Introduction
Vulvar carcinoma accounts for 3–5% of all female
genital cancers. Previously considered as a disease found in
elderly women, it is now recognized that the incidence among
younger women (<50 years old) is increasing. Radical vulvectomy
with bilateral inguinofemoral lymphadenectomy has been the standard
treatment for the majority of patients, but is associated with a
high risk of post-operative complications (1). Individualized therapy incurs fewer
complications and can improve outcome; the identification of novel
biomarkers for vulvar carcinoma progression will greatly aid the
development of such individualized therapies.
Galectins constitute a family of lectins defined by
shared consensus amino acid sequences and affinity for
β-galactose-containing oligosaccharides. To date, 14 mammalian
galectins have been identified and several have been shown to
exhibit important functions in physiological processes such as
embryonic development, apoptosis, wound healing, cell migration,
intercellular adhesion and immune responses (2). Galectins have also been indicated to be
involved in a number of pathological conditions, including
infectious diseases and cancer. Magnaldo et al (3) initially described galectin-7 (Gal-7) as
a marker of differentiation in stratified epithelia. Gal-7 exhibits
a wide range of biological functions, including the regulation of
cell growth, adhesion and apoptosis (4). There have been a number of studies
showing that the expression of Gal-7 is altered in tumors, with
upregulation and downregulation each being described in different
tumor types (3,5–8). However,
the precise role of Gal-7 in cancer development remains under
debate.
The expression of Gal-7 in VSCC has not been
previously reported and its clinical significance in patients with
VSCC remains unknown. Therefore, the present study investigated
whether abnormal Gal-7 expression is associated with VSCC malignant
progression using western blotting and immunohistochemistry (IHC),
and also assessed the degree of methylation at the Gal-7
(LGALS7) gene promoter using methylation-specific polymerase
chain reaction (MSP) to define the potential mechanisms of abnormal
Gal-7 expression.
Materials and methods
Sample collection and preparation
Tissue specimens were obtained from 50 patients with
VSCC who had undergone surgery in the Department of Gynecology of
The First Affiliated Hospital of China Medical University
(Shenyang, Liaoning, China) between 1998 and 2011. All patients
underwent curative resection, but were not treated with neoadjuvant
chemotherapy or radiotherapy. Immediately after surgery, the
specimen was divided into two sections: The first was stored at
−80°C and the second section was fixed in formalin. Normal vulvar
tissue specimens were also obtained from 20 patients undergoing
vulvar repair procedures. Tissue specimens were obtained after
obtaining informed consent from all patients and the study was
approved by the Research Ethical Committee of The First Affiliated
Hospital of China Medical University.
Patient and tissue
characteristics
The 50 patients with VSCC (mean age, 55.7±3.9 years;
range, 33–73 years) included 15 with Federation of International
Gynecologists and Obstetricians (1988) stage I disease, 22 with
stage II disease and 13 with stage III disease. In terms of
histological grade, 29 specimens were well-differentiated, 16 were
moderately-differentiated and 5 were poorly-differentiated. There
were 15 patients with lymphatic node metastasis across all
groups.
Western blot analysis
In total, 10 paired VSCC and corresponding
age-matched normal vulvar samples were selected for western blot
analysis. Equal quantities of protein from each sample were
separated by 15% SDS-PAGE and then transferred to polyvinylidene
fluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membranes were blocked in 5% skimmed milk in Tris-buffered
saline with Tween-20 buffer [20 mM Tris (pH 7.6), 137 mM NaCl and
0.05% Tween-20) at room temperature for 2 h, prior to incubation
with the primary antibodies for 2 h at room temperature. The
following primary antibodies were used: rabbit monoclonal
anti-Gal-7 (1:1,000; ab108623; Abcam, Cambridge, UK) and mouse
monoclonal anti-β-actin (1:1,000; Santa Cruz Biotechnology Inc.,
Dallas, TX, USA). Mouse anti-β-actin (1:1,000, Santa Cruz
Biotechnology) was used as a loading control. The membranes were
then incubated with their corresponding anti-mouse or anti-rabbit
secondary antibody for 1 h at room temperature. The protein bands
were visualized using an electrochemiluminescence detection kit
(Beyotime Institute of Biotechnology, Shanghai, China), and the
densities of the protein bands were determined by Quantity One
v.4.62 software (Bio-Rad Laboratories Inc.). Each analysis was
performed at least three times.
