Introduction
Cytotoxic T-lymphocyte (CTL) activation and
extension of the cell life span are necessary in order to enable
immunotherapy to perform effectively against cancer cells (1). CTLs are activated via the T-cell
receptor (TCR; signal one) (2), which
induces the proliferation of CTLs. Engagement of a second receptor,
cluster of differentiation 28 (CD28; signal two), by ligands on
antigen-presenting cells, is required to prevent anergy and the
apoptosis of CTLs (3). This results
in extension of the CTL lifespan. CTLs may be activated via the CD3
receptor [a component of the TCR (2)]
and the CD28 receptor using monoclonal antibodies specific for
their respective receptors (4). In
order to obtain an adequate number of CTLs for the effective
performance of immunotherapy, costimulation with anti-CD3 and
anti-CD28 has been utilized (5).
Costimulated lymphocytes cells have been demonstrated to exhibit
logarithmic growth and inhibit apoptosis via enhanced cytotoxicity
for the targeting of tumor cells (6).
The present study aimed to determine whether the mucin 1
(MUC1)-stimulated human mononuclear cells (M1SHMCs) of breast
cancer patients would demonstrate expanded levels of growth without
compromising their ability to kill cancer cells when costimulated
with anti-CD3 and anti-CD28.
Materials and methods
MUC1-variable number tandem repeat 1
(VNTR1) peptide
GSTAPPAHGVTSAPDTRPAP (7) peptide was synthesized by American
Peptide Co., Inc., (Sunnyvale, CA, USA) and Novartis International
AG (Basel, Switzerland).
Anti-CD3/CD28 antibody beads
Anti-CD28 antibodies were obtained from Murine
Hybridoma 9.3 (8), which was a gift
from Professor John Hansen (University of Washington, Seattle,
USA). Dynabeads® M-450 Tosylactivated (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) are superparamagnetic polystyrene beads,
which were activated using p-toluenesulfonyl chloride, according to
the manufacturer's instructions. Anti-CD3 (OKT3; Beckman Coulter,
Inc., Brea, CA, USA) and anti-CD28 (5 µg each) were subsequently
added to a centrifuge tube containing Buffer B Dynabeads kit
solution. The tube was then placed in a Dynabeads® Rotator Mixer
(Thermo Fisher Scientific, Inc.) and incubated for 24 h at 37°C
with 5% CO2. The following day, the tube was placed into
the Dynal® MPC-S magnet (Thermo Fisher Scientific, Inc.) for 3 min.
Supernatant was subsequently removed and discarded. The
anti-CD3/anti-CD28-coated Dynabeads were washed twice in Buffer D
[0.88 g NaCl, 0.1% bovine serum albumin, 80 ml 0.01 M Na-phosphate
(pH 7.4)] at 4°C. Following each wash, the tube was placed into the
Dynal magnet, and the supernatant was subsequently discarded.
Buffer E [2X; 2.42 g Tris and 80ml distilled H2O (pH
8.5)] was added, and incubated overnight at 37°C with 5%
CO2. The following day Dynabeads were placed into the
Dynal magnet, and the buffer was subsequently removed. The
anti-CD28/anti-CD3-coated Dynabeads were then stored in AIM-V®
medium (Gibco; Thermo Fisher Scientific, Inc.) at −20°C, for
storage over one day, or at 4°C for less than one day.
Human cells
The present study was approved by the Institutional
Review Board of Texas Tech University Health Sciences Center
(Amarillo, TX, USA). All human cells were obtained from expired
subjects in accordance with the Institutional Review Board criteria
of the Texas Tech University Health Sciences Center. Frozen human
peripheral blood hematopoietic stem cells were obtained from the
Bone Marrow Transplant Laboratory of the Harrington Cancer Center
(Amarillo, TX, USA) from expired, anonymous donors. Frozen human
peripheral blood mononuclear cells (PBMCs) were obtained from the
Bone Marrow Transplant Laboratory of the Harrington Cancer Center
(Amarillo, TX, USA) by apheresis from an expired, anonymous donor
with breast adenocarcinoma.
