Introduction
Collagenous and elastic fibers, the main components
of the extracellular matrix (ECM), are distributed widely among the
varying oral mucosa layers. While providing a protective screen for
the cells, tissues and organs it supports, the ETM is also involved
in cell proliferation, survival and apoptosis (1,2).
Clinically, a number of oral mucosal diseases are associated with
the remodeling of collagenous and elastic fibers (3,4). Multiple
studies have demonstrated that the fibrosis of the tongue mucosa
can be considered as a precancerous lesion (5,6). For this
reason, researchers are now focusing attention on the pathological
changes in the structure of oral mucosal fibers.
Matrix metalloproteinases (MMPs) are degradative
enzymes that exhibit a significant role in all aspects of tumor
progression via the enhancement of tumor-induced angiogenesis and
the destruction of the local tissue architecture, thus allowing
tumor invasion and metastasis (7).
Various MMPs are secreted during the growth, invasion, metastasis
and angiogenesis of tumors (8). These
MMPs affect the surrounding microenvironment, resulting in dynamic
changes in the structure of the ECM (9). Indeed, MMPs can degrade almost all
components of the ECM (8,10). MMP-1 (also known as collagenase-1) is
the principal human enzyme that cleaves native fibrillar collagen,
while MMP-2 (gelatinase) specifically degrades basement membrane
(BM) type IV collagen, but can also cleave other ECM components.
MMP-1 and MMP-2 have each been implicated in the degradation of the
ECM in oral diseases (10), and
through degradation of collagenous and elastic fibers, provide a
path for tumor cells to invade and migrate (11–14).
Studies have shown that during the invasion and metastasis period
of numerous cancers, activated MMP-1 is located in the peripheral
borders of tumor islands, where tumor cells possess invasion
ability (15–17). MMP-1 has been shown to promote
invasion and tumorigenesis through the degradation of the ECM as
the main component of connective tissue in the oral mucosal, while
MMP-2 has been associated with angiogenesis, tumor growth,
progression, invasiveness and metastasis in numerous cancer types
(18–22). Moreover, MMP-1 and −2 have been
suggested to be the most important MMPs in the invasion and
metastasis of oral mucosal cancer (23,24).
Recent studies have shown that MMPs and tissue
inhibitors of matrix metalloproteinases (TIMPs), which strictly
regulate MMP activity, are expressed in a relative equilibrium that
facilitates the maintenance of the dynamic balance between the
synthesis and degradation of the ECM (25,26).
Currently, 4 TIMP enzymes have been identified, namely TIMP-1, −2,
−3 and −4 (27). TIMP-1 and −2 have
been the most well studied of these TIMP enzymes. TIMP-1 interacts
with MMP-1 (28), while TIMP-2
selectively inhibits MMP-2 activity (29). To date, a number of studies have
suggested a positive role for TIMP-1 and −2 in the invasion and
metastasis of oral cancer (30), head
and neck squamous cell carcinoma (31), colon cancer (32) and breast cancer (22).
The current study investigated the importance of
MMP-1, MMP-2, TIMP-1 and TIMP-2 in the progression of tongue cancer
in a hamster model. Additionally, the changes in the expression of
MMP-1, MMP-2, TIMP-1 and TIMP-2, and the levels of collagenous and
elastic fibers were analyzed during different pathological stages
of tongue cancer, and computer-aided morphological analysis
software (33) was applied to analyze
the specific quantitative changes in the proteins during tongue
cancer development in an attempt to reveal the intrinsic nature of
the association between ECM metabolic disorders and tongue cancer
development.
Materials and methods
Experimental animals, grouping and
tissue preparation
All animal experiments were approved by the
Institutional Animal Care Committee of the Laboratory Animal
Committee of Harbin Medical University (Harbin, Heilongjiang,
China; approval no. HMU-IACUC 2006107). The animal grouping and
tongue cancer model were used as described in our previous study
(33). Briefly, 4-week-old male
hamsters (n=48; 35–55 g; SCXX2007-0005; Shanghai Laboratory Animal
Center, Shanghai, China) were randomized into two groups: The
control group (n=8), which was untreated, and the experimental
group (n=40), in which 1.5% DMBA acetone solution (Sigma-Aldrich,
St. Louis, MO, USA) was used to scratch the lingual mucosa 3 times
a week, for up to 8 weeks. Each animal received an intraperitoneal
injection of 40 mg/kg phenobarbital prior to sacrifice. Next, the
tongues were excised, and tissue was fixed in formalin, processed
in a standard manner by embedding in paraffin and sectioned into
4-µm thick sections for immunohistochemical, Gomori's trichrome and
Masson's trichrome staining. Histological sections were stained
with hematoxylin and eosin for pathological grading purposes
(33). Histological assessment was
performed by 2 pathologists blinded to the imaging results and
graded according to standards outlined by the World Health
Organization (34).