IHC
Formalin-fixed paraffin embedded sections (4-µm
thick) from the 50 cases of VSCC and the 20 samples of normal
vulvar tissue were analyzed by IHC. The sections were stained
according to the streptavidin-biotin-immunoperoxidase method
described by Ohno et al (9),
in 1998. Briefly, each section was deparaffinized with xylene,
rehydrated and washed with phosphate-buffered saline (PBS). Antigen
retrieval was performed by autoclaving the sections in citrate
buffer [0.016 M citric acid and 0.084 M sodium citrate (pH 6.0)] at
120°C for 2 min. The sections were allowed to cool to room
temperature and then washed three times for 5 min in PBS. The
sections were incubated in 0.3% hydrogen peroxide in absolute
methanol for 15 min to suppress endogenous peroxidase activity;
this was followed by incubation with 1% bovine serum albumin for 15
min to prevent non-specific binding. The sections were incubated
with the same primary antibodies that had been used for western
blotting (Gal-7; 1:800) at 4°C overnight. Subsequent to being
washed three times for 5 min in PBS, the sections were incubated
with their corresponding secondary antibodies, as for the western
blotting. The samples were then labeled with streptavidin
peroxidase for 15 min, treated with diaminobenzidine as a chromogen
for 5 min and counterstained with Mayers hematoxylin. The sections
were washed with PBS between each step of the procedure. Negative
controls were prepared by processing different sections following
the same procedure, but omitting the primary antibodies. Images of
5 randomly selected fields from non-necrotic areas in the VSCC
sections and from epidermal cell layers, including a region of the
dermis area in the normal vulvar tissues, were captured and
analyzed using an image analysis system (MetaMorph, Universal
Imaging Corporation, Dowington, PA, USA) and an Olympus digital
camera (DP10/Bx41; Olympus Corp., Tokyo, Japan) following the
manufacturers instructions. Analysis was performed after
hue-saturation-intensity transformation and color-deconvolution.
After evaluating several fields on positive control slides, the
intensity threshold for the immunostaining (brown) was set. The
mean integrated optical density (OD) was evaluated for each image
and the average change in OD between the positive areas in the VSCC
tissue and normal vulvar tissue was calculated.
DNA preparation and DNA modification
(bisulfite treatment)
A total of 30 paraffin-embedded VSCC tissues and 20
paraffin-embedded normal vulvar tissues were retrieved using xylene
and alcohol, and isolated using a DNA extraction kit (E.Z.N.A.®
Tissue DNA kit; Omega Nio-Tek Inc., Norcross, GA, USA).
DNA modification with sodium bisulfite causes
unmethylated cytosine bases to convert to uracil, while methylated
cytosine is resistant to conversion and remains unchanged (10). Therefore, following bisulfite
treatment, methylated alleles will have a different sequence
compared with unmethylated alleles. This can be used to design
allele-specific PCR primers to permit MSP. Genomic DNA (2 µg) was
first denatured by heating to 97°C for 10 min, followed by chilling
on ice at 0°C for 5 min, and was then incubated for 20 min at 48°C
with 3 M NaOH (2 µl). Bisulfite solution (2.5 M sodium
metabisulfite and 125 mM hydroquinone) was added and incubated for
12 h at 48°C in the dark. The bisulfite-modified DNA was then
purified using Wizard DNA purification resin (DNA Cleanup kit;
Promega Corporation, Madison, WI, USA) according to the
manufacturers instructions. Modified DNA was treated with 3 M NaOH
(5 µl) in 37°C for 10 min and precipitated with ammonium acetate 5
M (75 µl), 2.5 volumes 100% ethanol and 2 µl glycogen (20 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) and dissolved in 20 µl 5 mM Tris
(pH 8.0).
MSP
Since members of the galectin family have been shown
to be regulated by DNA methylation, the present study investigated
the methylation status of CpGs located in the promoter region (from
−200 bp upstream to −30 bp from the ATG start codon) of the human
Gal-7 gene in VSCC by MSP (11,12). The
modified DNA served as a template using primers specific for either
the methylated or bisulfite-modified unmethylated sequence. The
methylation specific primers were as follows: Forward,
5-GTTTTTAAGAAGAGGTGTTATTTTCG-3 and reverse,
5-AACCTACTAAAAACCTTAAATAAAAACA-3. The unmethylated DNA specific
primers were as follows: Forward, 5-AGTTTTTAAGAAGAGGTGTTATTTTTG-3
and reverse, 5-AACCTACTAAAAACCTTAAATAAAAACA-3. The PCR mixture
contained 2X GC buffer (Takara Biotechnology Co., Ltd., Dalian,
China) with 4 mmol/l MgCl2, 10 µmol/l of each primer,
6.25 mmol/l dNTPs and 1.5 unit LA Taq polymerase (Takara
Biotechnology Co., Ltd.). The PCR amplification of the modified DNA
samples consisted of one cycle of 94°C for 10 min, followed by 40
cycles of 94°C for 30 sec, 53°C for 30 sec and 72°C for 1 min, and
then one cycle of 72°C for 10 min. Human placental DNA was used as
a positive control for unmethylated DNA. In order to make a
positive control for methylated DNA, DNA from human placental
tissue was treated with M.SssI CpG methyltransferase (New England
BioLabs, Ipswich, MA, USA) prior to bisulfite treatment. The PCR
generated a 146-bp methylated product and a 147-bp unmethylated
product. Amplified PCR product (6 µl) were loaded onto 3% agarose
gels, stained with ethidium bromide and directly visualized under
ultraviolet illumination.
