Introduction
Esophageal carcinoma is the eighth most frequently
diagnosed cancer and the sixth leading cause of cancer mortality
worldwide, with an estimated 482,000 new cases and 407,000
mortalities in 2008. Patients with esophageal carcinoma have a
poorer prognosis in comparison to patients exhibiting any other
type of gastrointestinal tumor (1).
Lymph node involvement is an important prognostic factor for
survival in patients with esophageal carcinoma (2). Despite significant improvements in the
diagnosis and available therapeutic strategies for the disease,
survival rates remain low. For example, the 5-year survival rate of
patients exhibiting esophageal carcinoma with lymph node metastasis
who have undergone an esophagectomy and three-field lymphadenectomy
is only 15–39% (3).
Five members of the vascular endothelial growth
factor (VEGF) family (VEGF-A, VEGF-C, VEGF-D, VEGF-E and placental
growth factor) and their receptors [VEGF receptor (VEGFR)-1, VEGF-2
and VEGF-3] are important in the formation of the vascular network
(4). VEGF-C and VEGF-D have been
characterized as lymphangiogenic and angiogenic growth factors, and
have been demonstrated to signal through the receptors VEGFR-2 and
VEGFR-3 in various physiological and pathological processes
(5). A mouse model study demonstrated
that lymphatic spread and lymphangiogenesis are associated with the
expression of VEGF-D or VEGF-C by the tumor cells (6). Furthermore, VEGF-C and VEGF-D appear to
be involved in the origin and/or progression of lymphangiogenesis
in various different types of cancer, including gastric and
esophageal cancer, with overexpression correlated with nodal
metastasis and patient survival (7,8).
Podoplanin (PDPN) is 43-kDa mucin-type transmembrane
glycoprotein that is expressed in lymphatic endothelial cells, but
not in blood endothelial cells (9).
PDPN has previously been used to assess lymphatic vessel density
and invasion in various types of cancer, including esophageal
carcinoma (10,11). Thus, it may act as a mediator of tumor
cell invasion and metastasis (12).
The present study evaluated the association between
VEGF-C VEGF-D, VEGFR-3 and PDPN mRNA expression levels, and the
clinicopathological factors and survival of patients with
esophageal carcinoma.
Materials and methods
Patients and tissues
Tumor specimens were obtained from 84 patients with
primary esophageal cancer who underwent an esophagectomy at the
Department of Thoracic Surgery, Medical University of Białystok
(Białystok, Poland). No patients had received pre-operative
chemotherapy or radiotherapy. The study population consisted of 76
men (90.5%) and 8 women (9.5%), and the mean age at the time of
diagnosis was 63 years (range, 42–82 years). Pathological stage was
determined using the seventh edition of the American Joint
Committee on Cancer tumor-node-metastasis classification system
(13). Following surgery, all
patients underwent clinical follow-up evaluations every 3–6 months,
including a clinical history, physical examination, laboratory
analysis, fiberoptic esophagoscopy, ultrasound examination of the
neck and abdomen, barium esophagram, computed tomography (CT) scan,
endoscopic ultrasound, positron emission tomography-CT scan, and
endobronchial ultrasound if necessary. The mean follow-up time was
25 months (range, 3–101 months). Survival analysis was performed at
the termination of follow-up, including an overall survival (OS)
analysis. Non-malignant esophageal tissue samples were collected
from the same patients at a distance of 3–5 cm from the tumor (3–8
samples, per patient).
The present study was conducted in accordance with
the Declaration of Helsinki, the study protocol was approved by the
local Ethics Committee (approval no. R-1-002/28/2010) and written
informed consent was obtained from all participants prior to
analysis.
RNA extraction and complementary
(c)DNA synthesis
Tissue samples were collected intraoperatively.
Following macroscopic visual assessment, the samples of tumor
tissue and non-malignant esophageal tissue were frozen in liquid
nitrogen and stored at −80°C. Sections (4 µm) of frozen tissue
specimens were cut and stained with hematoxylin and eosin
(Cryotome™ FSE cryostat; Thermo Fisher Scientific, Inc., Hemel
Hempstead, UK). The presence of carcinoma cells was confirmed by
experienced pathologists. Only tumor samples composed of ≥50% tumor
cells upon microscopic analysis were used for subsequent
processing.
Total RNA was isolated and purified from the tissue
specimens using a mirVana™ miRNA Isolation kit (Ambion Life
Technologies, Austin, TX, USA), according to the manufacturer's
instructions. The resulting RNA extracts were stored at −80°C until
required. RNA quantity was assessed using a NanoDrop 2000c
Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). RNA quality, including 28S/18S ratio and RNA integrity
number, was measured using the 2100 Bioanalyzer (serial no.
DE72905449) and an RNA 6000 Nano Assay kit (Agilent Technologies
Inc., Santa Clara, CA, USA), according the manufacturer's
instructions. Total RNA (1 µg) was transcribed into cDNA using High
Capacity RNA-to-cDNA Master Mix with No-RT Control (Applied
Biosystems Life Technologies, Foster City, CA, USA) in a Labcycler
(model no. 1120240193; Sensoquest GmbH, Göttingen, Germany),
according to the manufacturers' instructions.