Cell culture conditions
Procedures were performed as previously described
(9). Mononuclear cells were not human
leukocyte antigen typed, as our previous studies (10–12) and
another literature study (13)
identified that cytotoxicity by M1SHMC may be non-major
histocompatibility complex-restricted. The cells were cultured at
2×106 cells/ml in AIM V® serum-free lymphocyte medium
(Gibco; Thermo Fisher Scientific, Inc.) and maintained in a 37°C
humidified 5% CO2 atmosphere (14). Interleukin-2 (100 IU/ml; Novartis
International AG) was added twice/week. The cells were stimulated
with MUC1-VNTR1 peptide on days zero and seven, at 1 µg/ml. The
cells were harvested on day eight.
Costimulation of mononuclear
cells
M1SHMCs (2.0×107 cells) were costimulated
by anti-CD3/anti-CD28 beads according to the manufacturers
instructions, at a 3:1 ratio of beads to cells (6) in AIM V® medium. This occurred at the
intervals shown in Fig. 1 (once,
twice or three times/week) or three times/week, as shown in
Figs. 2–4 (days 9, 11 and 13, with harvesting on day
14).
Growth of MCF-7 cell line
Human breast carcinoma MCF-7 cells were utilized as
the target cells (15). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Hyclone, Logan, UT, USA), 1% bovine insulin (Gibco Life
Technologies) and 1% L-glutamine (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. Medium was replaced
twice/week by removal of the DMEM and addition of 15 ml fresh DMEM
to the flask.
Chromium release assays
The MCF-7 cells were used as targets in a Chromium
51 release assay (16). The cells
were labeled with [51Cr] sodium chromate (200 µCi per
1×107 cells; New England Nuclear, PerkinElmer, Inc.,
Waltham, MA, USA) and added to microtiter plates at a concentration
of 5×103 target cells/well. Effector cells were tested
at the effector to target cell ratios of 2.5, 5.0 and 10, indicated
in figures 2 and 3. DMEM was added instead of effector cells
to the spontaneous 51Cr release control wells. The
maximum target control wells had 2% Triton X-100 (Sigma-Aldrich,
St. Louis, MO, USA) added in place of the effector cells, in order
to lyse the target cells. Assays were incubated for 18 h, which has
been identified to be superior to 4 h (9), at 37°C with 5% CO2, and each
assay was performed in triplicate. Plates were centrifuged at 200 ×
g, and one half of the 100 µl supernatant was harvested.
Radioactivity released into the supernatant was measured using
liquid scintillation or γ counting. The specific percentage lysis
was calculated using the formula below, where cpm represents
counts/minute:
In vivo protection experiment
Animal care was in accordance with the guidelines of
Texas Tech University Health Sciences Center (Amarillo, TX USA).
Mice were housed in individually ventilated cages supplied HEPA
filtered air, with a maximum of five mice per cage. Each animal
holding room was supplied with 10–15 air changes per hour, and
pressurized relative to the immunocompetance of the animals. The
light/dark cycle was set from 07:00–19:00 (light cycle-low
intensity). Diet and water was ad libitum, and the water was
de-ionized and autoclaved. The immunodeficient diet was Global
Irradiated Code 2918 (Harlan Teklad Labs, Houston, TX USA). Mice
were housed at a temperature of 68–79°F and humidity between
30–70%. Female non-obese diabetic severe combined immunodeficient
mice (Jackson Laboratory, Bar Harbor, ME, USA), at 6–12 weeks of
age, were injected subcutaneously (SC) in the back of the neck,
with 0.1 ml phosphate-buffered saline (PBS):Matrigel at a 1:1 ratio
(Gibco; Thermo Fisher Scientific, Inc.), containing
5×106 MCF-7 cells. An estrogen pellet (0.18 mg, 60 day
release; Innovative Research of America, Sarasota, FL, USA) was
injected SC into the posterior back of the mouse to allow tumor
growth. PBS, or anti-CD3/CD28 antibody beads or 5×107
M1SHMC were injected intraperitoneally (IP) on the same day as the
tumor cells. Control animals received PBS or anti-CD3/CD28 antibody
beads individually. The first group of 7 mice received MCF-7; the
second group of 1 mouse received anti-CD3/CD28 antibody beads IP;
the third group of 4 mice received 5×107 M1SHMC IP; and
the fourth group of 4 mice received M1SHMC and anti-CD3/CD28
antibody beads. Each mouse was examined for tumor development three
times/week for one month. Observed tumor development at 28 days
post-injection was used for statistical analysis. . The present
study adhered to the Guidelines for Ethical Conduct in the Care and
Use of Animals (https://www.aaalac.org/resources/theguide.cfm)
detailed by the American Psychological Association Board of
Scientific Affairs Committee on Animal Research and Ethics.