Statistical analyses
Statistical analyses of western blotting, IHC and
MSP data were performed using SPSS v.13.0 software (SPSS Inc.,
Chicago, IL, USA). The statistical differences between groups in
western blotting were compared using a paired sample t-test.
Students t-test was used to compare statistical differences in IHC
between groups, to permit correlation with clinicopathological
characteristics. The correlation between two variables in the MSP
test was evaluated using Pearsons χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
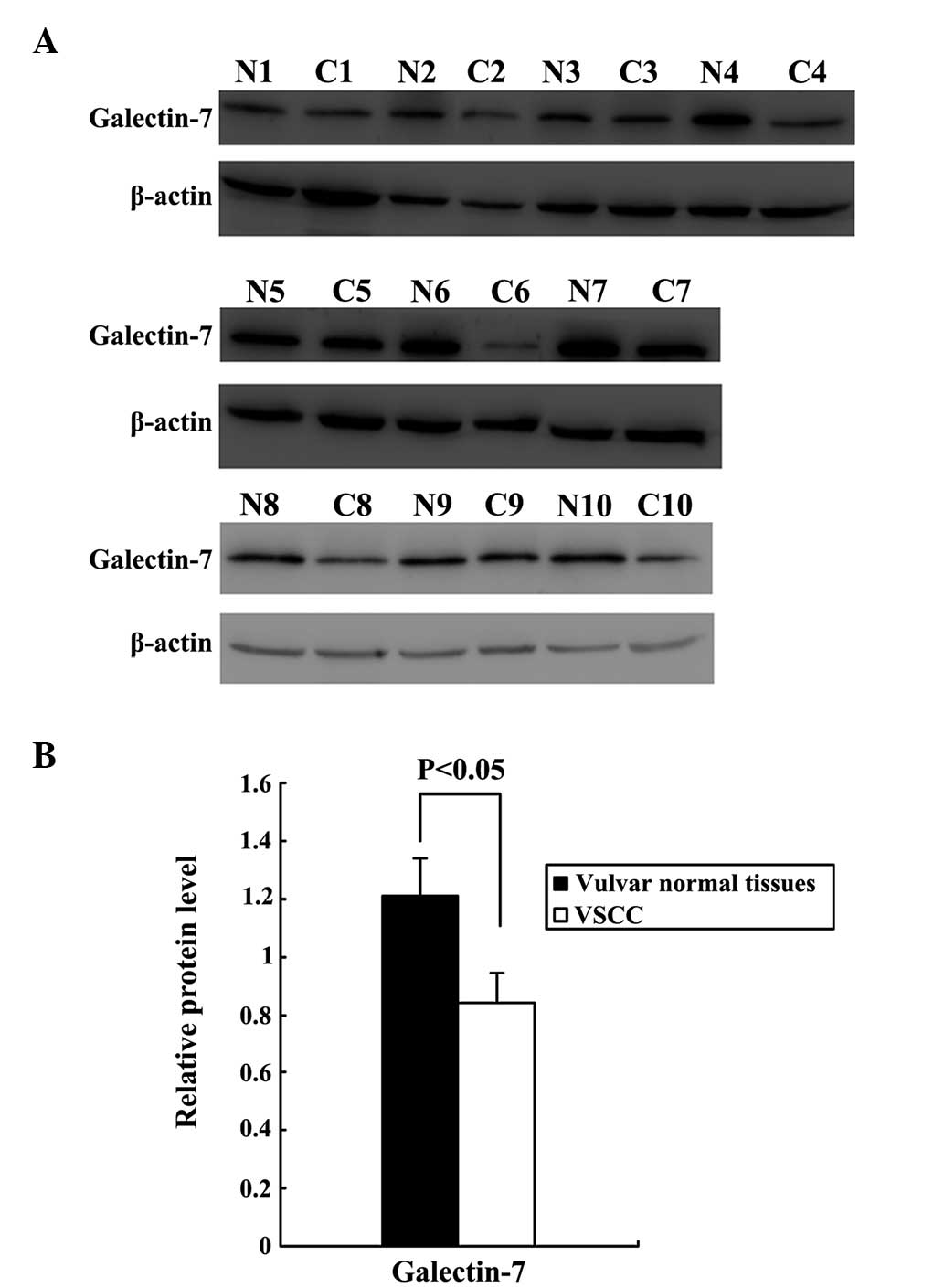
Gal-7 protein expression
Western blot analysis of total protein in the VSCC
tissues and normal vulvar tissues confirmed that Gal-7 expression
was significantly downregulated in VSCC relative to normal vulvar
tissues (P<0.05; Fig. 1, with
β-actin used as a loading control). The expression and localization
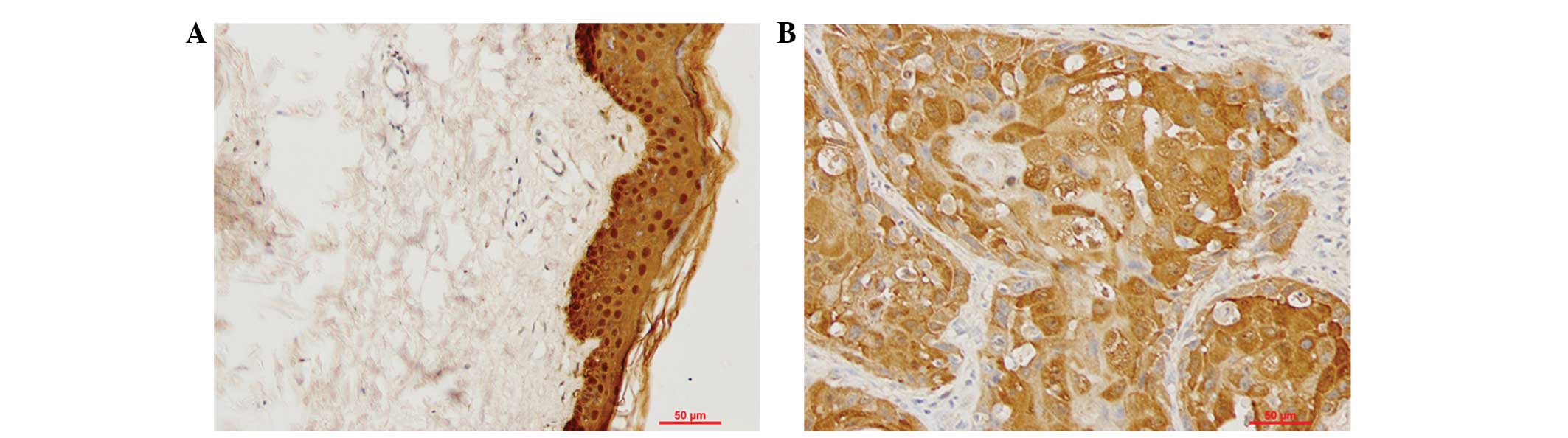
of Gal-7 was examined by IHC in tissue samples from 50 cases of
VSCC and 20 normal vulvar biopsies. Cytoplasmic and nuclear
staining was observed in the epithelial layers of all 20 samples of
normal vulvar tissue. Cytoplasmic staining was observed in the
cancer nests of 50 cases of VSCC. Statistical analysis indicated
that Gal-7 expression was significantly downregulated in the VSCC
tissues compared with the normal vulvar tissues (P<0.05;
Fig. 2; Table I).
 | Table I.Galectin-7 expression in VSCC and
vulvar normal tissues based on immunohistochemistry. |
Table I.
Galectin-7 expression in VSCC and
vulvar normal tissues based on immunohistochemistry.
| Group | n | Mean IOD | t | P-value |
|---|
| VSCC | 50 | 0.3380±0.0118 |
|
|
| Vulvar normal
tissue | 20 | 0.3441±0.0177 | 2.26 | 0.026 |
Next, the study investigated the potential
correlation between Gal-7 expression and clinicopathological
factors in patients with VSCC (Table
II). The age of the patients did not differ significantly
between the two groups. Gal-7 expression was significantly lower in
the patients with stage II and III disease compared with the
patients with stage I disease. Gal-7 expression was also
significantly lower in the patients with a well-differentiated
tumor histology compared with those patients who presented with
moderately- and poorly-differentiated malignancies, and was
similarly reduced in patients with lymph node metastases. Together,
these findings describe a correlation between downregulated Gal-7
expression and advanced clinical stage, poor tumor differentiation
and regional lymph node metastasis.
 | Table II.Correlation between the expression of
immunoreactivity to Gal-7 and clinicopathological features in 50
patients with vulvar squamous cell carcinoma. |
Table II.