Determining mRNA expression
levels
The mRNA expression levels of VEGF-C, VEGF-D,
VEGFR-3 and PDPN were evaluated in the tumor and paired
non-malignant esophageal tissues by performing comparative reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
using commercially available TaqMan® Gene Expression assays
(Applied Biosystems Life Technologies) (Table I). Amplification was performed in a
20-µl reaction mixture containing 10 µl TaqMan Gene Expression
Master Mix (Applied Biosystems Life Technologies), 1 µl appropriate
TaqMan Gene Expression assay solution and 2 µl cDNA solution. The
PCR cycle conditions were as follows: 50°C for 2 min and a hold at
95°C for 10 min, followed by 40 cycles of 95°C at 15 sec and 60°C
for 1 min. Each sample was analyzed in triplicate. The reaction was
conducted on an ABI PRISM® 7900HT Sequence Detection System (SDS;
Applied Biosystems Life Technologies) equipped with SDS software
(version 2.4) for performing baseline and cycle threshold (Ct)
calculations. Gene transcript expression levels were quantified as
Ct values normalized to a reference control gene (18S rRNA), using
the following equation: ΔCt = Ctgene - Ctref.
Gene expression levels were inversely proportional to the ΔCt
values and were based on a log2 scale. The reaction
mixture and cycle conditions for 18S rRNA cDNA amplification were
the same as those described for VEGF-C, VEGF-D, VEGFR-3 and PDPN
cDNA amplification.
 | Table I.Assays analyzed in the present
study. |
Table I.
Assays analyzed in the present
study.
| Gene symbol | Official gene product
name | Gene IDa | Assay IDb |
|---|
| VEGF-C | Vascular endothelial
growth factor C | 12682 | HS01099203_m1 |
| VEGF-D | Vascular endothelial
growth factor D | 3708 | Hs01047677_m1 |
| PDPN | Podoplanin | 29602 | Hs00366766_m1 |
| VEGFR-3 | Vascular endothelial
growth factor receptor 3 | 3767 | Hs01128659_m1 |
Tumor-associated fold-change (FC) in mRNA expression
level was calculated using the following equation: FC =
2−ΔΔCt, where ΔCt equals the difference between the
normalized expression of the gene in the tumor samples (Ctgene
T) and its normalized expression in the corresponding
non-malignant esophageal tissue (Ctgene N) (14). Logarithmically transformed FC values
[log2(FC)] were used for statistical analysis. A
log2(FC) value of 1.0 was used as the threshold to
categorize samples into low [log2(FC)<1.0] and high
[log2(FC)>1.0] gene expression groups.
Statistical analysis
Due to asymmetrical data distribution (as determined
by Shapiro-Wilk tests), non-parametric tests were used for all
statistical analyses. Categorical data were compared using the
χ2 or Fisher's exact probability test. Logistic
regression analysis was performed to identify univariable
predictors of lymph node metastasis. Significant univariable
predictors (and those that were clinically appropriate for
inclusion in a model to predict lymph node involvement) were
considered in a stepwise logistic regression model. OS times were
calculated from the date of surgery to the date of mortality or the
most recent follow-up. The Kaplan-Meier method was applied to
estimate the probability of survival as a function of time.
Differences in the survival of the subgroups of patients were
compared using the log-rank test. In addition, the prognostic value
of lymphatic vessel invasion was examined by performing univariate
and multivariate Cox's proportional hazard models. Statistical
analyses were performed using the Statistica (version 10.0;
StatSoft Inc., Tulsa, OK, USA) and Stata/IC (version 12.1;
StataCorp LP, College Station, TX, USA) software. P<0.05 was
considered to indicate a statistically significant difference.
Results
In the cancerous tissues, a high level of mRNA
expression for VEGF-C was observed in 44 patients (52.4%), for PDPN
in 44 patients (52.4%), for VEGFR-3 in 43 patients (51.2%) and for
VEGF-D in 27 patients (32.1%). The expression of PDPN was
significantly correlated with the histological type, tumor stage,
lymph node metastasis, depth of tumor invasion and tumor location
(P<0.05). However, there was no significant association between
PDPN mRNA expression and age, gender, tumor size, histological
grade or residual tumor (P>0.05). VEGF-C overexpression was
significantly associated with tumor depth, tumor stage and lymph
node metastasis (P<0.05). Furthermore, the expression of VEGF-D
was significantly associated with histological grade, tumor stage
and lymph node metastasis, and VEGFR-3 expression was significantly
correlated with tumor size (P<0.05) (Table II).
 | Table II.Association between PDPN, VEGF-C,
VEGF-D and VEGFR-3 mRNA expression levels, and clinicopathological
criteria. |
Table II.
