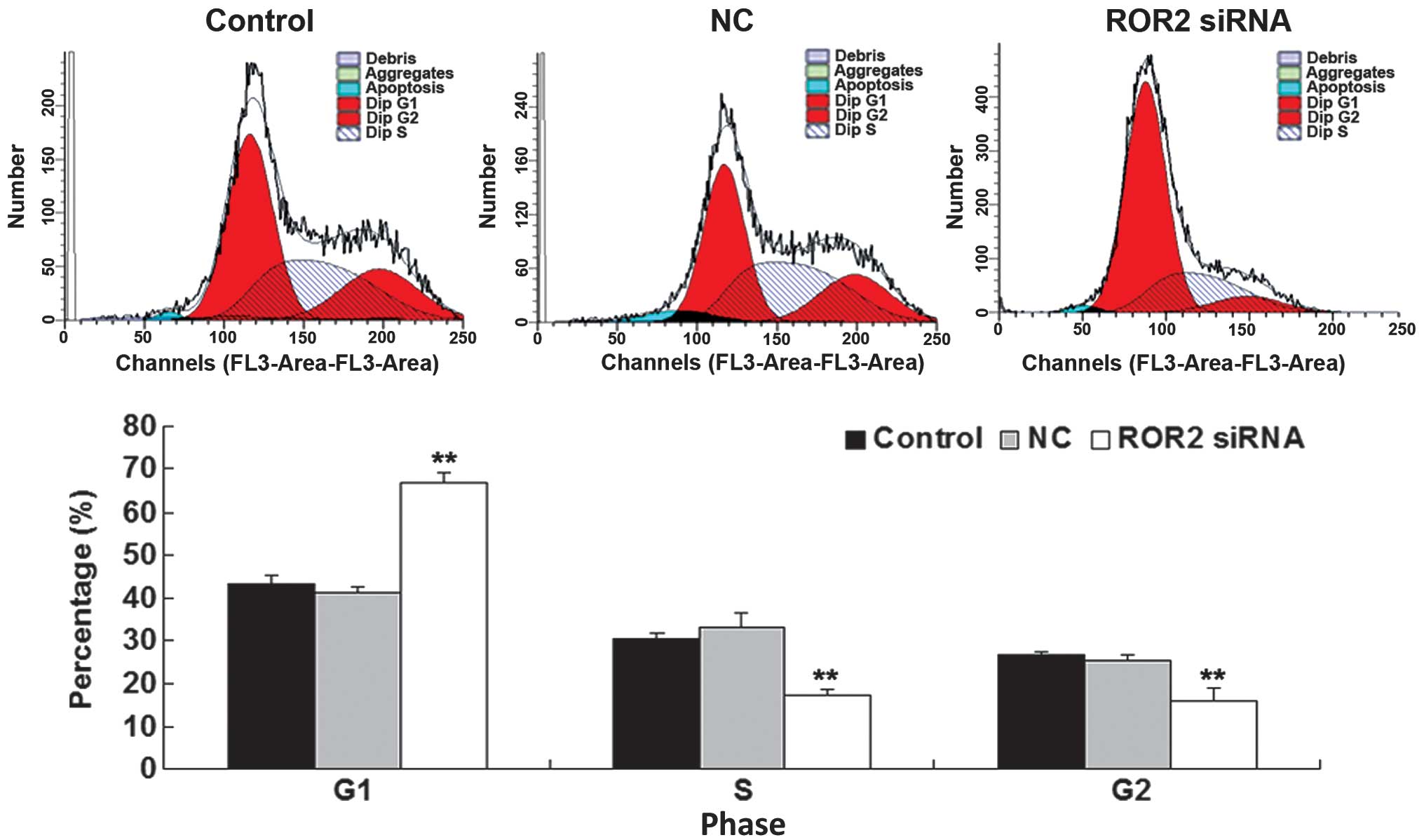
Downregulation of ROR2 expression
results in arrest of cell cycle progression of OS cells
To further investigate the molecular mechanism of
ROR2 in OS, cell cycle progression, which is closely associated
with cell proliferation and colony formation, was analyzed in U-2
OS cells in each group. Data from the cell cycle distribution assay
revealed an accumulation of ROR2-knockdown U-2 OS cells at G1
phase, and a decrease in S and G2 phases compared with the control
groups (Fig. 4). These findings
indicated that knockdown of ROR2 induced an arrest in cell cycle
progression, which may be the primary reason for the reduced
proliferative and colony-forming capacities of ROR2-knockdown U-2
OS cells.