Statistical analysis
GraphPad Prism 6 (GraphPad Software Inc., La Jolla,
CA, USA) statistical software package was utilized to analyze the
data. Data are expressed as the mean ± standard error. ANOVA,
Chi-square, and two-tailed Student's t test statistical analyses
were performed. P<0.05 was used to indicate statistical
significance.
Results
Growth of peripheral blood mononuclear
cells with anti-CD3/anti-CD28 costimulation
Monoclonal antibodies were bound onto beads in order
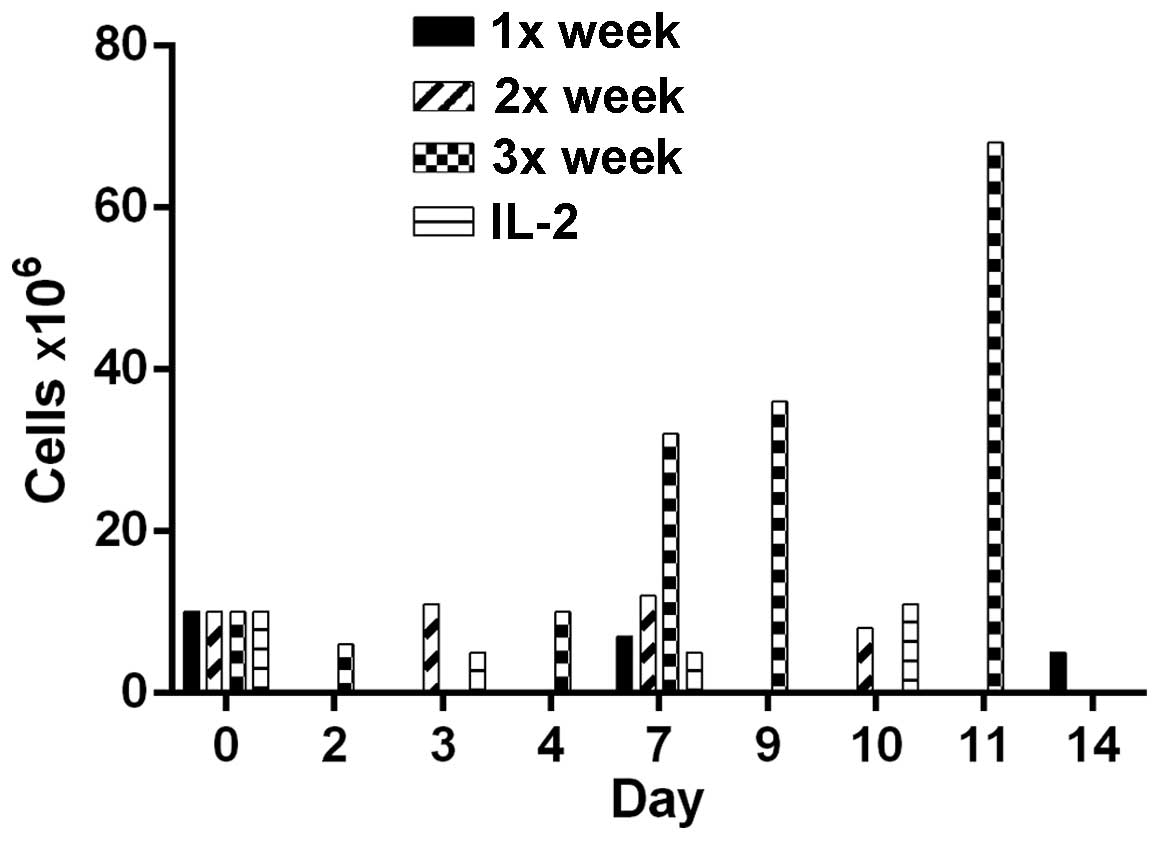
to costimulate patient lymphocytes. Cells underwent costimulation
by anti-CD28/anti-CD3 Dynabeads one, two or three times/week. Cells
costimulated three times/week demonstrated optimal proliferation
and an increased cell count, from 10 to 68 million (Fig. 1). Costimulation once or twice/week did
not significantly increase the cell number. Each bar represents a
single culture, thus no statistical analysis was performed. Thus,
M1SHMC were costimulated three times with anti-CD3/anti-CD28 beads
on days 9, 11 and 13, and harvested on day 14.
