Introduction
The literature reports that there are significant
regional differences in the incidence and mortality rates of
prostate cancer (PCa), particularly between Asian and European
countries (1). Worldwide, ~900,000
men (33 per 100,000 men) were estimated to have been diagnosed with
PCa in 2008, ~14% (122,000) of whom were diagnosed within the
Asia-Pacific region (10 per 100,000 men), with three-quarters of
these patients diagnosed in Japan (32%), China (28%) or Australia
(15%) (2). In the early stages of
PCa, the PCa cells depend on androgens for growth and survival, and
androgen-ablative therapy is an effective therapeutic treatment
(3,4).
However, in later stages, PCa is more invasive and evolves to
become androgen-independent, with a resistance to not only
androgen-ablative therapy, but also to various other chemotherapy
regimens (5–9). Currently, the treatment options for
late-stage PCa remain relatively inefficient. Therefore, anti-tumor
agents for PCa with high efficiency and safety are required.
Increasing evidence indicates that transient
receptor potential (TRP) ion channels play important roles in human
cancers (10,11). TRP melastatin 8 (TRPM8), a member of
the TRP family, is a thermally regulated and nonselective
Ca2+-permeable cation channel, and a novel specific
marker of the prostate (12,13). TRPM8 is expressed abundantly in the
prostate and its expression increases in PCa, suggesting that there
is a huge potential for the successful treatment of PCa by
specifically silencing or inhibiting TRPM8. The literature suggests
that knockdown or blockade of TRPM8 has therapeutic potential in
the treatment of several types of cancer, including prostate,
pancreatic and oral squamous cancers (14–17). Zhang
et al reported that knockdown of TRPM8 may lead to the
suppression of proliferation in androgen-sensitive human prostate
adenocarcinoma LNCaP cells (16).
Valero et al demonstrated that inhibition of TRPM8
expression, by small interfering RNA, or function, by specific
blockers such as AMTB and JNJ41876666, reduced the proliferation
rate and proliferative fraction in PCa cells, but not in normal
prostate cells (17). However, the
current literature does not refer to the precise molecular
mechanism underlying the action of TRPM8 gene silencing or its
antagonists.
The aim of the present study was to identify whether
N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide
(BCTC), a potent and specific antagonist of TRPM8 (18,19),
exerts an anti-tumor effect on the androgen-independent PCa DU145
cells, and the mechanism of how the inhibition functions. The
present study reports that BCTC exerts an anti-proliferative effect
on DU145 cells and induces tumor suppression through G0/G1 cell
cycle arrest, and inhibition of migration and invasion. This was
demonstrated by cell cycle-associated molecules, consisting of
phosphorylated protein kinase B (p-AKT), phosphorylated glycogen
synthase kinase (p-GSK-3β), cyclin D1, cyclin dependent kinase
(CDK) 2 and CDK6, and mobility-associated molecules, consisting of
phosphorylated focal adhesion kinase (p-FAK) and matrix
metalloproteinase (MMP) 2. These findings reveal that the blockade
of TRPM8 by BCTC has the potential to become a targeted therapeutic
strategy against PCa.
Materials and methods
Cell lines and chemicals
The LNCaP cell line, which was derived from a
metastatic site of the left supraclavicular lymph node, the DU145
cell line, which was derived from a metastatic site in the brain,
and the human immortalized prostatic cell line PNT1A were obtained
from American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Gibco RPMI-1640 medium containing 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. BCTC and vehicle dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
MTT assay
The cell viability was assessed using standard MTT
assay according to the manufacturer's instructions (Sigma-Aldrich).
The protocol was performed as follows: DU145 or PNT1A cells
(5×103 per well) were cultured in a 96-well plate
(Corning Incorporated, Corning, NY, USA). The cells were treated
with various concentrations of BCTC or vehicle (DMSO; maximum
concentration ≤0.5%), with 10 wells per group for statistical
analysis, following which the cells were cultured in drugs for 72
h, and 20 µl MTT solution (Sigma-Aldrich) was added subsequent to
drawing off the medium. The mixture was incubated for an additional
4 h at 37°C. The supernatant was removed and 150 µl DMSO added per
well. Using an ELISA kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), the optical density was measured at 490 nm. All experiments
were repeated in triplicate.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the DU145, PNT1A or
LNCaP cells using Invitrogen TRIzol reagent (Thermo Fisher
Scientific, Inc.). For RT analysis, 1 µg total RNA was reverse
transcribed, using the Moloney murine leukemia virus reverse
transcription system (Thermo Fisher Scientific). RT-PCR was
performed by adding 2 µl RT reaction mixture to make a final volume
of 20 µl. PCR was performed as follows: Pre-heating to 94°C for 2
min; 35 cycles of 94°C for 30 sec, 50°C for 30 sec and 72°C for 60
sec; and 1 cycle of final extension at 72°C for 10 min. The PCR
primers used were as follows: TRPM8 (first PCR) forward,
5′-TGTTTTGCCCAAGGAGGTGG-3′ and reverse,
5′-CAACCAGTTTCCAGACAAACG-3′; TRPM8 (second PCR) forward,
5′-ATGGGCAGCTGAAGCTTC-3′ and reverse, 5′-CTGCAGATTCCGGTACAC-3′; and
β-actin forward, 5′-TTAGTTGCGTTACACCCTTTC-3′ and reverse,
5′-GTCACCTTCACCGTTCCAGTT-3′.
Western blot analysis
The cells were washed twice with ice-cold phosphate
buffered saline (PBS) and solubilized in 1% Triton lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) on ice. The
protein samples were resolved in 7.5, 10, 12.5 or 15% SDS–PAGE
(Promoton Biotechnology, Shanghai, China) and analyzed with
specific antibodies for western blot analysis, as follows: p-Akt
(dilution, 1:1,000; catalog no. 4060), Akt (dilution, 1:1,000;
catalog no. 9272), p-GSK-3β (dilution, 1:1,000; catalog no. 9323),
GSK-3β (dilution, 1:1,000; catalog no. 12456), phosphorylated
extracellular signal-regulated kinase 1/2 (p-ERK1/2; dilution,
1:2,000; catalog no. 4370P), ERK1/2 (dilution, 1:2,000, catalog no.