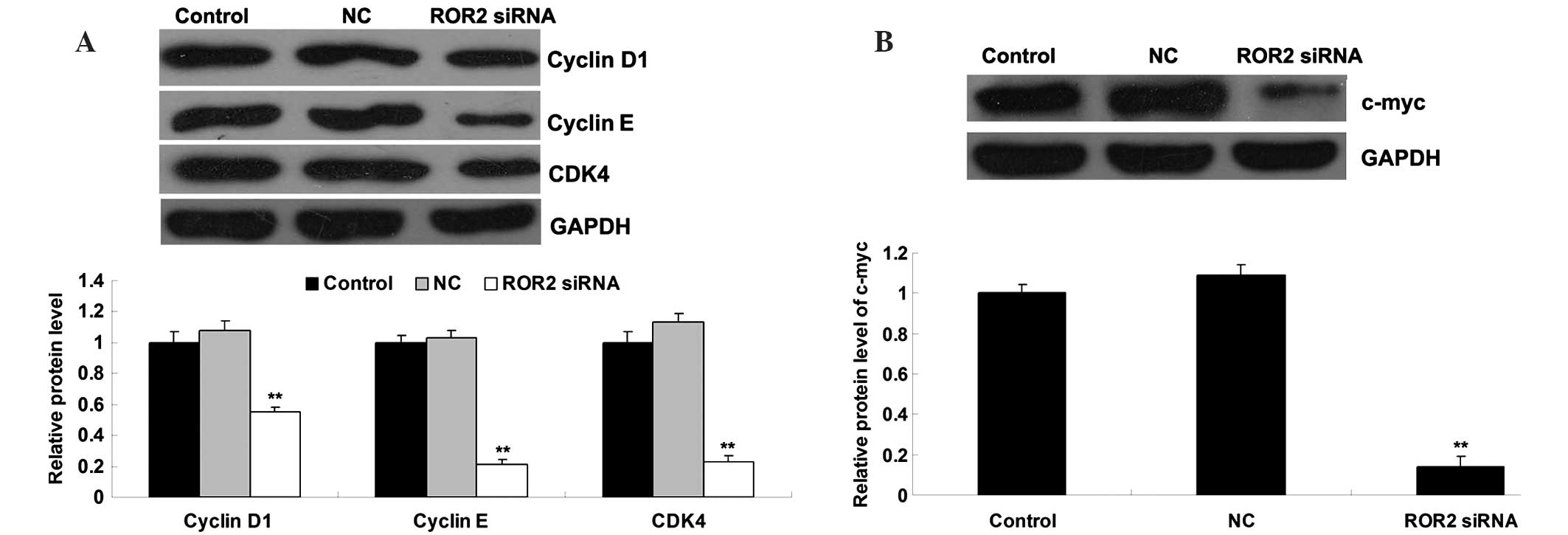
ROR2 knockdown leads to downregulation
of cell cycle proteins and c-myc
Consistent with the aforementioned data, the protein
expression levels of cyclin D1, cyclin E, and cyclin-dependent
kinase 4 (CDK4), which are involved in the cell cycle G1-S phase
transition, were significantly reduced in ROR2-knockdown OS cells
(Fig. 5A). In addition, c-myc, which
has been demonstrated to play a crucial role in cell proliferation,
was also found to have reduced expression in ROR2-knockdown OS
cells compared with the control groups (Fig. 5B).
Discussion
ROR2, a member of RTK family, is a type I
transmembrane protein that belongs to the RTK-like orphan receptor
subfamily of cell surface receptors (20). ROR2 is associated with the development
and progression of numerous types of human cancer, including OS
(14,15). ROR2 has been suggested to play a role
in the regulation of OS cell migration and invasion (21). However, the precise role of ROR2 in
the regulation of OS cell proliferation, as well as its underlying
mechanism, remains largely unclear. In the current study, the
expression of ROR2 was found to be significantly increased in OS
tissues and cell lines, and knockdown of ROR2 expression by
transfection with ROR2-specific siRNA markedly inhibited the
proliferation and colony formation of OS cells. These findings
suggest that ROR2 plays a promotive role in the regulation of OS
cell proliferation, and that knockdown of ROR2 expression may be
effective for inhibiting the development and growth of OS.
As the downregulation of cell proliferation and
colony formation induced by ROR2 knockdown may be due to the
inhibition of cell cycle progression, a cell cycle distribution
assay was also conducted. These findings revealed an accumulation
of ROR2-knockdown OS cells in G0/G1 phase, and a decrease in S and
G2/M phases, indicating that knockdown of ROR2 leads to an arrest
in cell cycle progression of OS cells. Further findings revealed
that the expression levels of cyclin D1, cyclin E and CDK4 were
significantly reduced in ROR2-knockdown OS cells. These molecules
are involved in the G0/G1-S transition of the cell cycle, and thus
the regulation of cell proliferation and colony formation (22).