Specific lysis of MCF-7 cells by
M1SHMCs was compared to M1SHMCs costimulated with
anti-CD3/anti-CD28 beads at the indicated effector to target
ratio
M1SHMCs were then compared with costimulated M1SHMCs
using an in vitro MCF-7 cell-killing assay and in an in
vivo MCF-7 tumor formation inhibition experiment. Costimulation
of M1SHMC with anti-CD3/anti-CD28 beads did not result in enhanced
killing of the MCF-7 cells. Reduced killing of MCF-7 cells was
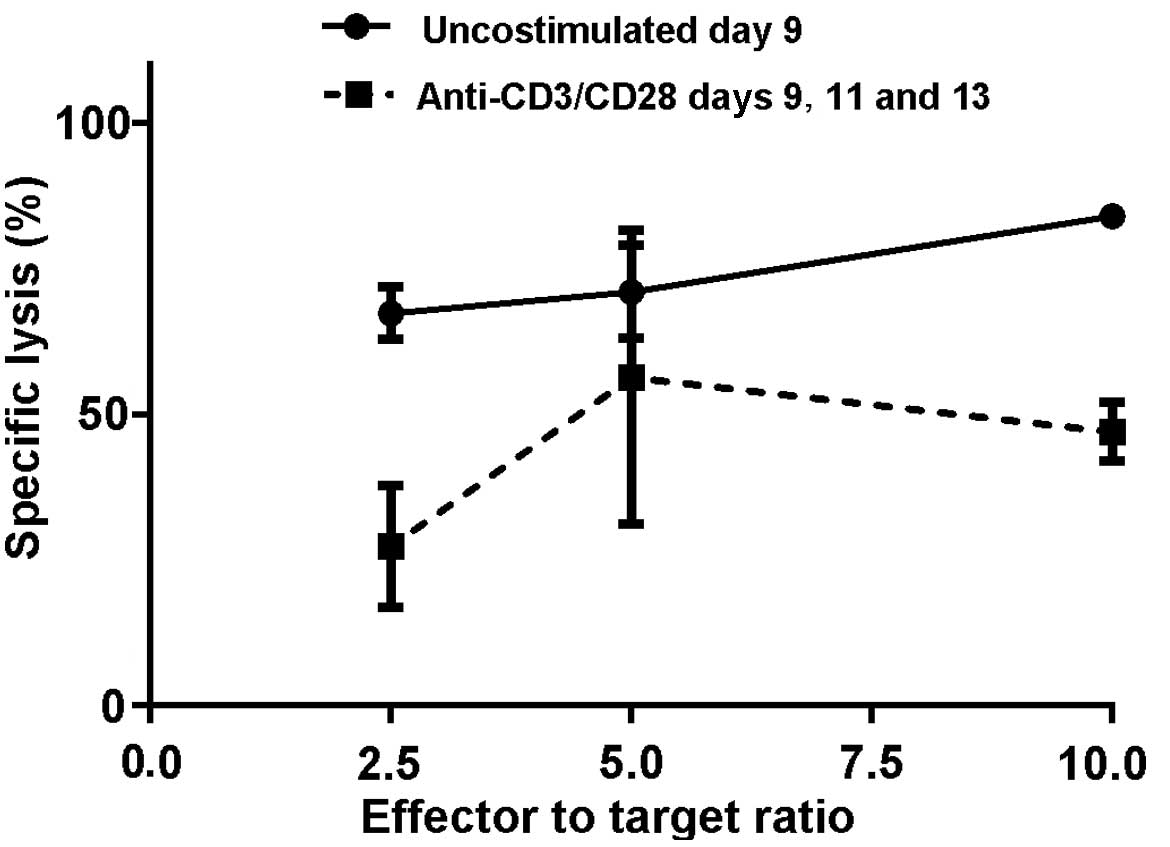
observed in two separate experiments (Figs. 2 and 3).