4695P), phosphorylated c-Jun N-terminal kinase (p-JNK; dilution,
1:1,000; catalog no. 4668P), JNK (dilution, 1:1,000; catalog no.
9258P), phosphorylated p38 (p-p38; dilution, 1:1,000; catalog no.
4511P), p38 (dilution, 1:1,000; catalog no. 9212P); p-FAK
(dilution, 1:1,000; catalog no. 8556), FAK (dilution, 1:1,000;
catalog no. 3285), cleaved caspase-3 (dilution, 1:500; catalog no.
8202) and MMP2 (dilution, 1:1,000; catalog no. 4022), all obtained
from Cell Signaling Technologies (Danvers, MA, USA); CDK2/4/6
(dilution, 1:1,000; catalog no. MS-299-P0; Neomarkers, Union City,
CA, USA); cyclin D1 (dilution, 1:1,000; catalog no. sc-735), cyclin
B1 (dilution, 1:1,000; catalog no. sc-245) and GAPDH (dilution,
1:1,000; catalog no. sc-166574), all obtained from Santa Cruz
Biotechnology (Dallas, TX, USA); B-cell lymphoma 2 (Bcl2; dilution,
1:1,000; catalog no. 12789-1-AP) and Bcl2-associated X protein
(Bax; dilution, 1:1,000; catalog no. 50599-2-Ig), obtained from
ProteinTech Group (Chicago, IL, USA); and TRPM8 (dilution, 1:500;
catalog no. ab3243; Abcam, Cambridge, UK).
Flow cytometry analysis of cell cycle
arrest and apoptosis
DU145 cells were synchronized by serum starvation in
a medium containing 0.1% serum for 24 h and induced to re-enter the
cell cycle by an exchange of 10% FBS with either vehicle (DMSO) or
various concentrations of BCTC. Subsequent to 24 h of incubation,
the cells were harvested and washed twice with PBS, then
re-suspended with pre-cooled 75% ethanol overnight, at 4°C. The
cells were washed again with PBS and centrifuged twice at 15,000 ×
g, treated with RNase A (100 mg/ml; BD Pharmingen, San Diego, CA,
USA) and propidium iodide (PI; 50 mg/ml; BD Pharmingen) at room
temperature for 30 min in darkness. Various phases of the cell
cycle were analyzed by flow cytometry (Becton-Dickinson, San Jose,
CA, USA). For analysis of apoptosis, following incubation with
various concentrations (0, 10, 100 µM) of BCTC for 48 h, the cells
were detached from monolayers with trypsin and incubated in a
binding buffer containing FITC-conjugated Annexin V (BD Pharmingen)
and PI at room temperature for 5 min in darkness, prior to analysis
by flow cytometry.
Scratch motility and Transwell
invasion assays
For the scratch motility assay, DU145 cells were
plated on a six-well plate to form confluent monolayers in complete
medium (RPMI-1640 and 10% FBS). A standard 200 µl pipette tip was
used to scratch across the wells, which were then washed with PBS.
The length of the scratch was monitored from either end when the
scratch was created and 24 h later, which is shorter than the time
required for DU145 cells to double in number. The migration rates
of the DU145 cells to colonize the scratch were calculated as the
proportion of the mean distance between the points of the scratch