Correlation between the expression of
immunoreactivity to Gal-7 and clinicopathological features in 50
patients with vulvar squamous cell carcinoma.
|
|
| Gal-7 |
|---|
|
|
|
|
|---|
| Clinicopathological
features | n | Mean IOD | P-value |
|---|
| Age, years |
|
| 0.469 |
| ≤45 | 13 | 0.3364±0.0095 |
|
|
>45 | 37 | 0.3386±0.0125 |
|
| Lymph node
metastasis |
|
| 0.048 |
|
Negative | 35 | 0.3399±0.0069 |
|
|
Positive | 15 | 0.3327±0.0083 |
|
| Clinical stage |
|
| 0.022 |
| I | 15 | 0.3409±0.0104 |
|
| II and
III | 35 | 0.3341±0.0064 |
|
| Histological
type |
|
| 0.040 |
|
Well-differentiated | 29 | 0.3422±0.0109 |
|
|
Moderately- and
poorly-differentiated | 21 | 0.3357±0.0098 |
|
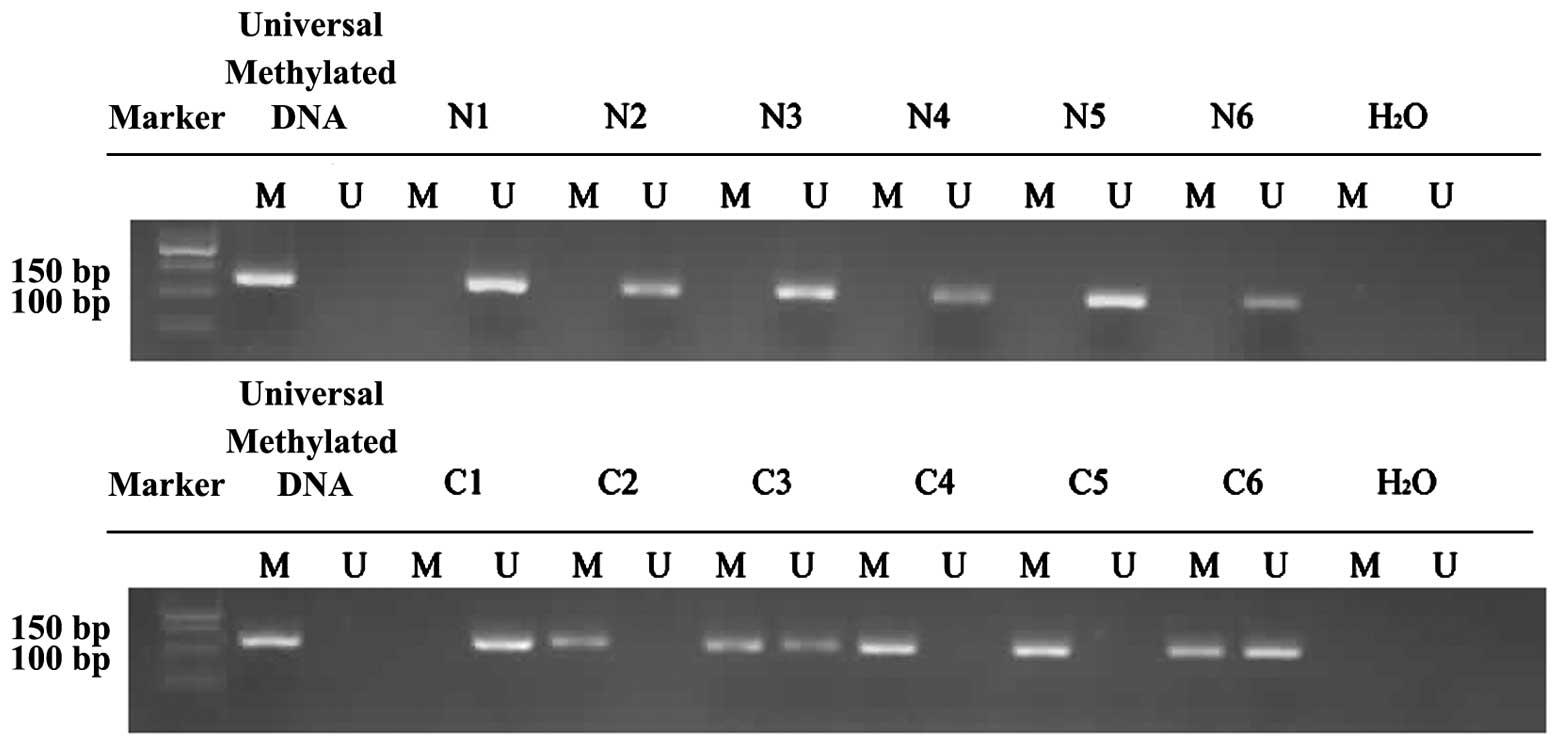
DNA hypermethylation in the promoter
of the Gal-7 gene in VSCC
The degree of methylation at the Gal-7 promoter was
significantly lower in the normal vulvar tissues compared with the
VSCC samples (P=0.023; Fig. 3). In
the 20 normal vulvar tissues, methylation was found at 30% of CpG
bases, compared with 60% in the 30 VSCC samples.