Furthermore, ROR2 is involved in the Wnt signaling
pathway (23). Wnt signaling is
important in normal embryonic pattern formation and cell
differentiation, as well as tumorigenesis (9,24), and may
therefore act as potential diagnostic or therapeutic target for
human malignancies. As a novel Wnt receptor, ROR2 provides the
potential to target the non-canonical Wnt pathway for cancer
treatments. Notably, ROR2 appears to possess dual roles as an
oncogene or tumor suppressor depending on tumor type (25). For instance, the protein expression of
ROR2 was reported to be significantly decreased in hepatocellular
carcinoma (HCC) tissues compared with adjacent non-tumorous
tissues, and loss of ROR2 expression was associated with poor
prognosis, suggesting that ROR2 may act as a tumor suppressor in
HCC (26). By contrast, ROR2 was
suggested to play an oncogenic role in certain other cancers,
including melanoma, renal cell cancer and oral squamous cell
carcinoma (27–29). ROR2 promotes the proliferation and
migration of renal cell carcinoma cells in vitro and in
vivo (30).
The role of Wnt/ROR2 signaling in the regulation of
OS development has been gradually elucidated. Wnt5b has been
identified as a ligand of ROR2, and the physiological interaction
of ROR2 and Wnt5b may enhance OS cell migration, suggesting that
Wnt/ROR2 signaling may be involved in OS metastasis (31). Furthermore, Wnt/ROR2 signaling was
found to promote OS cell invasion, at least in part, through the
activation of a Src-family protein tyrosine kinase as well as
upregulation of matrix metalloproteinase-13 expression (19,21). In
addition, Lu et al (17)
reported that ROR2 and Wnt5a were significantly upregulated in OS
tissues, and their expression levels were correlated with Enneking
surgical stage and tumor metastasis. In the current study, cyclin
D1, a target gene of Wnt signaling (32), was markedly downregulated following
knockdown of ROR2 in OS cells. In addition, c-myc, another target
gene of Wnt signaling that has been demonstrated to participate in
the regulation of cell proliferation (33), was found to have reduced expression in
ROR2-knockdown OS cells. Taken together, these findings suggest
that the inhibitory effect of ROR2 knockdown on OS cell
proliferation may be associated with the Wnt signaling pathway.
In summary, the present study revealed that the
expression level of ROR2 was significantly increased in OS tissues
and cell lines. We hypothesize that ROR2 is able to promote the
proliferation of OS cells through the regulation of cell cycle
progression, and that the Wnt signaling pathway is involved in
ROR2-mediated OS cell proliferation. Therefore, ROR2 may become a
potential molecular target for the treatment of OS.
Acknowledgements
This study was supported by the Scientific Research
Project of Education Department of Hunan Province (no. 11C1035),
Natural Science Fund Project of Hunan Province (no. 12JJ6074),
Research Project of Health Department of Hunan Province (no.
B2013-160) and the Introduction of Talent Research Project of
Jishou University (no. jsdxrcyjkyxm201110).
References
|
1
|
Valery PC, Laversanne M and Bray F: Bone
cancer incidence by morphological subtype: A global assessment.
Cancer Causes Control. 26:1127–1139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al: Italian and Scandinavian Sarcoma Groups: Neoadjuvant
chemotherapy with high-dose Ifosfamide, high-dose methotrexate,
cisplatin, and doxorubicin for patients with localized osteosarcoma
of the extremity: A joint study by the Italian and Scandinavian
Sarcoma Groups. J Clin Oncol. 23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mialou VI, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: prognostic
factors and long-term outcome - the French pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92:288–290. 2013.PubMed/NCBI
|
|
5
|
PosthumaDeBoer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundaram MV: Canonical RTK-Ras-ERK
signaling and related alternative pathways. WormBook. 1:1–38. 2013.
View Article : Google Scholar
|
|
7
|
Jiménez G, Shvartsman SY and Paroush Z:
The Capicua repressor-a general sensor of RTK signaling in
development and disease. J Cell Sci. 125:1383–1391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batchu SN and Korshunov VA: Novel tyrosine
kinase signaling pathways: Implications in vascular remodeling.
Curr Opin Nephrol Hypertens. 21:122–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katoh M: WNT signaling pathway and stem
cell signaling network. Clin Cancer Res. 13:4042–4045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green JL, Kuntz SG and Sternberg PW: Ror
receptor tyrosine kinases: Orphans no more. Trends Cell Biol.
18:536–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Bhat RA, Seestaller-Wehr LM,
Fukayama S, Mangine A, Moran RA, Komm BS, Bodine PV and Billiard J:
The orphan receptor tyrosine kinase Ror2 promotes osteoblast
differentiation and enhances ex vivo bone formation. Mol
Endocrinol. 21:376–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeChiara TM, Kimble RB, Poueymirou WT,
Rojas J, Masiakowski P, Valenzuela DM and Yancopoulos GD: Ror2,
encoding a receptor-like tyrosine kinase, is required for cartilage
and growth plate development. Nat Genet. 24:271–274. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehawej C, Chouery E, Maalouf D, Baujat G,
Le Merrer M, Cormier-Daire V and Mégarbané A: Identification of a
novel causative mutation in the ROR2 gene in a Lebanese family with
a mild form of recessive Robinow syndrome. Eur J Med Genet.