Immunohistochemistry
Immunohistochemical staining for MMP-1, MMP-2,
TIMP-1, TIMP-2 and type IV collagen was performed using 4-µm thick,
paraffin-embedded sections. Briefly, the sections were first
deparaffinized with xylene and rehydrated in graded ethanol.
Endogenous peroxidase activity was blocked by the immersion of the
slides in 3% hydrogen peroxide. To block non-specific antigens, 1%
bovine serum albumin was applied for 15 min. The sections were then
incubated with the respective rabbit anti-hamster polyclonal
primary antibodies [MMP-2 (cat no. BA0569; 1:300 dilution), MMP-9
(cat no. BA2202; 1:100 dilution), TIMP-1 (cat no. BA3727; 1:100
dilution), (TIMP-2 (cat no. BA0576; 1:50 dilution) and collagen,
type IV (cat no. BA2174; 1:500 dilution); all purchased from Boster
Bio-Engineering Co., Ltd., Wuhan, China] overnight in a humidified
chamber maintained at 4°C. Subsequently, the sections were
incubated with corresponding secondary antibodies (immediate-use
goat anti-rabbit IgG horseradish-peroxidase polymers; cat no.
PV-6001; Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing,
China) for 30 min at 37°C. The antibody reaction was visualized
using diaminobenzidine chromogen (Zhongshan Goldenbridge
Biotechnology Co., Ltd.). Finally, all the slides were
counterstained with hematoxylin. Sections incubated with
immunoglobulins of the same species at the same final
concentrations served as negative controls.
Gomori's aldehyde fuchsin (AF)
staining
Formalin-fixed, paraffin-embedded tissues were
sectioned, deparaffinized and rehydrated. AF-positive elastic
fibers stain bluish violet and are continuous filaments that are
widely distributed throughout the loose connective tissue. The
nuclei stain blue and the cytoplasm stains pink. Gomori's AF
staining (35) was used to observe
the distribution of elastic fibers per unit area.
Masson's trichrome staining
Formalin-fixed, paraffin-embedded tissues were
sectioned, deparaffinized, and rehydrated. Masson's trichrome
staining was used to observe deposited collagenous fiber (6). Collagen fibers stain blue and muscle
fibers stain red. Furthermore, the nuclei exhibit black staining
and the background exhibits red staining. The extent of collagenous
fiber expression was assessed per unit area.
Computer-aided morphological
analysis
Immunostaining reaction intensity and area [integral
optical density (IOD)] for each protein, and staining per unit area
in histological sections were separately assessed by 2 independent
pathologists who were blinded to the animal grouping using
computer-assisted morphological analysis. Slides that received
different assessments from the 2 pathologists were re-analyzed with
joint discussion. Image Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) was used to calculate the intensity and extent
of staining for the detected molecules, and the ratio of the
Gomori's AF/Masson's trichrome staining area to the total area of
the image in the different tissues. The per-area density of
staining was calculated in this manner to reflect the percentage of
the tissue that contained elastic and collagenous fibers, resulting
in a semi-quantitative analysis. A total of 5 microscopic fields
were randomly selected, and their images were cropped. The IOD
levels of the stained cells and the per-area staining of the
elastic and collagenous fibers in the tissue samples were then
calculated by image analysis. Results were expressed as the mean ±
standard deviation (SD) per tissue examined.
Statistical analysis
SPSS 18 software (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. All tests were 2-sided, and the
significance level was set at P≤0.05. Data are expressed as the
mean ± SD. Differences between the mean values of 2 sets were
assessed using the Student-Newman-Keuls q-test. Means, variances
and interquartile ranges were examined, and Levene's test was used
to test for homogeneity of variances, since the variances were
found to be unequal. The Wilcoxon rank sum test was used for post
hoc pair-wise analyses, and Spearman's rank correlation was used to
analyze correlations between different biomarkers and pathological
grades.
Results
Histological examination of hamster
tongue tissues using hematoxylin and eosin staining
In our previous study (33), lymphatic vessel changes were
morphometrically analyzed in hamsters using 5′-nucleotide
enzymes-alkaline phosphatase staining. The DMBA-induced lingual
mucosa tissue was harvested and divided into two blocks for frozen
and paraffin embedding in the previous and present study,
respectively. The lingual mucosa of 40 hamsters were treated with
1.5% DMBA. Ten hamsters were sacrificed every 2 weeks. Using
hematoxylin and eosin staining, it was observed that 16 hamsters
had atypical hyperplasia, 13 exhibited tongue squamous cell
carcinoma in situ and 11 had early tongue squamous cell
carcinoma. In addition, 8 hamsters were left untreated, with 2
hamsters from this group sacrificed every 2 weeks. No pathological
changes were observed in the untreated group using hematoxylin and
eosin staining. Thus, our previous study had no effect on the
current study and could be run concurrently.