Association between PDPN, VEGF-C,
VEGF-D and VEGFR-3 mRNA expression levels, and clinicopathological
criteria.
|
|
| PDPN | VEGFR-3 | VEGF-D | VEGF-C |
|---|
|
|
|
|
|
|
|
|---|
| Clinicopathological
criteria | n | Low n (%) | High n (%) | P-value | Low n (%) | High n (%) | P-value | Low n (%) | High n (%) | P-value | Low n (%) | High n (%) | P-value |
|---|
| Overall | 84 | 40 (47.6) | 44 (52.4) |
| 41 (48.8) | 43 (51.2) |
| 57 (67.9) | 27 (32.1) |
| 40 (47.6) | 44 (52.4) |
|
| Age, years |
|
|
| nsa |
|
| 0.064a |
|
| nsa |
|
| nsa |
|
<64 | 41 | 21 (51.2) | 20 (48.8) |
| 24 (58.5) | 17 (41.5) |
| 25 (61.0) | 16 (39.0) |
| 23 (56.1) | 18 (43.9) |
|
|
≥64 | 43 | 19 (44.2) | 24 (55.8) |
| 17 (39.5) | 26 (60.5 |
| 32 (74.4) | 11 (25.6) |
| 17 (39.5) | 26 (60.5) |
|
| Gender |
|
|
| nsa |
|
| nsa |
|
| nsa |
|
| nsa |
|
Female | 8 | 4
(50.0) | 4
(50.0) |
| 3
(37.5) | 5
(62.5) |
| 6
(75.0) | 2
(25.0) |
| 3
(37.5) | 5
(62.5) |
|
|
Male | 76 | 36 (47.4) | 40 (52.6) |
| 38 (50.0) | 38 (50.0) |
| 51 (67.1) | 25 (32.9) |
| 37 (48.7) | 39 (51.3) |
|
| Histological
type |
|
|
| 0.023a |
|
| nsa |
|
| nsa |
|
| nsa |
|
Sqcc | 40 | 14 (35.0) | 26 (65.0) |
| 19 (47.5) | 21 (52.5) |
| 28 (70.0) | 12 (30.0) |
| 18 (45.0) | 22 (55.0) |
|
|
Adc | 44 | 26 (59.1) | 18 (40.9) |
| 22 (50.0) | 22 (50.0) |
| 29 (65.9) | 15 (34.1) |
| 22 (50.0) | 22 (50.0) |
|
| Location |
|
|
| 0.037b |
|
| nsb |
|
| nsb |
|
| nsb |
|
Upper | 2 | 0 (0.0) |
2 (100.0) |
| 1
(50.0) | 1
(50.0) |
| 2
(100.0) | 0 (0.0) |
| 1
(50.0) | 1
(50.0) |
|
|
Midthoracic | 54 | 31 (57.4) | 23 (42.6) |
| 31 (57.4) | 23 (42.6) |
| 37 (68.5) | 17 (31.5) |
| 30 (55.5) | 24 (44.5) |
|
|
Lower | 28 | 9
(32.1) | 19 (67.9) |
| 9
(32.1) | 19 (67.9) |
| 18 (64.3) | 10 (35.7) |
| 9
(32.1) | 19 (67.9) |
|
| Tumor size, cm |
|
|
| nsa |
|
| 0.013a |
|
| nsa |
|
| nsa |
|
<4 | 38 | 14 (36.8) | 24 (63.2) |
| 13 (34.2) | 25 (65.8) |
| 24 (63.1) | 14 (36.9) |
| 18 (47.4) | 20 (52.6) |
|
| ≥4 | 46 | 26 (56.5) | 20 (43.5) |
| 28 (60.9) | 18 (39.1) |
| 33 (71.7) | 13 (28.3) |
| 22 (47.8) | 24 (52.2) |
|