In the first experiment, at a 10:1 ratio of effector to target
cells, the M1SHMCs possessed a specific lysis rate of 67±3% vs.
27±6% (P=0.004, two-tailed t test) for the anti-CD3/anti-CD28 bead
costimulated M1SHMCs (Fig. 2). In the
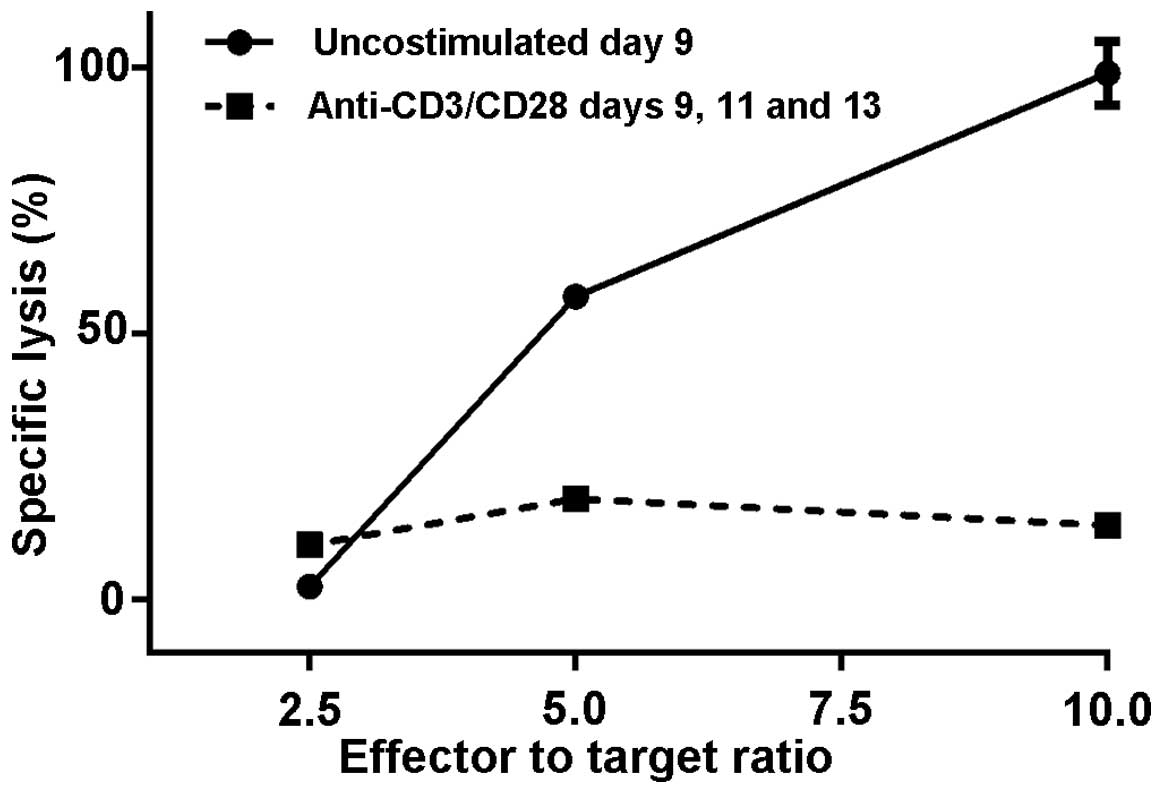
second experiment, the observed difference was even greater. At a
10:1 ratio of effector to target cells, the M1SHMCs possessed a
specific lysis rate of 99±3% vs. 14±0% + 0 (P<0.0001, two-tailed
t test) for the anti-CD3/anti-CD28 bead costimulated M1SHMC
(Fig. 3).