to the distance that remained cell-free subsequent to colonization.
These were normalized against the control (DMSO). The Transwell
invasion was performed using Matrigel-coated porous upper chamber
inserts (Becton and Dickinson, San Jose, CA, USA), which were
plated with 5×104 cells in 0.5% FBS medium, with 600 µl
complete medium in the lower chamber. Cotton-tipped swabs were used
to remove non-invasive cells in the interior of the inserts 48 h
later. The inserts were incubated with 500 µl 0.5% crystal violet
(Roche Biochemicals, Mannheim, Germany) for 20 min at room
temperature. Subsequent to thorough washing in PBS, the inserts
were observed using an Olympus IX70 inverted phase contrast
microscope (Olympus Corporation, Tokyo, Japan).
Statistical Analysis
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was
used for one-way analysis of variance tests for all the data. All
data are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
TRPM8 expression is increased in DU145
cells compared with PNT1A cells
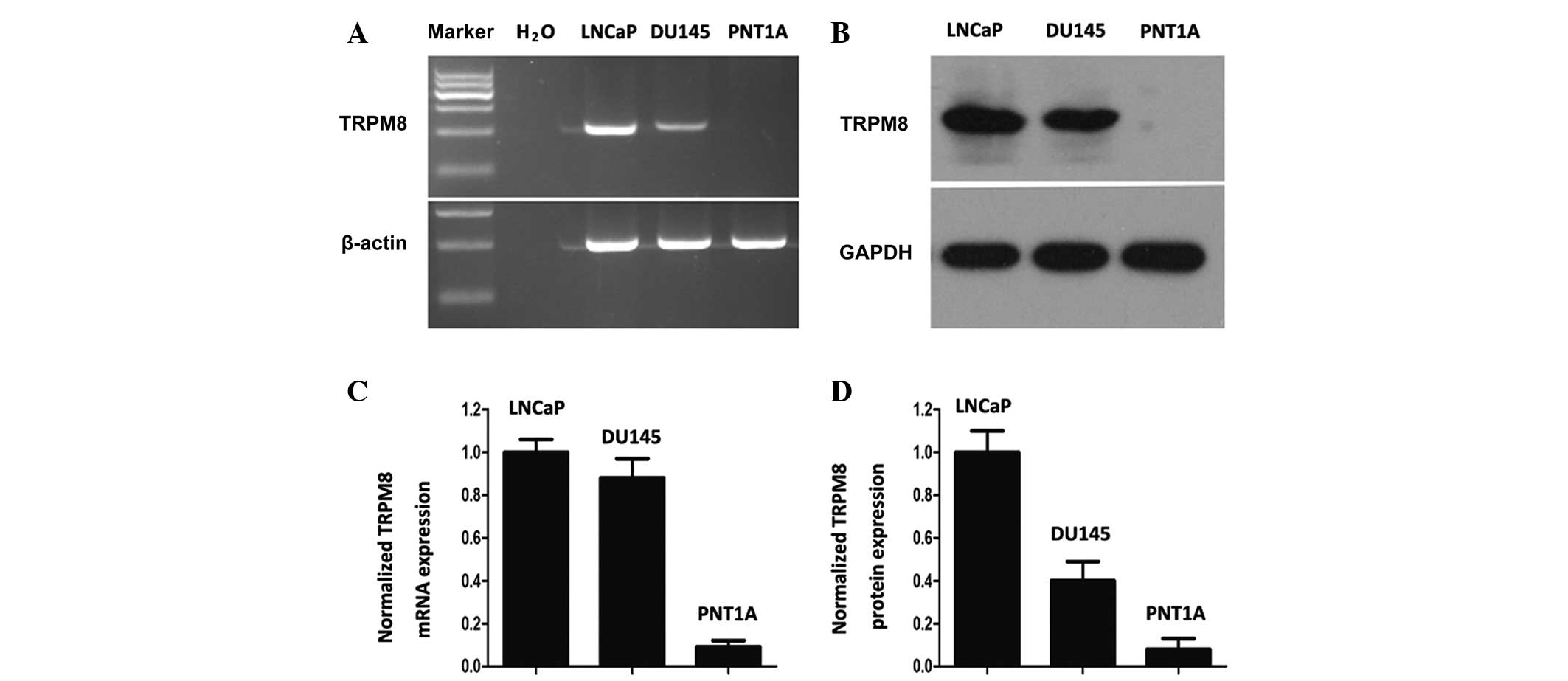
The expression of TRPM8 in DU145 cells was similar
to that in LNCaP cells at the mRNA and protein levels (Fig. 1A and B). However, the PNT1A cells
demonstrated little TRPM8 expression, as determined by grayscale
analysis with Image J software (Fig. 1C
and D). These results are mostly consistent with the results
obtained by Valero et al (17). The differential expression profile of
TRPM8 between tumor and non-tumor cells reveals the selective
cytotoxic effect of chemical drugs towards tumor cells.
BCTC arrests the growth of DU145
cells
Since DU145 cells demonstrated considerable
expression of TRPM8, the present study investigated whether the
potent TRPM8 antagonist BCTC exerted an anti-proliferative effect
in DU145 cells. The MTT assay established that BCTC reduced the
growth of DU145 cells in a dose-dependent manner (Fig. 2A). Dose response data demonstrated a
12.03 and 50.69% growth inhibition at 10 µM and 100 µM,
respectively, subsequent to a 72 h incubation. BCTC reduced
proliferation rates in DU145 cells, but not in PNT1A cells, which
indicated the potential for the highly selective anti-tumor
activity of BCTC towards PCa cells, with minimal effects on normal
prostate cells.
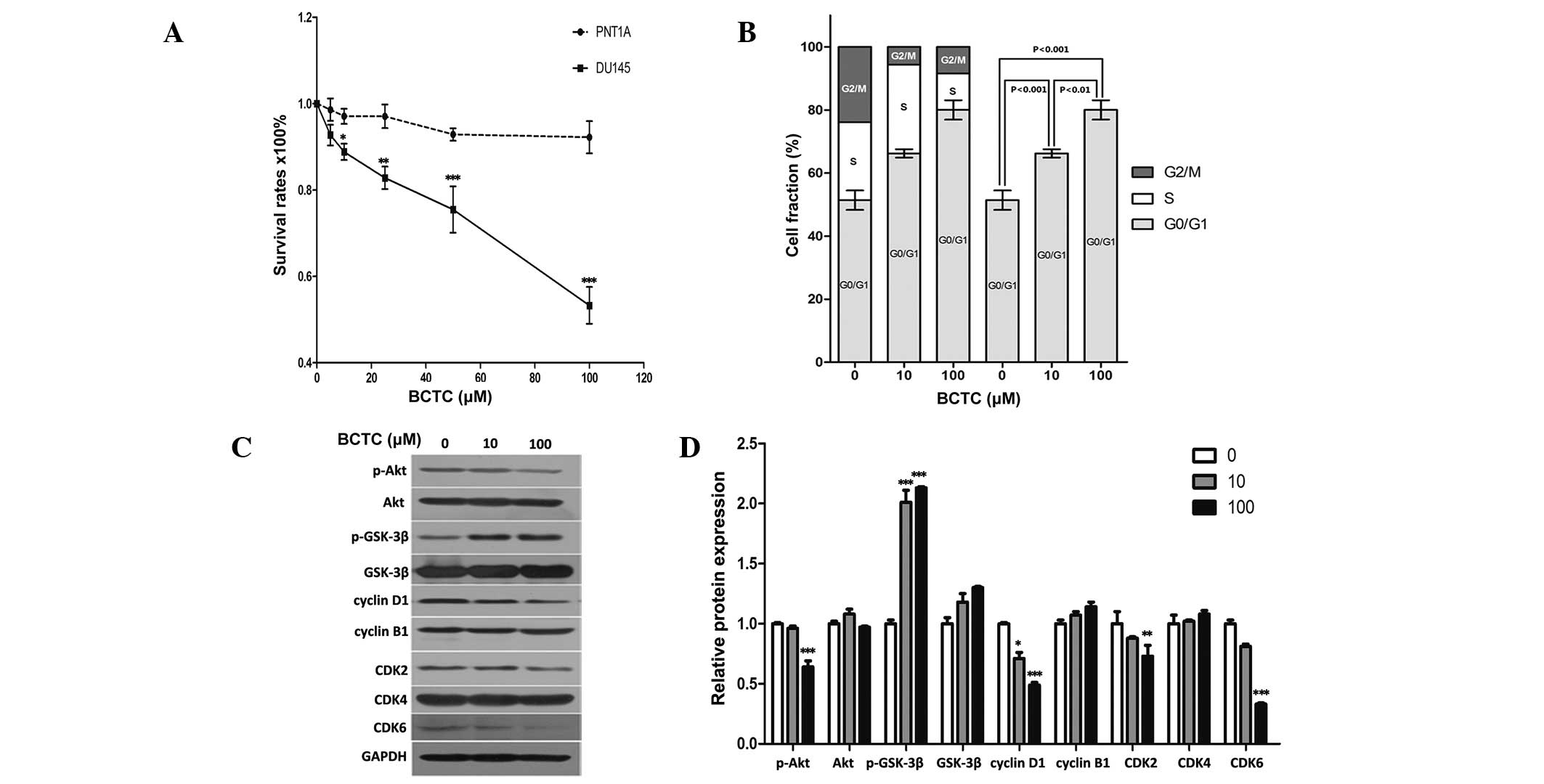 | Figure 2.BCTC suppressed the growth of DU145
cells and reduced G0/G1 phase cell cycle arrest. (A) BCTC reduced
the growth of DU145 cells in a dose-dependent manner by standard
MTT assay. Growth of DU145 cells was suppressed following
incubation with 0, 20, 40, 60, 80 or 100 µM BCTC for 72 h, compared
with PNT1A cells. MTT assays were conducted in triplicate. (B) BCTC
induced G0/G1 phase arrest. DU145 cells were harvested 48 h
subsequent to incubation with dimethyl sulfoxide, 10 µM BCTC or 100