| Histological
grade |
|
|
| nsb |
|
| nsb |
|
| 0.005b |
|
| nsb |
| G1 | 9 | 5
(55.5) | 4
(44.5) |
| 4
(44.4) | 5
(55.6) |
| 2
(22.2) | 7
(77.8) |
| 7
(77.8) | 2
(22.2) |
|
| G2 | 37 | 20 (54.0) | 17 (46.0) |
| 19 (51.3) | 18 (48.7) |
| 29 (78.4) | 8
(21.6) |
| 16 (43.2) | 21 (56.8) |
|
| G3 | 38 | 15 (39.5) | 23 (60.5) |
| 18 (47.4) | 20 (52.6) |
| 26 (68.4) | 12 (31.6) |
| 17 (44.7) | 21 (55.3) |
|
| Tumor stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
I+II | 38 | 23 (60.5) | 15 (39.5) | 0.026a | 19 (50.0) | 19 (50.0) | nsa | 21 (55.3) | 17 (44.7) | 0.022a | 23 (60.5) | 15 (39.5) | 0.026a |
|
III | 46 | 17 (37.0) | 29 (63.0) |
| 22 (47.8) | 24 (52.2) |
| 36 (78.3) | 10 (21.7) |
| 17 (37.0) | 29 (63.0) |
|
| Depth of tumor
invasion |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
T1+T2 | 28 | 18 (64.3) | 10 (35.7) | 0.026a | 15 (53.6) | 13 (46.4) | nsa | 16 (57.1) | 12 (42.9) | nsa | 19 (67.8) | 9 (32.2) | 0.008a |
|
T3+T4 | 56 | 22 (39.3) | 34 (60.7) |
| 26 (46.4) | 30 (53.6) |
| 41(73.2) | 15 (26.8) |
| 21 (37.5) | 35 (62.5) |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| N0 | 30 | 19 (63.3) | 11 (36.7) | 0.027a | 14 (46.7) | 16 (53.3) | nsa | 16 (53.3) | 14 (46.7) | 0.031a | 19 (63.3) | 11 (36.7) | 0.027a |
| N1 | 54 | 21 (38.9) | 33 (61.1) |
| 27 (50.0) | 27 (50.0) |
| 41(75.9) | 13 (24.1) |
| 21 (38.9) | 33 (61.1) |
|
| Residual tumor |
|
|
|
|
|
|
|
|
|
|
|
|
|
| R0 | 72 | 37 (51.4) | 35 (48.6) | nsa | 36 (50.0) | 36 (50.0) | nsa | 51 (70.8) | 21 (29.2) | nsa | 36 (50.0) | 36 (50.0) | nsa |
|
R1+R2 | 12 | 3
(25.0) | 9
(75.0) |
| 5
(41.7) | 7
(58.3) |
| 6
(50.0) | 6
(50.0) |
| 4
(33.3) | 8
(66.7) |
|
To investigate the risk factors associated with
lymph node metastasis, univariate and multivariate regression
analyses of gender, tumor location, tumor size, depth of invasion,
and PDPN, VEGFR-3, VEGF-C and VEGF-D mRNA expression were
conducted. Logistic univariate analysis identified that tumor size,
depth of invasion, and VEGF-C, VEGF-D and PDPN mRNA expression were
all significantly associated with lymph node metastasis