Effect of costimulation with
anti-CD3/anti-CD28 on the inhibition of tumor production by
M1SHMCs
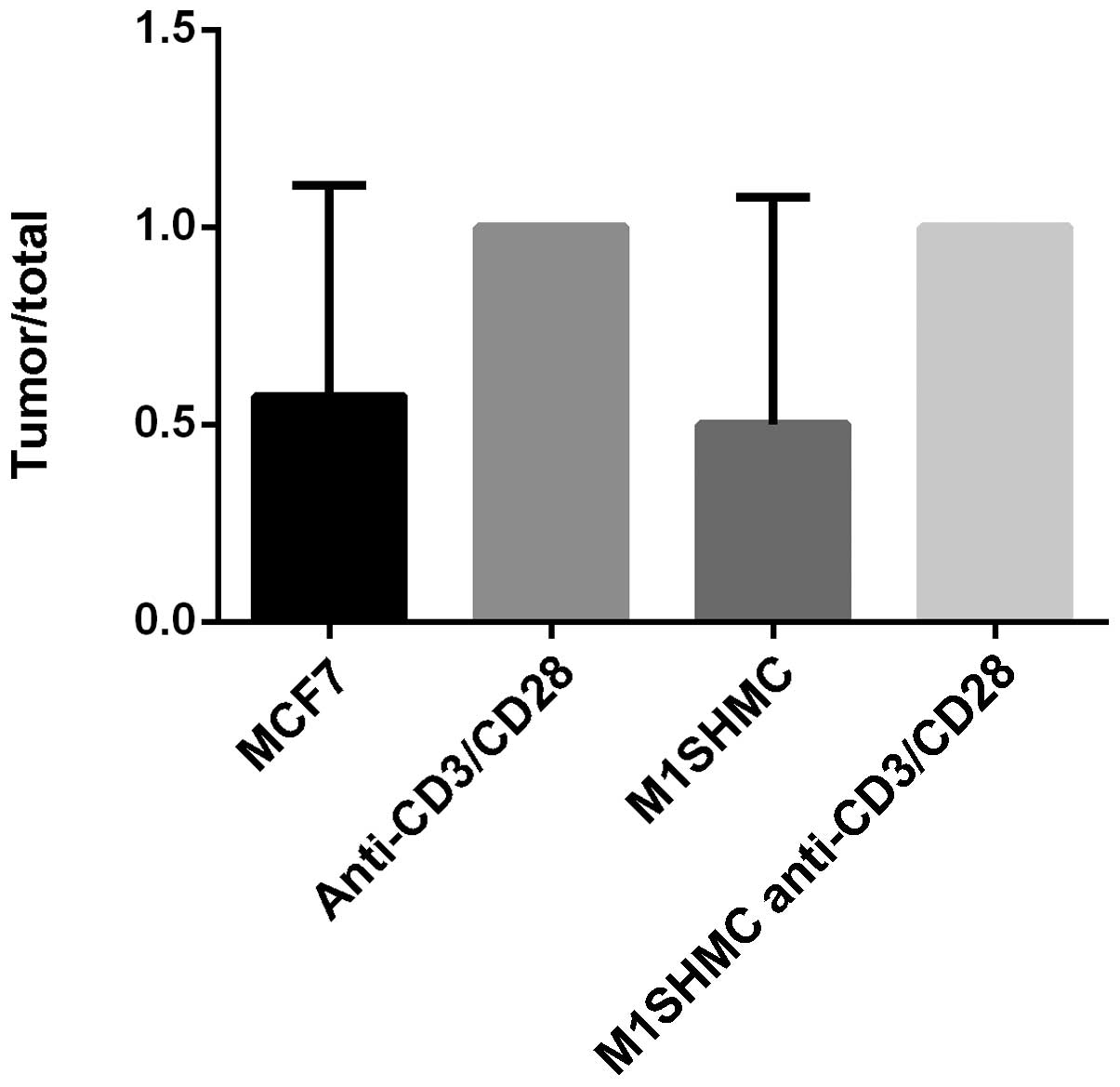
The in vivo results were consistent with
those observed in vitro. There was enhanced tumor formation
in the animals injected with breast cancer patients PBMCs,
costimulated with anti-CD3 and anti-CD28 beads with or without MUC1
stimulation (Fig. 4). In the first
group of 7 mice that received MCF-7, 4 developed tumors (57%); in
the second group of 1 mouse that received anti-CD3/CD28 antibody
beads, a tumor developed (100%); in the third group of 4 mice that
received M1SHMC, two mice developed tumors (50%); and in the fourth
group of 4 mice that received M1SHMC and anti-CD3/CD28 antibody
beads, all mice developed tumors (100%; P=0.4 using Chi-square,
group 3 vs. group 4).