The correlation between Gal-7 gene promoter
methylation and clinicopathological factors in the patients with
VSCC was also examined (Table II).
Increased Gal-7 promoter methylation was correlated with advanced
clinical stage, poor tumor differentiation and regional lymph node
metastasis (P<0.05). There was no association between patient
age and Gal-7 promoter methylation.
Discussion
Gal-7 is an important member of the galectin family
that is mainly expressed in stratified epithelia, particularly in
the epidermis, and also in various types of cancer. Gal-7 is
associated with the differentiation and maturation of epithelial
tissue, and with the regeneration process of damaged skin tissue
(13). The biological functions of
Gal-7 are diverse and include the regulation of cell growth, cell
adhesion and apoptosis (14–16).
In recent years, numerous studies have shown that
Gal-7 plays an important role in tumorigenesis, although the
mechanisms underlying this remain under debate. Overexpression of
Gal-7 has been reported in several tumors, including pancreatic
cancer, esophageal squamous cell carcinoma, buccal squamous cell
carcinoma, and hypopharyngeal and laryngeal squamous cell carcinoma
(7,8,17,18). Park et al (19) reported that Gal-7 induces the
expression of matrix metalloproteinase-9 (MMP-9) through the p38
mitogen-activated protein kinase signaling pathway, promoting tumor
metastasis in human cervical adenocarcinoma. Saussez et al
(18) also found that the
overexpression of Gal-7 could induce the expression of MMP-9 in
hypopharyngeal and laryngeal squamous cell carcinoma.
Downregulation of Gal-7 in ovarian cancer cell lines using small
interfering RNA results in the inhibition of ovarian cancer cell
proliferation (20), suggesting that
the overexpression of Gal-7 in these tumors may promote
proliferation and metastasis. By contrast, the underexpression of
Gal-7 has also been reported in several tumors, including gastric
cancer and neuroblastoma (15,21). Kim
et al (21) treated gastric
cancer cell lines using the demethylating agent, 5-azacytidine,
with a resulting marked increase in Gal-7 expression, suggesting
that Gal-7 expression is regulated by DNA methylation in gastric
cancer cell lines. Kopitz et al (15) reported that Gal-7 acts as a lectin on
the cell surface, and that it could inhibit proliferation by
binding to ganglioside GM1 on the surface of neuroblastoma
cells.
Gal-7 is also able to act as a pro-apoptotic protein
that is induced by p53. The induction of apoptosis by Gal-7 is
believed to involve c-Jun N-terminal kinase activation, release of
mitochondrial cytochrome c and B cell lymphoma-2 binding in
the mitochondrial membrane (14,22–24).
Ectopic expression of Gal-7 in cancer cells increases their
susceptibility to apoptosis and thereby suppresses tumor growth
(25). In addition, Gal-7 has also
been described as enhancing immune function, with the study by
Rossi et al (26) finding that
Gal-7 single glycosylation recognition sites could bind to the αβ
T-cell antigen receptor-cluster of differentiation 3 complex on the
surface of T lymphocytes, suggesting a role in T-cell activation
and proliferation. Menkhorst et al (16) reported that pre-treatment of
trophoblasts with soluble Gal-7 enhanced adhesion to endometrial
epithelial cells, indicating a potential role in embryo attachment
to endometrial cells and perhaps a more general function in cell
adhesion.