µM BCTC, and the cell cycle distribution was examined. The G0/G1
phase rates of DU145 cells treated with various concentrations of
BCTC were fractioned for statistical analysis. (C) Cell
cycle-associated proteins, consisting of pAKT, AKT, pGSK-3β,
GSK-3β, cyclin B1 and D1, and CDK2, 4 and 6 were detected by
western blot analysis. Cells were harvested 48 h subsequent to the
indicated drug incubation, and western blot analysis was performed.
(D) Western blot analysis results were quantified and one-way
analysis of variance was performed. *P<0.05; **P<0.01;
***P<0.001. Error bars indicate the mean ± standard deviation.
BCTC,
N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide;
MTT assay, colorimetric assay; AKT, protein kinase B; GSK, glycogen
synthase kinase; CDK, cyclin-dependent kinase; p-AKT,
phosphorylated protein kinase B; p-GSK-3β, phosphorylated glycogen
synthase kinase. |
BCTC induces G0/G1 phase arrest rather
than apoptosis in DU145 cells
Consistent with the results of the MTT assay, flow
cytometric analysis of BCTC-treated DU145 cells indicated an
increased proportion of cells in the G0/G1 phases of the cell cycle
(Fig. 2B). Cell cycle-associated
proteins, including Akt, GSK-3β, cyclin B1, cyclin D1 and CDK2/4/6,
were detected by western blot analysis (Fig. 2C and D). p-Akt was downregulated by
BCTC, while p-GSK-3β was upregulated, with each of their
unphosphorylated forms unchanged. Cyclin D1, the most relevant
protein in the cell cycle (20), was
significantly downregulated, while cyclin-B1 remained unchanged.
The expression levels of CDK2, 4 and 6 were also tested, and the
expression of CDK2 and CDK6 was decreased by BCTC, but CDK4 levels
remained unchanged. These results indicate that BCTC induced G0/G1
arrest by selectively modulating the expression levels of a subset
of cell cycle regulator proteins.
The ability of BCTC to trigger apoptosis was
investigated through a series of experiments. It was found that
BCTC did not elevate LDH or caspase-3 activity at concentrations of
5 and 10 nM, in comparison to docetaxel, a well-known and
broad-spectrum chemotherapy agent (21), that acted as the positive control
(Fig. 3A and B). Flow cytometric
analysis revealed that BCTC did not increase the apoptotic rate of
DU145 cells (docetaxel acted as the positive control and increased
apoptosis) (Fig. 3C). Accordingly, to
ascertain the mechanism of these results, western blot analysis was
performed. The levels of proteins associated with apoptosis,
consisting of Bcl2, Bax, and cleaved caspase-3, did not change in
BCTC-treated DU145 cells, but in docetaxel-treated cells the levels
of apoptosis-associated proteins changed (Fig. 3D and E). Overall, this data
demonstrated that BCTC possesses the ability to induce cell cycle
arrest, without triggering apoptosis.