Discussion
This present study was performed in order to
investigate the optimum interval of time for the costimulation of
M1SHMCs with anti-CD3/CD28 antibody beads, to promote the
proliferation of CTLs and the killing of breast cancer cells,
thereby preventing tumor growth. With regard to lymphocyte cell
growth, the most frequent intervals of costimulation with
anti-CD3/CD28 antibody beads provided the optimal rate of cell
proliferation. However, the anti-CD3/CD28 bead costimulation of
M1SHMCs resulted in a significant decrease in breast cancer cell
killing activity. This led to enhanced tumor cell growth. Whilst
costimulation with anti-CD3/CD28 antibody beads may be utilized for
the activation of lymphocytes (17),
the results of the present study suggested that costimulated
M1SHMCs, whilst exhibiting higher rates of proliferation, possess a
reduced ability to kill cancer cells, and thus this method of
treatment may not be advisable following antigen activation of
lymphocytes under the conditions used here. We have previously
shown that continued stimulation of CTL rendered them anergic
(9). In support of this, constitutive
proliferating CAR T cells showed inferior antitumor effect
(18). In addition, repetitive
signaling rendered CAR T cells susceptible to activation-induced
cell death (AICD) (19).
In conclusion, whilst CTL activation and extension
of the cell life span may be necessary in order to enable
immunotherapy to perform effectively against cancer cells (1), excessive proliferation and signaling of
the T cells may inhibit their antitumor activity. This resulting
immune suppression may be prevented by using a lower anti-CD3/CD28
bead: T-cell ratio (20), which
should reduce the T cells signaling through the CD3 complex, and
reduce activation-induced cell death. Another alternative is
altering the anti-CD3/CD28 ratios (21), where a lower anti-CD3/CD28 ratio
should reduce activation-induced cell death and reduce apoptosis
through CD28 engagement. A more physiological method that could be
utilized for costimulation may be artificial antigen-presenting
cells (22,23), with the addition of additional
costimulatory and pro-survival molecules.
Acknowledgements
The authors would like to thank the Coffee Memorial
Blood Center (Amarillo, TX, USA) and the Harrington Cancer Center
for providing the PBMCs, and those mentioned in the text for
providing materials and/or services. Deena C. Victor, research
assistant, Texas Tech University Health Sciences Center,
participated in the initial phase of the studies. The present study
was supported in part by VA Medical Research funds (0006),
Harrington Research Foundation (Amarillo, TX, USA) and the Women's
Health Research Institute (Texas Tech University Health Sciences
Center).
References
|
1
|
Barrett DM, Singh N, Porter DL, Grupp SA
and June CH: Chimeric antigen receptor therapy for cancer. Annu Rev
Med. 65:333–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lechler R and George AJT: 21.
Transplantation and rejection: Immunology. Roitt I, Bristoff J and
Male D: Immunology (5th). (Philadelphia, NJ). Mosby. 1998.
|
|
3
|
Boise LH, Minn AJ, Noel PJ, et al: CD28
costimulation can promote T cell survival by enhancing the
expression of Bcl-XL. Immunity. 3:87–98. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riddell SR and Greenberg PD: The use of
anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand
human antigen-specific T cells. J Immunol Methods. 128:189–201.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levine BL, Ueda Y, Craighead N, Huang ML
and June CH: CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce
long-term autocrine growth of CD4+ T cells and induce
similar patterns of cytokine secretion in vitro. Int
Immunol. 7:891–904. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine BL, Cotte J, Small CC, et al:
Large-scale production of CD4+ T cells from HIV-1-infected donors
after CD3/CD28 costimulation. J Hematother. 7:437–448. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gendler S, Taylor-Papadimitriou J, Duhig
T, Rothbard J and Burchell J: A highly immunogenic region of a
human polymorphic epithelial mucin expressed by carcinomas is made
up of tandem repeats. J Biol Chem. 263:12820–12823. 1988.PubMed/NCBI
|
|
8
|
Hansen J, Martin P and Nowinski R:
Monoclonal antibodies identifying a novel T-Cell antigen and Ia
antigens of human lymphocytes. Immunogenetics. 10:247–260. 1980.