Levels of elastic fibers during
different stages of carcinoma progression
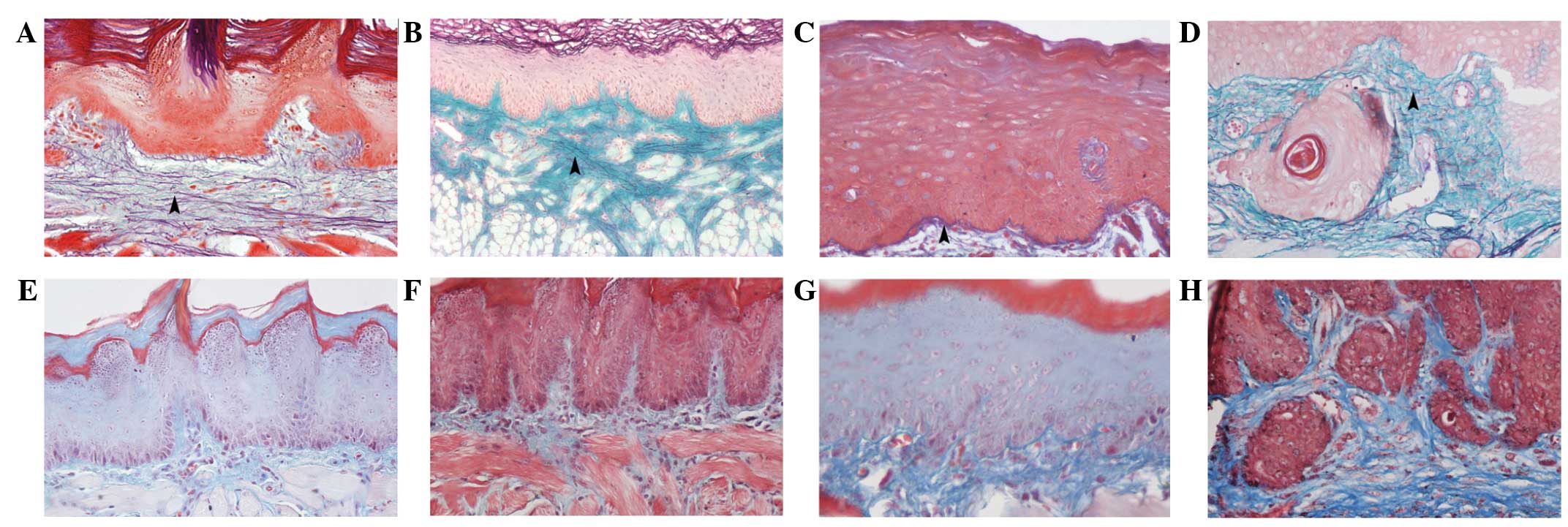
Numerous AF-positive elastic fibers were distributed
throughout the lamina propria of the normal tongue. The elastic
fibers within atypical hyperplastic tissues did not differ
significantly in morphology compared with the normal tongue mucosa
(Fig. 1A and B). AF-positive elastic
fibers in the lamina propria demonstrated intermittent fracturing,
shortening and distribution sparseness in the tissues from in
situ carcinomas (Fig. 1C).
Additionally, fewer AF-positive elastic fibers were found in the
lamina propria layer of the invasive carcinoma tissues (Fig. 1D).
Correlations between per-area staining of elastic
fibers and different tumor progression stages were analyzed using
Spearman's correlation test. The results showed that the expression
levels of elastic fibers decreased gradually with the malignant
progression of hamster tongue carcinoma (r=-0.566; P<0.01).
Levels of collagenous fiber during
different stages of carcinoma progression
Masson's trichrome-positive collagenous fibers were
long and thin, with a straight, flat orientation in the normal
lamina propria (Fig. 1E). In the
atypical hyperplastic tissues, the morphology of the collagenous
fibers did not change significantly (Fig.
1F). Moreover, the in situ carcinoma tissues exhibited
thicker, compact collagenous fiber bundles in the lamina propria
(Fig. 1G). In invasive carcinoma,
collagenous fiber levels were increased, and fibers appeared
compactly distributed, with a deeper staining color (Fig. 1H). The results showed that the
expression levels of collagenous fiber was positively correlated
with the progression of the tumor (r=0.619, P<0.01).