It is therefore clear that Gal-7 has diverse
cellular roles. In agreement with this, the present results showed
that the expression of Gal-7 was lower in VSCC samples compared
with normal vulvar tissues, with downregulation of Gal-7
correlating with advanced clinical stage, poor differentiation and
lymph node metastasis in VSCC. As such, Gal-7 downregulation can be
observed to have a deleterious effect in VSCC.
The expression of certain members of the galectin
family has been demonstrated to be under the control of epigenetic
regulatory mechanisms (11,12). It is unclear whether Gal-7 expression
in VSCC is similarly controlled. Kim et al (21) reported that the expression of Gal-7
was downregulated in gastric cancer, and that this was the
consequence of alterations in DNA methylation status. Demers et
al (27) and Moisan et al
(28) found that treatment of T
lymphoma cell lines with 5-aza-2-deoxycytidine was sufficient to
induce Gal-7 expression, although these cells do not express Gal-7
under normal conditions. We hypothesized that the methylation
status of the Gal-7 gene could be clinically relevant in
determining its expression in VSCC, with promoter CpG methylation
inhibiting transcription of the Gal-7 gene and demethylation
resulting in an increase in its expression. Thus, the Gal-7 gene
promoter methylation status was analyzed in the VSCC tissues and
normal vulvar tissues by MSP. The results showed that the Gal-7
gene promoter in the VSCC tissues was significantly more likely to
be methylated compared with that in the normal vulvar tissues; an
observation that is consistent with the lower Gal-7 expression in
VSCC samples compared with normal controls. The present data also
revealed significantly more Gal-7 promoter methylation in cases
with poor differentiation, advanced clinical stage and lymph node
metastasis. Thus, the malignant potential of cells was associated
with Gal-7 gene promoter methylation. This is of particular
relevance in the development of novel therapeutic approaches to
this condition, and suggests that the demethylation of the Gal-7
gene promoter may be of benefit in reducing the development of
VSCC.
We anticipate that these findings will provide a
logical basis for the design and development of further studies
examining demethylation in VSCC.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30973190).
References
|
1
|
Coulter J and Gleeson N: Local and
regional recurrence of vulvar cancer: Management dilemmas. Best
Pract Res Clin Obstet Gynaecol. 17:663–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magnaldo T, Fowlis D and Darmon M:
Galectin-7, a marker of all types of stratified epithelia.
Differentiation. 63:159–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang RY and Liu FT: Galectins in cell
growth and apoptosis. Cell Mol Life Sci. 60:267–276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Wu TC, Chen WQ, Zhou LJ, Wu Y, Zeng
L and Pei HP: Roles of galectin-7 and S100A9 in cervical squamous
carcinoma: Clinicopathological and in vitro evidence. Int J Cancer.
132:1051–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rorive S, Eddafali B, Fernandez S,
Decaestecker C, André S, Kaltner H, Kuwabara I, Liu FT, Gabius HJ,
Kiss R and Salmon I: Changes in galectin-7 and cytokeratin-19
expression during the progression of malignancy in thyroid tumors:
Diagnostic and biological implications. Mod Pathol. 15:1294–1301.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takata T, Ishigaki Y, Shimasaki T,
Tsuchida H, Motoo Y, Hayashi A and Tomosugi N: Characterization of
proteins secreted by pancreatic cancer cells with anticancer drug
treatment in vitro. Oncol Rep. 28:1968–1976. 2012.PubMed/NCBI
|
|
8
|
Zhu X, Ding M, Yu ML, Feng MX, Tan LJ and
Zhao FK: Identification of galectin-7 as a potential biomarker for
esophageal squamous cell carcinoma by proteomic analysis. BMC
Cancer. 10:2902010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohno T, Nakano T, Niibe Y, Tsujii H and
Oka K: Bax protein expression correlates with radiation-induced
apoptosis in radiation therapy for cervical carcinoma. Cancer.
83:103–110. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polyak K, Xia Y, Zweier JL, Kinzler KW and
Vogelstein B: A model for p53-induced apoptosis. Nature.