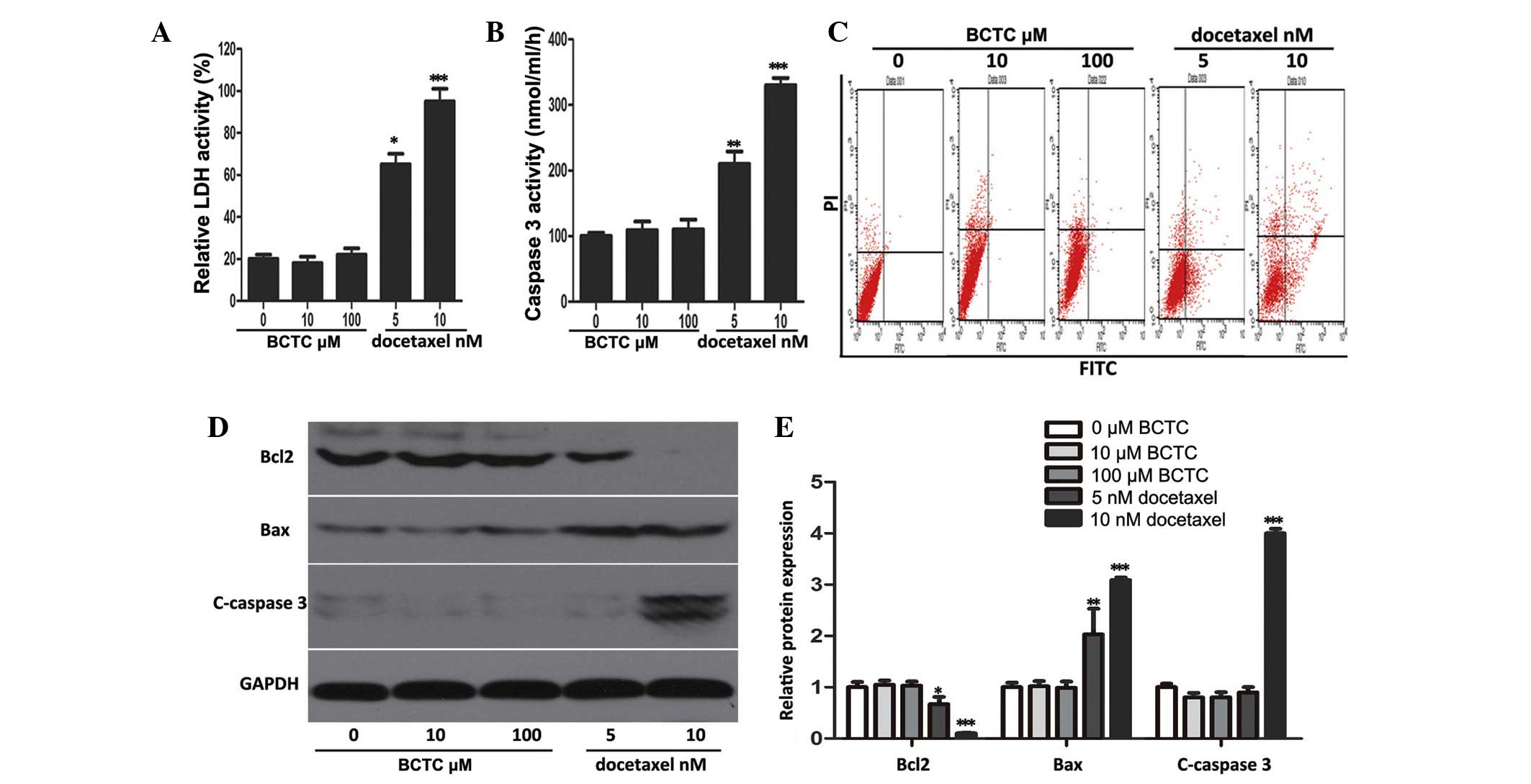 | Figure 3.BCTC did not induce apoptosis in DU145
cells. (A and B) LDH release and caspase-3 activity were detected
in BCTC-treated DU145 cells. The cells were treated with various
concentrations of BCTC (0, 10 and 100 µM) or docetaxel (5 or 10 nM;
positive control) for 72 h prior to performing LDH release and
caspase-3 activity assays. LDH release and caspase-3 activity are
expressed as a relative value to that of the untreated cells, which
is set to 100%. (C) Cell apoptosis was analyzed by Annexin V-FITC
assay. (D) Expression of apoptosis-associated proteins consisting
of Bcl2, Bax, C-caspase-3, were investigated by western blot
analysis. (E) Western blot analysis was quantified and one-way
analysis of variance was performed. *P<0.05; **P<0.01;
***P<0.001. Error bars indicate the mean ± standard deviation.
BCTC,
N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide;
LDH, lactate dehydrogenase; FITC, fluorescein isothiocyanate; Bcl2,
B-cell lymphoma 2; c-caspase-3, cleaved-caspase-3; Bax,
Bcl2-associated X protein. |
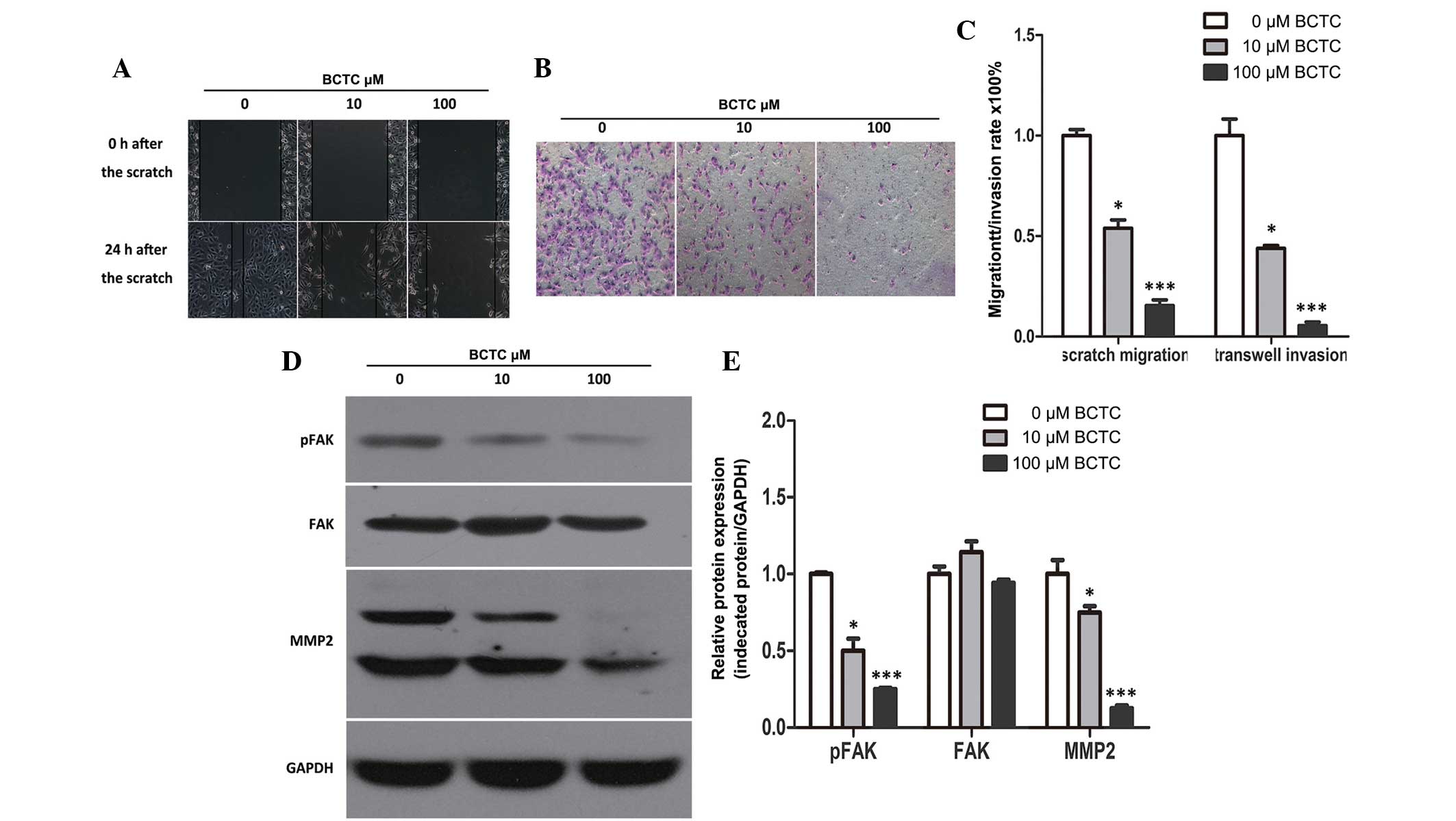
BCTC inhibits the migration and
invasion of DU145 cells
To investigate the potential effect of BCTC on the
migration of DU145, a standard scratch motility assay was
performed. Compared to the non-treated group, the migration of
BCTC-treated DU145 cells was significantly reduced (Fig. 4A and C). Furthermore, BCTC reduced the
invasion of DU145 cells in Matrigel-coated Transwell assays
(Fig. 4B and C). Western blot
analysis demonstrated the alteration of motility-associated protein
levels in DU145 cells following incubation with BCTC. MMP2 and