Expression of MMP-1 and TIMP-1 during
different stages of carcinoma progression
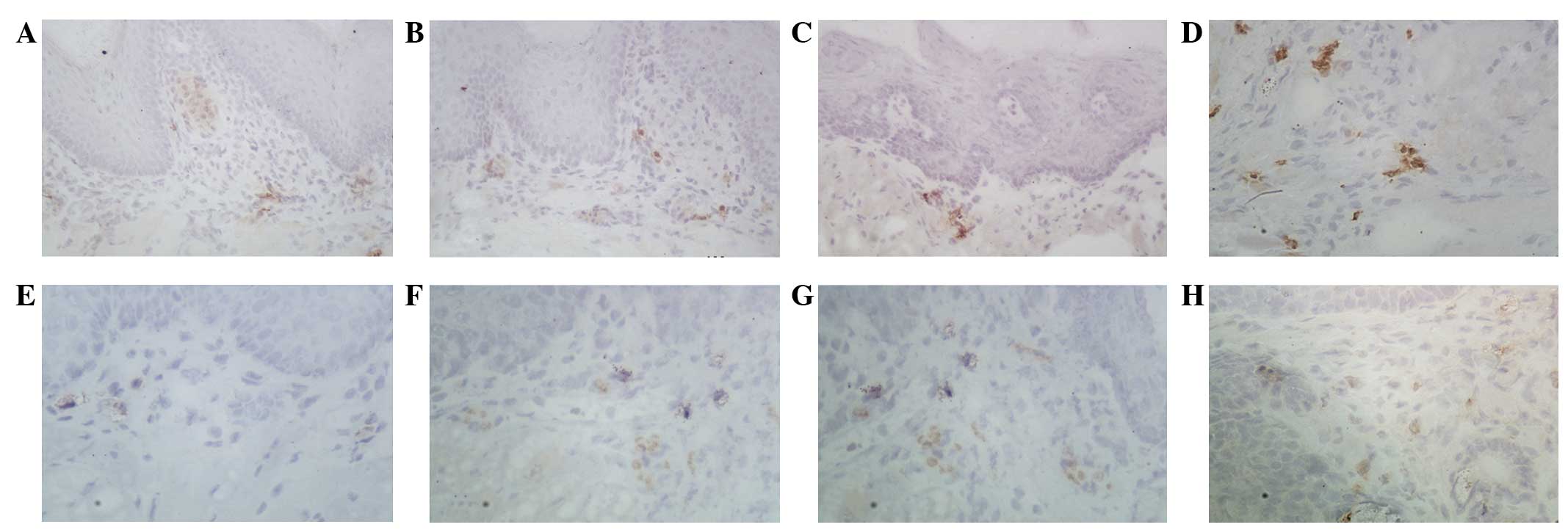
In the normal and atypical hyperplastic tissues,
MMP-1 was only expressed in a few epithelial and stromal cells as
brownish granules (Fig. 2A and B). In
the in situ carcinoma tissues, the expression of MMP-1 was
mainly found in stromal cells surrounding the epithelial nests of
the carcinoma (Fig. 2C). In tongue
invasive carcinoma, MMP-1 was expressed in significantly increased
levels in the cytoplasm of the stromal cells of cancer nests and
around the blood vessels (Fig. 2D).
Similarly, the expression of TIMP-1 was extremely weak in the
normal tongue mucosa and atypical hyperplastic tissues (Fig. 2E and F). In the in situ
carcinoma tissues, TIMP-1 expression was mainly observed in the
stromal cells surrounding the epithelial nests. Positive expression
of TIMP-1 was mainly observed in the cytoplasm of the cancer and
stromal cells (Fig. 2G and H).
The expression of MMP-1 increased with the
progression of hamster tongue carcinogenesis (P>0.05).
Additionally, the expression of TIMP-1 was highly correlated with
carcinogenic progression (r=0.705, P<0.01; r=0.759,
P<0.01).