(P<0.05). Among these factors, PDPN mRNA expression (P=0.022),
increasing tumor size (P=0.001) and increasing depth of invasion
(P=0.002) were significant independent risk factors for lymph node
metastasis. The other factors were not predictive for lymph node
metastasis (Table III).
 | Table III.Univariate and multivariate analysis
of risk factors for lymph node metastases. |
Table III.
Univariate and multivariate analysis
of risk factors for lymph node metastases.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Gender |
3.400 | 0.752–15.379 | ns |
|
| ns |
| Tumor location |
0.712 | 0.297–1.706 | ns |
|
| ns |
| Tumor size | 15.667 | 5.144–47.713 | <0.001 | 8.286 | 2.355–29.158 | 0.001 |
| Depth of tumor
invasion |
6.531 | 2.408–17.718 | <0.001 | 10.272 | 2.277–46.331 | 0.002 |
| VEGFR-3 mRNA
expression |
0.875 | 0.358–2.139 | ns |
|
| ns |
| VEGF-C mRNA
expression |
2.714 | 1.079–6.827 |
0.034 |
|
| ns |
| VEGF-D mRNA
expression |
0.362 | 0.140–0.939 |
0.036 | 0.315 | 0.084–1.184 | ns |
| PDPN mRNA
expression |
2.714 | 1.079–6.827 |
0.034 | 5.980 | 1.301–27.481 | 0.022 |
The median patient follow-up period was 31 months
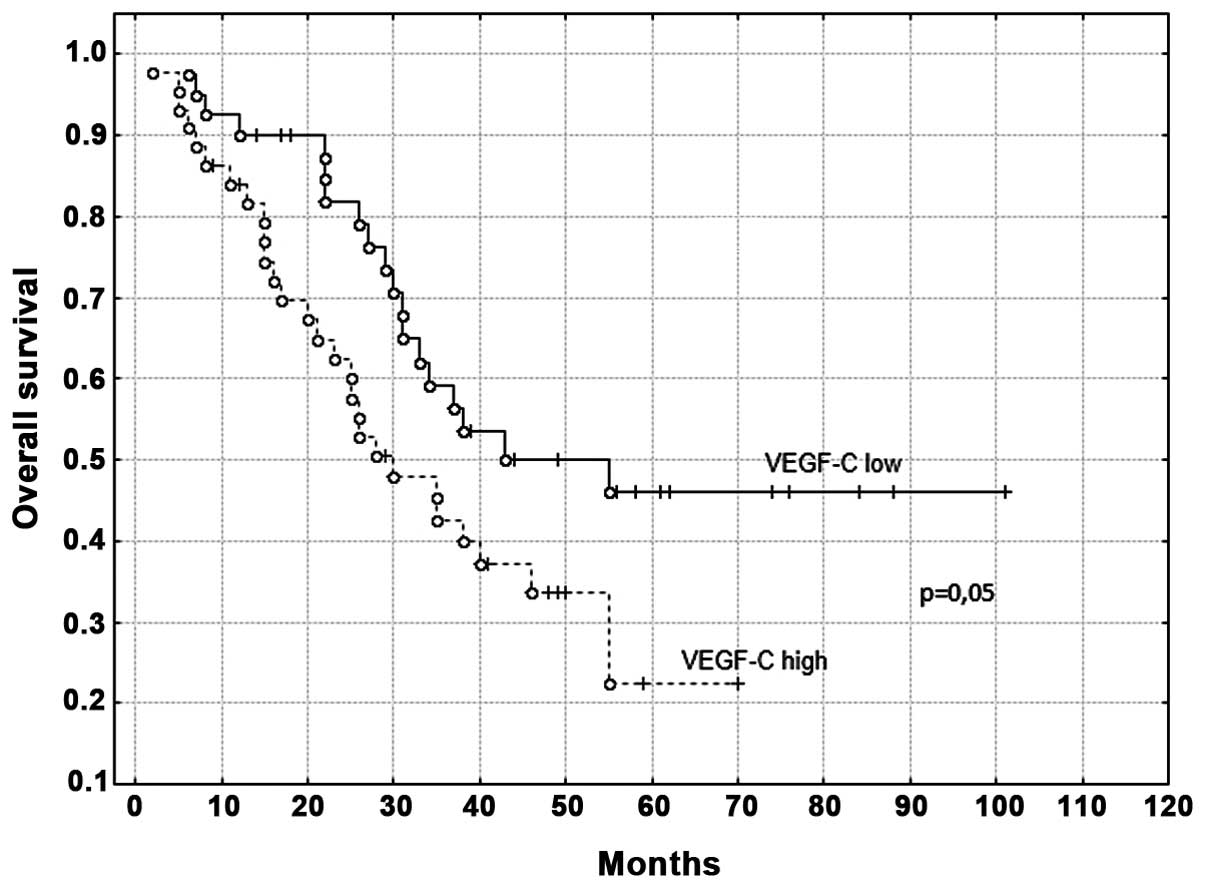
(range, 2–101 months). For VEGF-C mRNA expression, the median OS
time of the patients was 37 months in the low expression group [95%
confidence interval (CI), 29–44 months] and 27 months in the high
expression group (95% CI, 20–38 months; Fig. 1). For VEGF-D mRNA expression, the
median OS time of the patients was 31 months in the low expression
group (95% CI, 26–38 months) and 30 months in the high expression
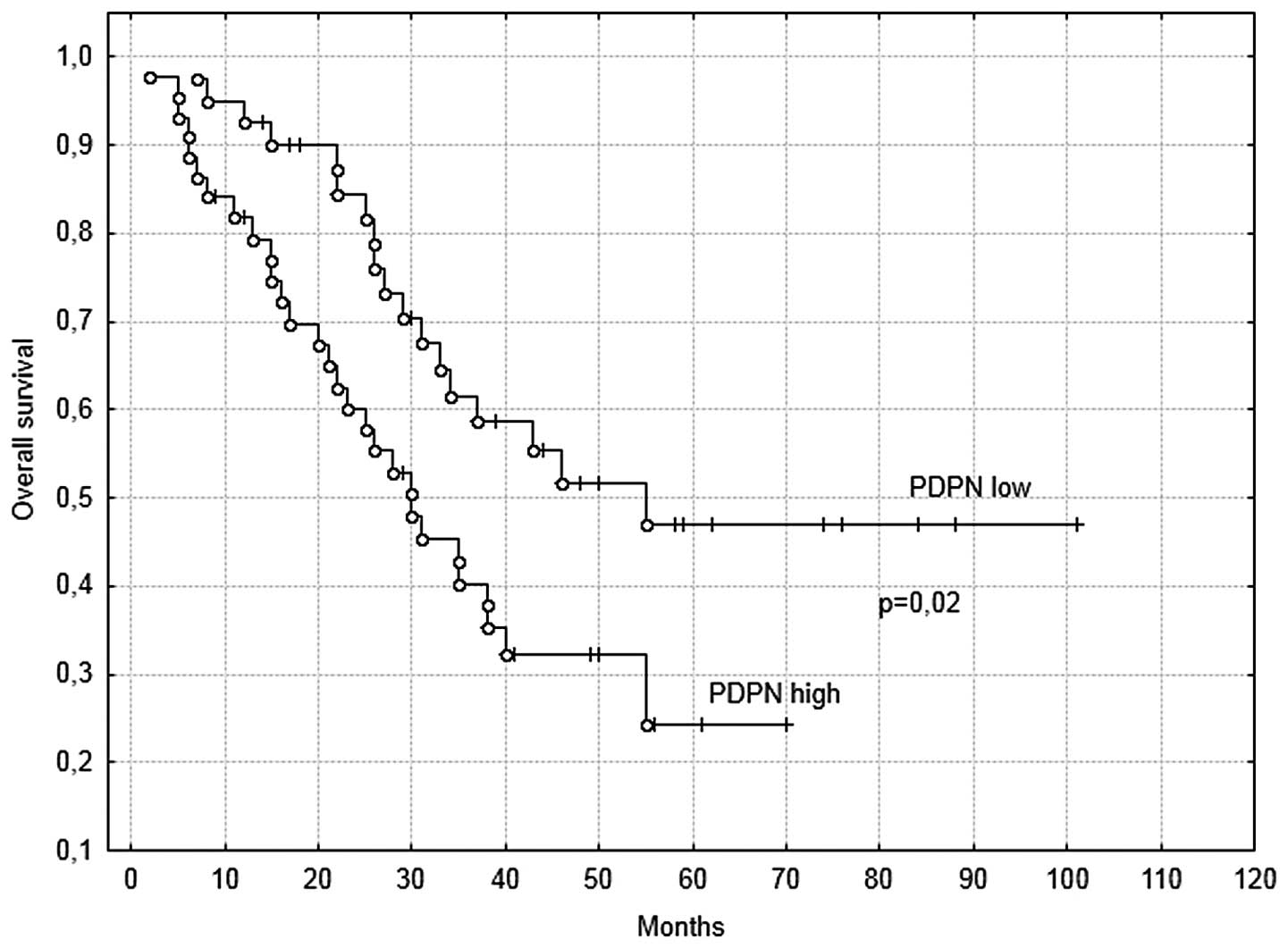
group (95% CI, 14–50). For PDPN mRNA expression, the median OS time
was 37 months in the low expression group (95% CI, 27–46 months)
and 28.5 months in the high expression group (95% CI, 20–38 months)
(Fig. 2). Furthermore, for VEGFR-3
mRNA expression, the median OS time of the patients was 34 months
in the low expression group (95% CI, 26–40 months) and 29 months in
the high expression group (95% CI, 21–41 months).