p-FAK were found to be downregulated by BCTC, which verified that
BCTC inhibited the migration and invasion of DU145 cells (Fig. 4D and E).
MAPK signal pathways may partially
participate in the anti-tumor activity of BCTC
Western blot analysis revealed that p-ERK1/2 was
downregulated substantially BCTC-treated DU145 cells, while p-p38
and p-JNK increased in comparison to the non-treated group, with
all of their unphosphorylated forms unchanged (Fig. 5A and B). In addition, specific
inhibitors of p38 and JNK (SB203580 and SP600125, respectively)
attenuated the inhibition of proliferation of DU145 cells by BCTC
(Fig. 5C), suggesting that MAPK
pathways partially participate in the anti-tumor activity of
BCTC.
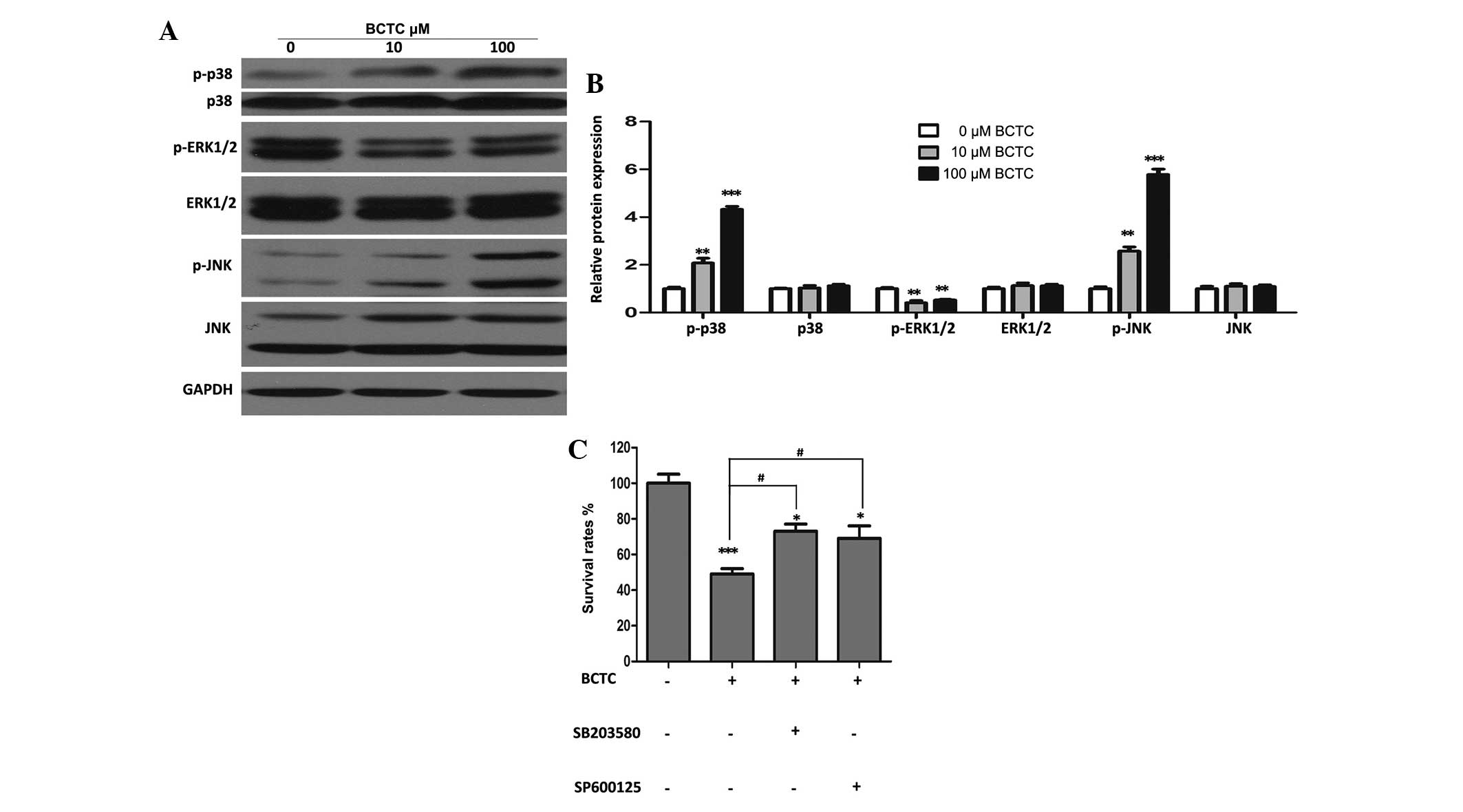 | Figure 5.Mitogen-activated protein kinase
signal pathways may partially be involved in the anti-tumor
activity of BCTC towards DU145 cells. (A) DU145 cells were treated
with various concentrations of BCTC for 48 h. Western blot analysis
was performed to investigate the expression of p-p38, p38,
p-ERK1/2, ERK1/2, p-JNK and JNK. (B) Western blot analysis was
quantified and a one-way analysis of variance was performed. (C)
DU145 cells were pre-treated with a p38 inhibitor (SB203580; 20µM)
and a JNK inhibitor (SP600125; 10 µM) for 4 h, and then treated
with 100 µM BCTC. After 48 h treatment, cell viability was
determined by a MTT assay. Experiments were performed in
triplicate. Western blot analysis was quantified and one-way
analysis of variance was performed. Error bars indicate the mean ±
standard deviation. *P<0.05, **P<0.01 and ***P<0.001 vs.