Expression of MMP-2 and TIMP-2 during
the progression of hamster tongue carcinoma
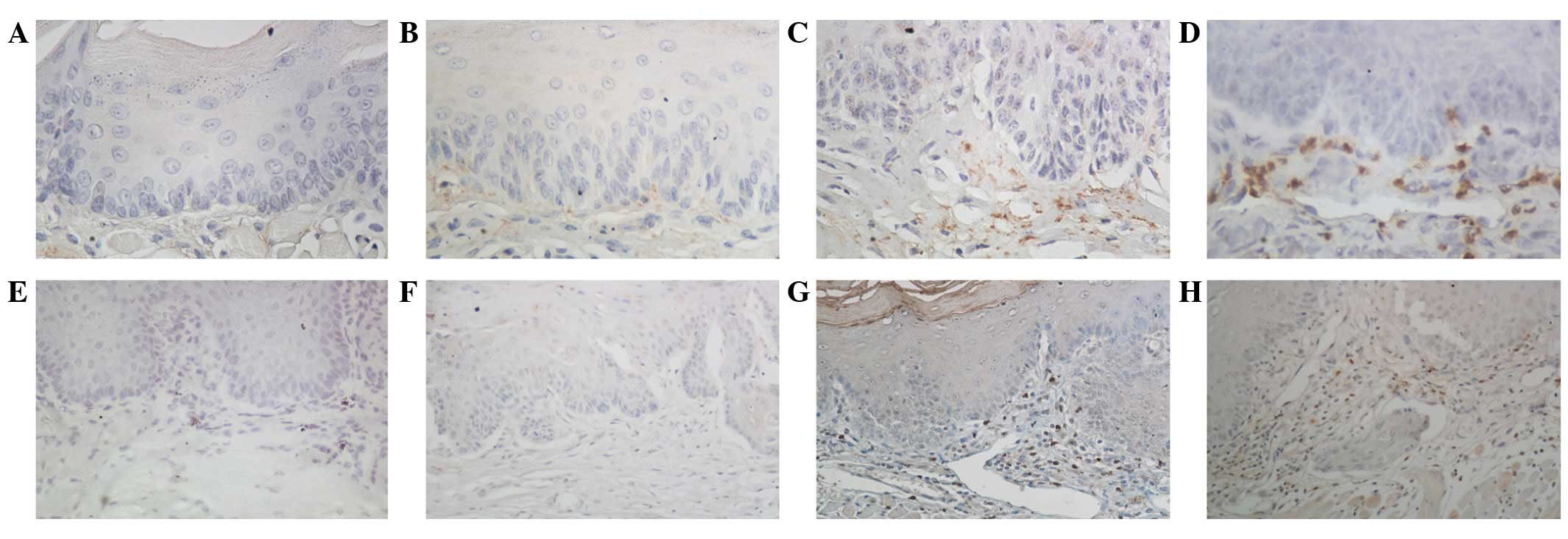
The expression of MMP-2 in the normal tongue mucosal
tissues was negative (Fig. 3A). In
the atypical hyperplastic and in situ carcinoma tissues, the
expression of MMP-2 was significantly increased in the epithelial
and cancer cells (Fig. 3B and C). In
invasive carcinoma, the expression of MMP-2 was mainly distributed
in the stromal and cancer cells of the lamina propria, and
relatively weak expression was found in the basement cells around
the cancer nests (Fig. 3D). TIMP-2
expression was negative or weakly positive in the normal tongue
mucosa and atypical hyperplastic tissues (Fig. 3E and F). In the in situ
carcinoma tissues, the expression of TIMP-2 was mainly found in the
stromal cells of the lamina propria (Fig.
3G). In the invasive tissues, TIMP-2 expression was found
mainly in the cytoplasm of the cancer and stromal cells as brown or
dark-brown granules (Fig. 3H).
Moreover, it was found that the expression levels of
MMP-2 and/or TIMP-2 were positively correlated with the progression
of the tongue tumor (r=0.633, P<0.01; r=0.751, P<0.01,
respectively). Additionally, by analyzing the associations between
type IV collagen expression (substrate of MMP-2) and the
progression of hamster tongue cancer, it was found that the
expression of type IV collagen was gradually reduced with
carcinogenic progression (r=-0.881, P<0.001).
Correlation of elastic and collagenous
fibers with MMP and TIMP expression during the progression of
hamster tongue carcinoma
High levels of proteases facilitate the degradation
of the BM and ECM, providing channels that allow tumor cells to
migrate and metastasize through the vasculature system (36). For this reason, the present study next
determined whether the levels of the components of the ECM were
correlated with the expression of MMPs and/or TIMPs. As shown in
Table I, the level of elastic fibers
was negatively correlated with the expression of MMP-1 and MMP-2
(r=-0.257, P<0.05; and r=-0.267, P<0.05, respectively). The
results also showed that the level of collagenous fibers was
positively correlated with MMP-1 and MMP-2 protein expression
(r=0.364, P<0.01; and r=0.297, P<0.05, respectively). The
results also showed type IV collagen expression to be negatively
correlated with MMP-2 protein expression (r=-0.552, P<0.01).
 | Table I.Association between collagenous fiber
and elastic fiber levels, and MMP and TIMP expression levels in
hamster tongue carcinoma by Spearman's correlation. |
Table I.