The patients in the high VEGF-C expression group
were associated with a significantly shorter OS time following
surgery compared with the patients in the low expression group
(P=0.05; Fig. 1). Furthermore, the OS
time of the patients in the high PDPN expression group was
significantly shorter than that in the low expression level
(P=0.02; Fig. 2). By contrast, no
association was identified between VEGFR-3 and VEGF-D expression
levels and OS time.
In univariate analysis, the following parameters
significantly affected OS: Tumor size, depth of tumor invasion,
lymph node metastasis, histological grade, tumor stage, residual
tumor, and VEGF-C and PDPN mRNA expression. In multivariate
analysis, tumor size, residual tumor and PDPN mRNA expression were
identified as independent prognostic factors for a poor OS time in
esophageal cancer (Table IV).
 | Table IV.Cox regression analysis of
independent factors affecting overall survival. |
Table IV.
Cox regression analysis of
independent factors affecting overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Depth of tumor
invasion | 3.765 | 1.802–7.87 | <0.001 | 2.191 | 0.957–5.014 | ns |
| Lymph node
metastasis | 7.72 | 3.099–19.231 | <0.001 |
|
| ns |
| Histological
grade | 1.832 | 1.156–2.905 |
0.010 |
|
| ns |
| Tumor stage | 3.937 | 2.025–7.654 | <0.001 |
|
| ns |
| Tumor size | 2.277 | 1.236–4.197 |
0.008 | 1.955 | 1.002–3.811 | 0.049 |
| Residual tumor | 4.227 | 2.131–8.384 | <0.001 | 3.784 | 1.858–7.707 | <0.001 |
| VEGFR-3 mRNA
expression | 1.015 | 0.572–1.800 | ns |
|
| ns |
| VEGF-C mRNA
expression | 1.768 | 0.982–3.183 |
0.050 |
|
| ns |
| VEGF-D mRNA
expression | 0.845 | 0.445–1.604 | ns |
|
| ns |
| PDPN mRNA
expression | 1.95 | 1.079–3.524 |
0.027 | 2.081 | 1.121–3.865 | 0.020 |
Discussion
Metastasis is directly or indirectly responsible for
>90% of all cancer mortalities (15). In numerous types of carcinoma, the
presence of tumor cells in the lymph nodes is the initial
manifestation of metastasis and one of the most important factors
of a poor prognosis. The rapid growth and invasive character of
esophageal tumors has often been associated with the lymphatic
spread of the disease at diagnosis (3).
The induction of lymphangiogenesis by tumors is
mediated by growth factors. The most widely investigated factors
are members of the VEGF family (VEGF-C, VEGF-D and VEGF-A) and
their receptors (16,17). Furthermore, previous studies have
indicated that PDPN may be associated with lymphatic dissemination
and prognosis in patients with esophageal cancer (18).
The aim of the present study was to analyze the
association between the mRNA expression of VEGF-C, VEGF-D, VEGFR-3
and PDPN, and the clinicopathological factors and outcomes of
patients with esophageal cancer. Overexpression of VEGF-C, VEGF-D,
VEGFR-3 and PDPN was observed in the cancerous tissue samples.
These findings are in accordance with the results of studies by
Kimura et al (19) and Okazawa
et al (20), and our previous
study (8), which used
immunohistochemistry to demonstrate that esophageal tumors express
VEGF-C and VEGF-D. In addition, Tanaka et al (21) used RT-qPCR to demonstrate that
esophageal cancer cells express VEGF-C, and Tong et al
(22) and Rahadiani et al
(23) identified PDPN overexpression
in esophageal carcinoma.