55:103–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Debebe Z and Rathmell WK: Ror2 as a
therapeutic target in cancer. Pharmacol Ther. 150:143–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rebagay G, Yan S, Liu C and Cheung NK:
ROR1 and ROR2 in human malignancies: Potentials for targeted
therapy. Front Oncol. 2:342012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Ying J, Tong X, Zhong L, Su X, Xiang
T, Shu X, Rong R, Xiong L, Li H, et al: Epigenetic identification
of receptor tyrosine kinase-like orphan receptor 2 as a functional
tumor suppressor inhibiting β-catenin and AKT signaling but
frequently methylated in common carcinomas. Cell Mol Life Sci.
71:2179–2192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D,
Yan CM, Wang DJ and Sun JY: Expression of WNT-5a and ROR2
correlates with disease severity in osteosarcoma. Mol Med Rep.
5:1033–1036. 2012.PubMed/NCBI
|
|
18
|
Ren D, Minami Y and Nishita M: Critical
role of Wnt5a-Ror2 signaling in motility and invasiveness of
carcinoma cells following Snail-mediated epithelial-mesenchymal
transition. Genes Cells. 16:304–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enomoto M, Hayakawa S, Itsukushima S, Ren
DY, Matsuo M, Tamada K, Oneyama C, Okada M, Takumi T, Nishita M and
Minami Y: Autonomous regulation of osteosarcoma cell invasiveness
by Wnt5a/Ror2 signaling. Oncogene. 28:3197–3208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stricker S and Mundlos S: FGF and ROR2
receptor tyrosine kinase signaling in human skeletal development.
Curr Top Dev Biol. 97:179–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamagata K, Li X, Ikegaki S, Oneyama C,
Okada M, Nishita M and Minami Y: Dissection of Wnt5a-Ror2 signaling
leading to matrix metalloproteinase (MMP-13) expression. J Biol
Chem. 287:1588–1599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang HR, Lian JD, Lo CW, Chang YC, Yang
MY and Wang CJ: Induction of urothelial proliferation in rats by
aristolochic acid through cell cycle progression via activation of
cyclin D1/cdk4 and cyclin E/cdk2. Food Chem Toxicol. 44:28–35.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Chen H, Hu L, Xing Y, Sasaki T,
Villosis MF, Li J, Nishita M, Minami Y and Minoo P: Ror2 modulates
the canonical Wnt signaling in lung epithelial cells through
cooperation with Fzd2. BMC Mol Biol. 9:112008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lerner UH and Ohlsson C: The WNT system:
Background and its role in bone. J Intern Med. 277:630–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ford CE, Ma Qian SS, Quadir A and Ward RL:
The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in
human carcinogenesis. Int J Cancer. 133:779–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wright TM, Brannon AR, Gordan JD, Mikels
AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen
E, et al: Ror2, a developmentally regulated kinase, promotes tumor
growth potential in renal cell carcinoma. Oncogene. 28:2513–2523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ
and Liu XH: Loss of Wnt5a and Ror2 protein in hepatocellular
carcinoma associated with poor prognosis. World J Gastroenterol.
18:1328–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Connell MP, Fiori JL, Xu M, Carter AD,
Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier
M, et al: The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A
signaling in metastatic melanoma. Oncogene. 29:34–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wright TM, Brannon AR, Gordan JD, Mikels
AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen
E, et al: Ror2, a developmentally regulated kinase, promotes tumor
growth potential in renal cell carcinoma. Oncogene. 28:2513–2523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobayashi M, Shibuya Y, Takeuchi J, Murata
M, Suzuki H, Yokoo S, Umeda M, Minami Y and Komori T: Ror2
expression in squamous cell carcinoma and epithelial dysplasia of
the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
107:398–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morioka K, Tanikawa C, Ochi K, Daigo Y,
Katagiri T, Kawano H, Kawaguchi H, Myoui A, Yoshikawa H, Naka N, et
al: Orphan receptor tyrosine kinase ROR2 as a potential therapeutic
target for osteosarcoma. Cancer Sci. 100:1227–1233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng BQ, Jiang Y, Zhu Q and Lin WG:
Wnt/β-catenin aids in regulating the proliferation of hepG2 cells
mediated by thy-1. Genet Mol Res. 13:5115–5127. 2014. View Article : Google Scholar : PubMed/NCBI
|