Association between collagenous fiber
and elastic fiber levels, and MMP and TIMP expression levels in
hamster tongue carcinoma by Spearman's correlation.
|
| MMP-1 | MMP-2 | MMP-1/TIMP-1 | MMP-2/TIMP-2 |
|---|
|
|
|
|
|
|
|---|
| Component | R | P-value | R | P-value | R | P-value | R | P-value |
|---|
| Elastic fiber | −0.257 | 0.047a | −0.267 | 0.039a | – | – | −0.613 | 0.002b |
| Collagenous
fiber |
0.364 |
0.004b |
0.297 | 0.021a | −0.549 | <0.001b | – | – |
MMP activity is controlled by changes in the
delicate balance between the expression and synthesis of MMPs and
their major endogenous inhibitors, TIMPs. The catalytic competence
of MMPs is controlled through the activation of MMP pro-enzymes and
their inhibition or activation by TIMPs (37). Therefore, the associations between the
ratios of MMP1/TIMP1, MMP2/TIMP2 and ECM components were analyzed
(Table I). The results showed that a
correlation existed between collagenous fiber level and the ratio
of MMP-1/TIMP-1 (r=-0.549, P<0.01). The associations between
elastic fiber level and the MMP-2/TIMP-2 ratio were also
significant (r=-0.613, P<0.01).
Discussion
In the lamina propria of the tongue, elastic fibers
provide lingual tissue flexibility, while collagenous fibers
provide toughness and support. Type IV collagen, the main component
of the BM, can effectively prevent harmful substances from invading
the lamina propria. As the main component of the ECM, collagenous
fibers are widely distributed throughout the layers of the tongue
tissue, and the changes in the structure and content of the ECM
directly affect the physiological and/or pathological processes of
tissue repair and tumor metastasis (5,38,39).
The present study observed the gradual reduction,
damage, fracturing or even disappearance of elastic fibers with
DMBA-induced hamster tongue cancer progression. Additionally, the
papillae layer of the lamina propria gradually lost its structure
during cancer progression. The results showed that the elastic
fiber content was lower in the invasive carcinoma tissues than in
the normal tissues, and we hypothesize that fracturing of the
elastic fibers in the lamina propria allows cancerous cells to move
deeper into the tissue, accelerating the process of cancer
formation. At the same time, it was also observed that the
expression of MMP-2 the in invasive carcinoma tissues, which have
low elastic fiber levels, was higher than that in the atypical
hyperplastic tissues, which have high elastic fiber levels. TIMP-2
protein expression also increased; however, the difference was not
as significant as that observed for MMP-2. Additionally, the
results showed MMP-2 expression to be negatively correlated with
the level of elastic fibers. An inverse correlation was also
demonstrated between elastic fiber content and TIMP-2 expression,
suggesting that the MMP-2/TIMP-2 ratio could represent an
independent index with which to evaluate cancer progression and
invasion. Moreover, the results suggested that the expression of
type IV collagen was gradually reduced with carcinogenic
progression, and was decreased with increasing MMP-2 expression.
These results suggested that damage of the BM components of the
epithelial tissues could provide proper channels for tumor cell
invasion, which was similar to previously published results
(38,40,41). Thus,
we speculate that the overexpression of MMP-2 in cancer tissues can
accelerate the degradation of type IV collagen, leading to
fractures and defects in the BM, thereby reducing its barrier
function and promoting the invasion of cancer cells into the lamina
propria. So, from the present study and others, we believe that it
is the mutual effects of MMP-2 and type IV collagen that create
appropriate conditions for tumor cell invasion and metastasis.
Oral submucous fibrosis, a type of collagenosis,
results in a precancerous lesion (6,42). The
formation of numerous new collagenous fibers was observed in the
present study during early-stage carcinogenesis, and this may be
the reason that the lamina propria appeared to thicken. Although
the collagenous fiber content in the lamina propria was high, the
fibers were damaged as the cancer cells broke through the BM during
late-stage carcinogenesis. The degradation substrates of MMP-1 are
mainly type I and type III collagen. Moreover, MMP-1 expression has
been shown to be increased in oral mucosal fibrosis tissues and
oral cancer tissues (43). The
present study found that MMP-1 expression increased with the
progression of tongue cancer; however during the period between
carcinoma in situ and invasive cancer, MMP-1 expression was
retained at a relatively high level, without continuing to
increase. Further analysis showed that the MMP1/TIMP1 ratio was
reduced with increased collagenous fiber content. Therefore, we
speculate that the increase in MMP-1 activity in the ECM,
stimulating the production of high levels of TIMP-1, would lead to
the inhibition of MMP-1 in a negative feedback loop. Thus, while
changes in the expression of MMP-1 and TIMP-1 may lead to the
deposition of collagenous fibers in the oral mucosa, they are not
the only reason for this phenomenon.