Previous studies have demonstrated that increased
VEGF-C and VEGF-D expression is correlated with increased tumor
cell dissemination to the regional lymph nodes in a range of
primary human carcinomas, including esophageal cancer (8,20,21). The present study identified that
VEGF-D mRNA overexpression was associated with histological grade,
tumor stage and lymph node metastasis. The study by Tzao et
al (24) and our previous study
(8) obtained similar results. In the
current study, a high expression level of VEGF-C was significantly
correlated with tumor stage, depth of tumor invasion and lymph node
metastasis. This is in accordance with previous studies by Okazawa
et al (20), Tanaka et
al (21) and Kitadai et al
(25), which identified a close
correlation between VEGF-C expression and depth of tumor invasion,
tumor stage and lymph node metastasis. In the patients with
esophageal cancer, VEGFR-3 expression was only correlated with
tumor size.
The current study identified that PDPN
overexpression was correlated with tumor stage, depth of tumor
invasion, lymph node metastasis, tumor location and histological
type. The current findings are in agreement with those obtained by
Nakayama et al (18),
Rahadiani et al (23) and Tong
et al (22), which
demonstrated a significant correlation between PDPN tumor
expression and pathological stage, depth of tumor invasion and
lymph node metastasis in esophageal carcinoma.
Furthermore, the present study demonstrated that
VEGF-C, VEGF-D and PDPN mRNA expression, tumor size, and depth of
tumor invasion were associated with lymph node metastasis by
performing univariate regression analysis. Multivariate logistic
regression analysis also revealed PDPN mRNA expression, increasing
tumor size and increasing depth of tumor invasion to be independent
factors affecting lymph node metastasis. These findings indicate
that PDPN, VEGF-C and VEGF-D mRNA expression were more
significantly associated with lymphatic spread than hematogenous
metastasis, highlighting their possible efficacy in predicting the
nodal status of patients with esophageal cancer.
In the present study, a poor OS time was positively
correlated with the overexpression of VEGF-C and PDPN in the
esophageal cancer cells. Multivariate analysis identified tumor
size, residual tumor and mRNA PDPN expression as independent
factors of patient prognosis. Furthermore, the present study used
the Kaplan-Meier method to determine that high VEGF-C expression
was associated with a significantly shorter OS time compared with
low VEGF-C expression. In agreement with this finding, Kitadai
et al (25), Kimura et
al (26) and our previous study
(8) used immunohistochemistry to
demonstrate that the prognosis of patients with VEGF-C-positive
tumors was significantly worse than that of patients with
VEGF-C-negative tumors. Similarly, Okazawa et al (20) identified a significant difference in
survival rates between groups with or without VEGF-C overexpression
in patients with esophageal cancer. In addition, this study
performed a multivariate analysis that determined that gender, age,
VEGF-C expression and lymphatic invasion were all prognostic
determinants in esophageal cancer. Using univariate survival
analysis, Tanaka et al (21)
determined a significant difference in OS between high and low
VEGF-C mRNA expression in patients with esophageal cancer. Tong
et al (22) performed
immunohistochemical analysis and, using univariate and multivariate
analysis, identified that overexpression of PDPN was both a
prognostic factor and independent prognostic factor for 5-year
disease-free survival. Furthermore, Rahadiani et al
(23) demonstrated that PDPN
overexpression was a prognostic factor for OS and disease-free
survival, and an independent prognostic factor for disease-free
survival.
The present results indicated that, as they are
secreted by esophageal cancer cells, VEGF-C and PDPN may be able to
induce and mediate tumor cell invasion, spread cancer cells beyond
the primary tumor, and form a metastatic focus in the lymph
nodes.
In conclusion, the current study identified that the
expression of VEGF-C, VEGF-D and PDPN mRNA was significantly
correlated with lymph node metastasis and tumor stage. In
particular, PDPN overexpression was significantly associated with
patients at a high risk of regional lymph node metastasis. Thus,
VEGF-C and PDPN overexpression may be useful as possible indicators
of poor prognosis, and PDPN overexpression may be applied as an
independent prognostic marker in patients with esophageal cancer
that have undergone potentially curative esophagectomy.
Acknowledgements
The present study was partially conducted within the
Cancer/Mutagenesis research area of the Leading National Research
Centre (KNOW). The study was supported by a grant obtained from the
National Science Centre (no. N N403 282739).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eloubeidi MA, Desmond R, Arguedas MR, Reed
CE and Wilcox CM: Prognostic factors for the survival of patients
with esophageal carcinoma in the US: The importance of tumor length
and lymph node status. Cancer. 95:1434–1443. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilson M, Rosato EL, Chojnacki KA,
Chervoneva I, Kairys JC, Cohn HE, Rosato FE Sr and Berger AC:
Prognostic significance of lymph node metastases and ratio in
esophageal cancer. J Surg Res. 146:11–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veikkola T and Alitalo K: VEGFs, receptors
and angiogenesis. Semin Cancer Biol. 9:211–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Achen MG and Stacker SA: Molecular control
of lymphatic metastasis. Ann NY Acad Sci. 1131:225–234. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stacker SA, Caesar C, Baldwin ME, Thornton
GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H and Achen
MG: VEGF-D promotes the metastatic spread of tumor cells via the
lymphatics. Nat Med. 7:186–191. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shida A, Fujioka S, Ishibashi Y, Kobayashi
K, Nimura H, Mitsumori N, Suzuki Y, Kawakami M, Urashima M and
Yanaga K: Prognostic significance of vascular endothelial growth
factor D in gastric carcinoma. World J Surg. 29:1600–1607. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kozlowski M, Naumnik W, Niklinski J,
Milewski R, Dziegielewski P and Laudanski J: Vascular endothelial
growth factor C and D exprossion correlates with lymph node
metastasis and poor prognosis in patients with resected esophageal
cancer. Neoplasma. 58:311–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler
E, Alitalo K, et al: Angiosarcomas express mixed endothelial
phenotypes of blood and lymphatic capillaries: Podoplanin as a
specific marker for lymphatic endothelium. Am J Pathol.