View Article : Google Scholar
|
|
9
|
Wright SE, Khaznadar R, Wang Z, Quinlin
IS, Rewers-Felkins KA, Phillips CA and Patel S: Generation of
MUC1-stimulated mononuclear cells using optimized conditions. Scand
J Immunol. 67:24–29. 2008.PubMed/NCBI
|
|
10
|
Wright SE, Rewers-Felkins KA, Quinlin IS,
Eldridge PW, Zorsky PE, Klug PP, Phillips CA and Philip R: Adoptive
immunotherapy of mucin1 expressing adenocarcinomas with mucin1
stimulated human peripheral blood mononuclear cells. Int J Mol Med.
9:401–404. 2002.PubMed/NCBI
|
|
11
|
Wright SE, Kilinski L, Talib S, Lowe KE,
Burnside JS, Wu JY, Dolby N, Dombrowski KE, Lebkowski JS and Philip
R: Cytotoxic T lymphocytes from humans with adenocarcinomas
stimulated by native MUC1 mucin and a mucin peptide mutated at a
glycosylation site. J Immunother. 23:2–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright SE, Rewers-Felkins K A, Quinlin IS,
Fogler WE, Phillips CA, Townsend M, Robinson W and Philip R:
MHC-unrestricted lysis of MUC1-expressing cells by human peripheral
blood mononuclear cells. Immunol Invest. 37:215–225. 2008.
|
|
13
|
Alajez NM, Schmielau J, Alter MD, Cascio M
and Finn OJ: Therapeutic potential of a tumor-specific,
MHC-unrestricted T-cell receptor expressed on effector cells of the
innate and the adaptive immune system through bone marrow
transduction and immune reconstitution. Blood. 105:4583–4589. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanof ME and Smith PD: Preparation of
human mononuclear cell populations and subpopulations. Current
Protocols in Immunology. Coligan JE: (New York, NY, USA). John
Wiley & Sons, Inc. 71994.
|
|
15
|
Zhai YF, Esselman WJ, Oakley CS, Chang CC
and Welsch CW: Growth of MCF-7 human breast carcinoma in severe
combined immunodeficient mice: Growth suppression by recombinant
interleukin-2 treatment and role of lymphokine-activated killer
cells. Cancer Immunol Immunother. 35:237–245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wunderlich J: Chromium Release Cytoxicity
Assay. Current Protocols in Immunology. Coligan JE: (New York, NY,
USA). John Wiley & Sons, Inc. 31994.
|
|
17
|
Levine BL, Bernstein WB, Connors M,
Craighead N, Lindsten T, Thompson CB and June CH: Effects of CD28
costimulation on long-term proliferation of CD4+ T cells in the
absence of exogenous feeder cells. J Immunol. 159:5921–5930.
1997.PubMed/NCBI
|
|
18
|
Frigault MJ, Lee J, Basil MC, Carpenito C,
Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE,
Posey AD Jr, et al: Identification of chimeric antigen receptors
that mediate constitutive or inducible proliferation of T cells.
Cancer Immunol Res. 3:356–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Künkele A, Johnson AJ, Rolczynski LS,
Chang CA, Hoglund V, Kelly-Spratt KS and Jensen MC: Functional
Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL
Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent
AICD. Cancer Immunol Res. 3:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalamasz D, Long SA, Taniguchi R, Buckner
JH, Berenson RJ and Bonyhadi M: Optimization of human T-cell
expansion ex vivo using magnetic beads conjugated with
anti-CD3 and Anti-CD28 antibodies. J Immunother. 27:405–418. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y and Kurlander RJ: Comparison of
anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for
expanding human T cells: Differing impact on CD8 T cell phenotype
and responsiveness to restimulation. J Transl Med. 8:1042010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maus MV, Thomas AK, Leonard DGB, Allman D,
Addya K, Schlienger K, Riley JL and June CH: Ex vivo
expansion of polyclonal and antigen-specific cytotoxic T
lymphocytes by artificial APCs expressing ligands for the T-cell
receptor, CD28 and 4-1BB. Nat Biotechnol. 20:143–148. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh H, Figliola MJ, Dawson MJ, Olivares
S, Zhang L, Yang G, Maiti S, Manuri P, Senyukov V, Jena B, et al:
Manufacture of clinical-grade CD19-specific T cells stably
expressing chimeric antigen receptor using Sleeping Beauty system
and artificial antigen presenting cells. PLoS One. 8:e641382013.
View Article : Google Scholar : PubMed/NCBI
|