In the present DMBA-induced tongue cancer hamster
model, which reproduces a number of the characteristics of the ECM
in tongue tissues during carcinogenesis, elastic fiber and type IV
collagen degradation, as well as collagenous fiber deposition, was
able to be reproducibly measured in the tongue mucosal tissues. The
experiments revealed, for the first time, the occurrence of changes
in the morphology and content of collagenous and elastic fibers,
and type IV collagen during tongue cancer progression in a hamster
model. Changes of these matrix components directly or indirectly
altered tissue structures, leading to tissue fibrosis. Indeed, the
data demonstrated that MMP-2 and TIMP-2 have significant roles in
the degradation of the BM and elastic fibers, and that they are
intimately involved in tongue cancer invasion. In addition, MMP-1
and TIMP1 also appear to be involved in tongue cancer progression.
Thus, the present study provides a novel visual field for the study
of tongue cancer pathology and suggests the importance of fully
understanding the molecular mechanisms of tumorous tissue
remodeling.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 305400083).
References
|
1
|
Souza LF, Souza VF, Silva LD, Santos JN
and Reis SR: Expression of basement membrane laminin in oral
squamous cell carcinomas. Braz J Otorhinolaryngol. 73:768–774.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakamoto S and Kyprianou N: Targeting
anoikis resistance in prostate cancer metastasis. Mol Aspects Med.
31:205–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajalalitha P and Vali S: Molecular
pathogenesis of oral submucous fibrosis-a collagenous metabolic
disorder. J Oral Pathol Med. 34:321–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reichart PA, van Wyk CW, Becker J and
Schuppan D: Distribution of procollagenous type III, collagenous
type VI and tenascin in oral submucous fibrosis (OSF). J Oral
Pathol Med. 23:394–398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin HJ and Lin JC: Treatment of oral
submucous fibrosis by collagenousase: Effects on oral opening and
eating function. Oral Dis. 13:407–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sumeth Perera MW, Gunasinghe D, Perera PA,
Ranasinghe A, Amaratunga P, Warnakulasuriya S and Kaluarachchi K:
Development of an in vivo mouse model to study oral submucous
fibrosis. J Oral Pathol Med. 36:273–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerkelä E and Saarialho-Kere U: Matrix
metalloproteinases in tumor progression: Focus on basal and
squamous cell skin cancer. Exp Dermatol. 12:109–125. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sorsa T, Tjäderhane L and Salo T: Matrix
metalloproteinases (MMPs) in oral diseases. Oral Dis. 10:311–318.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seguier S, Gogly B, Bodineau A, Godeau G
and Brousse N: Is collagenous breakdown during periodontitis linked
to inflammatory cells and expression of matrix metalloproteinases
and tissue inhibitors of metalloproteinases in human gingival
tissue? J Periodontol. 72:1398–1406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stetler-Stevenson WG: Tissue inhibitors of
metalloproteinases in cell signaling: Metalloproteinase-independent
biological activities. Sci Signal. 1:re62008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
George A, Ranganathan K and Rao UK:
Expression of MMP-1 in histopathological different grades of oral
squamous cell carcinoma and in normal buccal mucosa-an
immunohistochemical study. Cancer Biomark. 7:275–283.
2010.PubMed/NCBI
|
|
17
|
Zhou J, Brinckerhoff C, Lubert S, et al:
Analysis of matrix metalloproteinase-1 gene polymorphisms and
expression in benign and malignant breast tumors. Cancer Invest.
29:599–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durlik M and Gardian K: Metalloproteinase
2 and 9 activity in the development of pancreatic cancer. Pol
Przegl Chir. 84:377–382. 2012.PubMed/NCBI
|
|
19
|
Itoh T, Tanioka M, Yoshida H, Yoshioka T,
Nishimoto H and Itohara S: Reduced angiogenesis and tumor
progression in gelatinase A-deficient mice. Cancer Res.
58:1048–1051. 1998.PubMed/NCBI
|
|
20
|
Uhlmann ME, Georgieva M, Sill M, Linnemann
U and Berger MR: Prognostic value of tumor progression-related gene
expression in colorectal cancer patients. J Cancer Res Clin Oncol.
138:1631–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J and Cai Y: Matrix metalloproteinase
2 polymorphisms and expression in lung cancer: A meta-analysis.
Tumour Biol. 33:1819–1828. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Teng XD, Guo XX, Li ZG, Han JG
and Yao L: Expression of tissue levels of matrix metalloproteinases
and their inhibitors in breast cancer. Breast. 22:330–334. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurahara S, Shinohara M, Ikebe T, et al:
Expression of MMPS, MT-MMP and TIMPs in squamous cell carcinoma of
the oral cavity: Correlations with tumor invasion and metastasis.