154:385–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kozłowski M, Naumnik W, Nikliński J,
Milewski R, Lapuć G and Laudański J: Lymphatic vessel invasion
detected by the endothelial lymphatic marker D2-40 (podoplanin) is
predictive of regional lymph node status and an independent
prognostic factor in patients with resected esophageal cancer.
Folia Histochem Cytobiol. 49:90–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kozłowski M, Niklińska W, Naumnik W,
Laudański W, Milewski R and Nikliński J: Intratumoral lymphatic
vessel density and intratumoral and peritumoral lymphatic vessel
invasion as predictive factors of lymph node metastasis and
prognostic factors in esophageal cancer. Kardiochir Torakochir Pol.
10:120–129. 2013.
|
|
12
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofor G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: Esophagus and esophagogastric junction.
AJCC cancer staging manual. Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: (7th). (New York, NY). Springer. 103–107.
2010.
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sporn MB: The war on cancer. Lancet.
347:1377–1381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirakawa S, Brown LF, Kodama S, Paavonen
K, Alitalo K and Detmar M: VEGF-C-induced lymphangiogenesis in
sentinel lymph nodes promotes tumor metastasis to distant sites.
Blood. 109:1010–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kozłowski M, Laudański W, Mroczko B,
Szmitkowski M, Milewski R and Łapuć G: Serum tissue inhibitor of
metalloproteinase 1 (TIMP-1) and vascular endothelial growth factor
A (VEGF-A) are associated with prognosis in esophageal cancer
patients. Adv Med Sci. 58:227–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakayama Y, Matsumoto K, Nagato M, Inoue
Y, Katsuki T, Minagawa N, Shibao K, Tsurudome Y, Hirata K, Higure
A, et al: Significance of lymphangiogenesis as assessed by
immunohistochemistry for podoplanin in patients with esophageal
carcinoma. Anticancer Res. 27:619–625. 2007.PubMed/NCBI
|
|
19
|
Kimura H, Kato H, Tanaka N, Inose T,
Faried A, Sohda M, Nakajima M, Fukai Y, Miyazaki T, Masuda N, et
al: Preoperative serum vascular endothelial growth factor-C
(VEGF-C) levels predict recurrence in patients with esophageal
cancer. Anticancer Res. 28:165–169. 2008.PubMed/NCBI
|
|
20
|
Okazawa T, Yoshida T, Shirai Y, Shiraishi
R, Harada T, Sakaida I, Abe T and Oka M: Expression of vascular
endothelial growth factor C is a prognostic indicator in esophageal
cancer. Hepatogastroenterology. 55:1503–1508. 2008.PubMed/NCBI
|
|
21
|
Tanaka T, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Shiozaki M, Naganawa Y, Fujii Y and Takeyama
H: Vascular endothelial growth factor C (VEGF-C) in esophageal
cancer correlates with lymph node metastasis and poor patient
prognosis. J Exp Clin Cancer Res. 29:832010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong L, Yuan S, Feng F and Zhang H: Role
of podoplanin expression in esophageal squamous cell carcinoma: A
retrospective study. Dis Esophagus. 25:72–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahadiani N, Ikeda JI, Makino T, Tian T,
Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K and Morii E: Tumorigenic
role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg
Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tzao C, Lee SC, Tung HJ, Hsu HS, Hsu WH,
Sun GH, Yu CP, Jin JS and Cheng YL: Expression of hypoxia-inducible
factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D
as outcome predictors in resected esophageal squamous cell
carcinoma. Dis Markers. 25:141–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitadai Y, Amioka T, Haruma K, Tanaka S,
Yoshihara M, Sumii K, Matsutani N, Yasui W and Chayama K:
Clinicopathological significance of vascular endothelial growth
factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J
Cancer. 93:662–666. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura Y, Watanabe M, Ohaga T, Saeki H,
Kakeji Y, Baba H and Maehera Y: Vascular endothelial growth factor
C expression correlates with lymphatic involvement and poor
prognosis in patients with esophageal squamous cell carcinoma.
Oncol Rep. 10:1747–1751. 2003.PubMed/NCBI
|