389:300–305. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benvenuto G, Carpentieri ML, Salvatore P,
Cindolo L, Bruni CB and Chiariotti L: Cell-specific transcriptional
regulation and reactivation of galectin-1 gene expression are
controlled by DNA methylation of the promoter region. Mol Cell
Biol. 16:2736–2743. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruebel KH, Jin L, Qian X, Scheithauer BW,
Kovacs K, Nakamura N, Zhang H, Raz A and Lloyd RV: Effects of DNA
methylation on galectin-3 expression in pituitary tumors. Cancer
Res. 65:1136–1140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gendronaneau G, Sidhu SS, Delacour D, Dang
T, Calonne C, Houzelstein D, Magnaldo T and Poirier F: Galectin-7
in the control of epidermal homeostasis after injury. J Mol Biol
Cell. 19:5541–5549. 2008. View Article : Google Scholar
|
|
14
|
Bernerd F, Sarasin A and Maagnaldo T:
Galectin-7 overexpression is associated with the apoptotic process
in UVB-induced sunburn keratinocaytes. Proc Natl Acad Sci USA.
96:11329–11334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kopitz J, André S, von Reitzenstein C,
Versluis K, Kaltner H, Pieters RJ, Wasano K, Kuwabara I, Liu FT,
Cantz M, et al: Homodimeric galectin-7 (p53-induced gene 1) is a
negative growth regulator for human neuroblastoma cells. Oncogene.
22:6277–6288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menkhorst EM, Gamage T, Cuman C,
Kaitu'u-Lino TJ, Tong S and Dimitriadis E: Glectin-7 acts as an
adhesion molecule during implantation and increased expression is
associated with miscarriage. Placenta. 35:195–201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, He QY, Yuen AP and Chiu JF:
Proteomics of buccal squamous cell carcinoma: The involvement of
multiple pathways in tumorigenesis. Proteomics. 4:2465–2475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saussez S, Decaestecker C, Lorfevre F,
Chevalier D, Mortuaire G, Kaltner H, André S, Toubeau G, Gabius HJ
and Leroy X: Increased expression and altered intracellular
distribution of adhesion/growth-regulatory lectins galectins-1 and
−7 during tumour progression in hypopharyngeal and laryngeal
squamous cell carcinomas. Histopathology. 52:483–493. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JE, Chang WY and Choa M: Induction of
matrix ametalloproteinase-9 by galectin-7 through p38 MAPK
signaaling in HeLa human cervical epithelial adenocarcinoma cells.
Oncol Rep. 22:1373–1379. 2009.PubMed/NCBI
|
|
20
|
Kim HJ, Jeon HK, Lee JK, Sung CO, Do IG,
Choi CH, Kim TJ, Kim BG, Bae DS and Lee JW: Clinical significance
of galectin-7 in epithelial ovarian cancer. Anticancer Res.
33:1555–1561. 2013.PubMed/NCBI
|
|
21
|
Kim SJ, Hwang JA, Ro JY, Lee YS and Chun
KH: Galectin-7 is epigenetically-regulated tumor suppressor in
gastric cancer. Oncotarget. 4:1461–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuwabara I, Kuwabara Y, Yang R, Schuler M,
Green D, Zuraw B, Hsu D and Liu F: Galectin-7 (PIG1) exhibits
pro-apoptotic function through JNK activation and mitochondrial
cytochrome c release. J Biol Chem. 277:3487–3497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villeneuve C, Baricault L, Canelle L,
Barboule N, Racca C, Monsarrat B, Magnaldo T and Larminat F:
Mitochondrial proteomic approach reveals galectin-7 as a novel
BCL-2 binding protein in human cells. Mol Biol Cell. 22:999–1013.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barkan B, Cox AD and Kloog Y: Ras
inhibition boosts galectin-7 at the expense of galectin-1 to
sensitize cells to apoptosis. Oncotarget. 4:256–268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda S, Kuwabara I and Liu FT: Suppression
of tumor growth by galectin-7 gene transfer. Cancer Res.
64:5672–5676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rossi NE, Reiné J, Pineda-Lezamit M,
Pulgar M, Meza NW, Swamy M, Risueno R, Schamel WW, Bonay P,
Fernández-Malavé E and Regueiro JR: Differential antibody binding
to the surface alphabetaTCR. CD3 complex of CD4+ and CD8+ T
lymphocytes is conserved in mammals and associated with
differential glycosylation. Int Immunol. 20:1247–1258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Demers M, Couillard J, Giglia-Mari G,
Magnaldo T and St-Pierre Y: Increased galectin-7 gene expression in
lymphoma cells is under the control of DNA methylation. Biochem
Biophys Res Commun. 387:425–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moisan S, Demers M, Mercier J, Magnaldo T,
Potworowski EF and St-Pierre Y: Upregulation of galectin-7 in
murine lymphoma cells is associated with progression toward an
aggressive phenotype. Leukemia. 17:751–759. 2003. View Article : Google Scholar : PubMed/NCBI
|