Head Neck. 21:627–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakano K, Siar CH, Nagai N, et al:
Distribution of basement membrane type IV collagenous alpha chains
in ameloblastoma: An immunofluorescence study. J Oral Pathol Med.
31:494–499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hojilla CV, Mohammed FF and Khokha R:
Matrix metalloproteinases and their tissue inhibitors direct cell
fate during cancer development. Br J Cancer. 89:1817–1821. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stetler-Stevenson WG: The tumor
microenvironment: Regulation by MMP-independent effects of tissue
inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 27:57–66.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagase H: Activation mechanisms of matrix
metalloproteinases. Biol Chem. 378:151–160. 1997.PubMed/NCBI
|
|
29
|
Stetler-Stevenson WG, Krutzsch HC and
Liotta LA: Tissue inhibitor of metalloproteinase (TIMP-2). A new
member of the metalloproteinase inhibitor family. J Biol Chem.
264:17374–17378. 1989.PubMed/NCBI
|
|
30
|
Pradhan-Palikhe P, Vesterinen T, Tarkkanen
J, et al: Plasma level of tissue inhibitor of matrix
metalloproteinase-1 but not that of matrix metalloproteinase-8
predicts survival in head and neck squamous cell cancer. Oral
Oncol. 46:514–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burduk PK, Bodnar M, Sawicki P, et al:
Expression of metalloproteinases 2 and 9 and tissue inhibitors 1
and 2 as predictors of lymph node metastases in oropharyngeal
squamous cell carcinoma. Head Neck. 37:418–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giaginis C, Nikiteas N, Margeli A, et al:
Serum tissue inhibitor of metalloproteinase 1 and 2 (TIMP-1 and
TIMP-2) levels in colorectal cancer patients: Associations with
clinicopathological variables and patient survival. Int J Biol
Markers. 24:245–252. 2009.PubMed/NCBI
|
|
33
|
Chen D, Zheng J, Li H, Wang Q and Jiao X:
Computer-assisted morphometric analysis of lymphatic vessel changes
in hamster tongue carcinogenesis. J Oral Pathol Med. 39:518–524.
2010.PubMed/NCBI
|
|
34
|
Thompson L: World health organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
35
|
Chatterjee S, Mark ME, Wooley PH, et al:
Increased dermal elastic fibers in the tight skin mouse. Clin Exp
Rheumatol. 22:617–620. 2004.PubMed/NCBI
|
|
36
|
Ali FM, Patil A, Patil K and Prasant MC:
Oral submucous fibrosis and its dermatological relation. Indian
Dermatol Online J. 5:260–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong Q, Jun T, Lei J, Xiling J and
Tamamura R: Expression and clinical significance of matrix
metalloproteinase-2 and its inhibitor TIMP-2 in oral squamous cell
carcinoma. J Hard Tissue Biol. 2:54–60. 2006. View Article : Google Scholar
|
|
38
|
Santos-García A, Abad-Hernández MM,
Fonseca-Sánchez E, Julián-González R, Galindo-Villardón P,
Cruz-Hernández JJ and Bullón-Sopelana A: E-cadherin, laminin and
collagenous IV expression in the evolution from dysplasia to oral
squamous cell carcinoma. Med Oral Patol Oral Cir Bucal.
11:E100–E105. 2006.(In Spanish). PubMed/NCBI
|
|
39
|
Utsunomiya H, Tilakaratne WM, Oshiro K, et
al: Extracellular matrix remodeling in oral submucous fibrosis: Its
stage-specific modes revealed by immunohistochemistry and in situ
hybridization. J Oral Pathol Med. 34:498–507. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krecicki T, Zalesska-Krecicka M, Jelen M,
et al: Expression of type IV collagenous and matrix
metalloproteinase-2 (type IV collagenousase) in relation to nodal
status in laryngeal cancer. Clin Otolaryngol Allied Sci.
26:469–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lazaris ACH, Tzoumani AN, Thimara I, et
al: Immunohistochemical assessment of basement membrane components
in colorectal cancer: prognostic implications. J Exp Clin Cancer
Res. 22:599–606. 2003.PubMed/NCBI
|
|
42
|
Shieh TY and Yang JF: Collagenousase
activity in oral submucous fibrosis. Proc Natl Sci Counc Repub
China B. 16:106–110. 1992.PubMed/NCBI
|
|
43
|
Yen CY, Chen CH, Chang CH, et al: Matrix
metalloproteinases (MMP) 1 and MMP10 but not MMP12 are potential
oral cancer markers. Biomarkers. 14:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|