control; #P<0.05 vs. BCTC group. BCTC,
N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide;
p-ERK, phosphoryalted extracellular signal-regulated kinase; p-JNK,
phosphorylated c-Jun N-terminal kinase; p-p38, phosphorylated
p38. |
Discussion
TRPM8 is a receptor-activated non-selective cation
channel that is highly expressed in PCa cells, and in previous
years has emerged as a promising prognostic marker and putative
therapeutic target in PCa (11,16,17,22).
Notably, TRPM8 induces tumor suppression when it is inhibited and
also when activated. Since TRPM8 plays a key role in
Ca2+ homeostasis of prostate epithelial cells, either
over-expression or repression of TRPM8 will lead to a loss of
Ca2+ homeostasis, and also to the degradation of cell
viability (16,22,23).
Previous studies have provided clear evidence that over-expression
of TRPM8 in the androgen-independent PC3 cell line derived from
metastatic sites in bone or the activation of TRPM8 using the
classical TRPM8 agonist menthol in DU145 cells suppressed PCa cell
viability (24,25). By contrast, Zhang et al and
Valero et al demonstrated the anti-tumor effect of the
knockdown or blockade of TRPM8 in PCa cells (16,17). In
particular, Valero et al provided evidence that knockdown
and antagonists of TRPM8, including BCTC, possessed the ability to
inhibit the proliferation, cell cycle progression and migration of
PCa cells (17). However, this study
presented only the anti-tumor phenomena and the precise molecular
mechanism was not discerned, which consequently was a major aim of
the present study. To further explore the feasibility of targeting
TRPM8, the present study chose the potent and specific antagonist
BCTC to determine the precise mechanism of repressing PCa cells by
inhibiting TRPM8.
In the majority of the literature associated with
TRPM8, the dose of BCTC is usually <10 µM; however, the present
study used 10 µM and 100 µM. In addition, in the literature, BCTC
was mainly used to inhibit the internal Ca2+ flux of
cells induced by exogenous TRPM8 agonists or cold temperature, and
not to disturb the normal function of TRPM8 at physiological
conditions (18,26,27). The
techniques performed most often were Ca2+ imaging and
patch clamp technique. However, these detect the immediate changes
of Ca2+ flux or membrane potential. A higher dose of
BCTC would be required if continuing changes of Ca2+
flux or membrane potential are expected to affect the normal
function of cells. The present study revealed that BCTC did not
clearly affect the proliferation of PNT1A cells, even at the dose
of 100 µM, thereby demonstrating the safety and selectivity of this
drug.
The present study demonstrated that BCTC induced
evident G0/G1 cell cycle arrest in DU145 cells, through selectively
modulating the expression of a subset of cell cycle regulators,
including cyclin D1, CDK2 and CDK6. However, BCTC did not induce
apoptosis of DU145 cells, which was verified by a series of
experiments, including LDH and caspase-3 activity testing, flow
cytometric analysis and determining the expression of
apoptosis-associated proteins by western blot analysis.
In addition to cell cycle deregulation, metastasis
is another important manifestation of cancer (25). FAK is a non-receptor protein tyrosine
kinase that regulates adhesion-dependent cell signaling and is
required for the invasion and metastasis of cancer cells (28,29). The
MMP family plays a central role in cell migration, and the
literature has indicated that stromal connective tissue and
basement membranes are mostly degraded by the MMP family of
proteins, which comprise >20 zinc-dependent endopeptidases
(30). The present study indicated
that blockade of TRPM8 by BCTC, through the downregulation of MMP2
and p-FAK, reduced the motility of DU145 cells.
MAPK family members are known to control cell cycle
progression at various stages in a cell type- and context-specific
manner (31). The present study
revealed that p-ERK1/2 was substantially downregulated, while p-p38
and p-JNK were upregulated in BCTC-treated DU145 cells.
Furthermore, specific inhibitors of p38 and JNK attenuated the
inhibition of proliferation by BCTC, which suggests that MAPK
pathways are partially involved in the anti-tumor activity of BCTC
towards DU145 cells.
In summary, the present study demonstrated that
blockade of TRPM8 by BCTC reduced proliferation, cell cycle
progression, migration and invasion of DU145 cells, which is
partially attributed to the alteration of MAPK signal pathways.
These findings indicate that the TRPM8 inhibitor BCTC is a
potential therapeutic strategy against PCa and provide novel
insight into the understanding of PCa biology.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant nos.81172734 and 81202027) and the
Specialized Research Fund for the Doctoral Program of Higher
Education (grant no. 20120141120052).
References
|
1
|
Akaza H: Asian trends in primary androgen
depletion therapy on prostate cancer. Cancer Biol Med. 10:187–191.
2013.PubMed/NCBI
|
|
2
|
Baade PD, Youlden DR, Cramb SM, Dunn J and
Gardiner RA: Epidemiology of prostate cancer in the Asia-Pacific
region. Prostate Int. 1:47–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenthal SA and Sandler HM: Treatment
strategies for high-risk locally advanced prostate cancer. Nat Rev
Urol. 7:31–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Clegg NJ and Scher HI:
Anti-androgens and androgen-depleting therapies in prostate cancer:
New agents for an established target. Lancet Oncol. 10:981–991.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Damber JE and Aus G: Prostate cancer.
Lancet. 371:1710–1721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taplin ME: Drug insight: Role of the
androgen receptor in the development and progression of prostate
cancer. Nat Clin Pract Oncol. 4:236–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sullivan GF, Amenta PS, Villanueva JD,
Alvarez CJ, Yang JM and Hait WN: The expression of drug resistance
gene products during the progression of human prostate cancer. Clin
Cancer Res. 4:1393–1403. 1998.PubMed/NCBI
|
|
8
|
Chen CD, Welsbie DS, Tran C, Baek SH, Chen
R, Vessella R, Rosenfeld MG and Sawyers CL: Molecular determinants
of resistance to antiandrogen therapy. Nat Med. 10:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agus DB, Cordon-Cardo C, Fox W, Drobnjak
M, Koff A, Golde DW and Scher HI: Prostate cancer cell cycle
regulators: Response to androgen withdrawal and development of
androgen independence. J Natl Cancer Inst. 91:1869–1876. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wissenbach U, Niemeyer BA and Flockerzi V:
TRP channels as potential drug targets. Biol Cell. 96:47–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prevarskaya N, Zhang L and Barritt G: TRP
channels in cancer. Biochim Biophys Acta. 1772:937–946. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsavaler L, Shapero MH, Morkowski S and
Laus R: Trp-p8, a novel prostate-specific gene, is up-regulated in
prostate cancer and other malignancies and shares high homology
with transient receptor potential calcium channel proteins. Cancer
Res. 61:3760–3769. 2001.PubMed/NCBI
|
|
13
|
Henshall SM, Afar DE, Hiller J, Horvath
LG, Quinn DI, Rasiah KK, Gish K, Willhite D, Kench JG,
Gardiner-Garden M, et al: Survival analysis of genome-wide gene
expression profiles of prostate cancers identifies new prognostic
targets of disease relapse. Cancer Res. 63:4196–4203.
2003.PubMed/NCBI
|
|
14
|
Okamoto Y, Ohkubo T, Ikebe T and Yamazaki
J: Blockade of TRPM8 activity reduces the invasion potential of
oral squamous carcinoma cell lines. Int J Oncol. 40:1431–1440.
2012.PubMed/NCBI
|
|
15
|
Yee NS, Brown RD, Lee MS, Zhou W, Jensen
C, Gerke H and Yee RK: TRPM8 ion channel is aberrantly expressed
and required for preventing replicative senescence in pancreatic
adenocarcinoma: Potential role of TRPM8 as a biomarker and target.
Cancer Biol Ther. 13:592–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L and Barritt GJ: Evidence that
TRPM8 is an androgen-dependent Ca2+ channel required for
the survival of prostate cancer cells. Cancer Res. 64:8365–8373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valero ML, de Queiroz Mello F, Stühmer W,
Viana F and Pardo LA: TRPM8 ion channels differentially modulate
proliferation and cell cycle distribution of normal and cancer
prostate cells. PloS One. 7:e518252012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madrid R, Donovan-Rodríguez T, Meseguer V,
Acosta MC, Belmonte C and Viana F: Contribution of TRPM8 channels
to cold transduction in primary sensory neurons and peripheral
nerve terminals. J Neurosci. 26:12512–12525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mergler S, Mertens C, Valtink M, Reinach
PS, Székely VC, Slavi N, Garreis F, Abdelmessih S, Türker E, Fels G
and Pleyer U: Functional significance of thermosensitive transient
receptor potential melastatin channel 8 (TRPM8) expression in
immortalized human corneal endothelial cells. Exp Eye Res.
116:337–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trudeau ME: Docetaxel: a review of its
pharmacology and clinical activity. Can J Oncol. 6:443–457.
1996.PubMed/NCBI
|
|
22
|
Kulkarni P: TRPM8 and prostate cancer: To
overexpress or repress, that is the question-comment on “Effects of
TRPM8 on proliferation and motility of prostate cancer PC-3 cells”
by Yang ZH et al in Asian Journal of Andrology. Asian J
Androl. 11:150–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flourakis M and Prevarskaya N: Insights
into Ca2+ homeostasis of advanced prostate cancer cells.
Biochim Biophys Acta. 1793:1105–1109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang ZH, Wang XH, Wang HP and Hu LQ:
Effects of TRPM8 on the proliferation and motility of prostate
cancer PC-3 cells. Asian J Androl. 11:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Wang X, Yang Z, Zhu G, Chen D and
Meng Z: Menthol inhibits the proliferation and motility of prostate
cancer DU145 cells. Pathol Oncol Res. 18:903–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mälkiä A, Madrid R, Meseguer V, de la Peña
E, Valero M, Belmonte C and Viana F: Bidirectional shifts of TRPM8
channel gating by temperature and chemical agents modulate the cold
sensitivity of mammalian thermoreceptors. J Physiol. 581:155–174.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valero M, Morenilla-Palao C, Belmonte C
and Viana F: Pharmacological and functional properties of TRPM8
channels in prostate tumor cells. Pflugers Arch. 461:99–114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parsons JT, Slack-Davis J, Tilghman R and
Roberts WG: Focal adhesion kinase: Targeting adhesion signaling
pathways for therapeutic intervention. Clin Cancer Res. 14:627–632.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slack JK, Adams RB, Rovin JD, Bissonette
EA, Stoker CE and Parsons JT: Alterations in the focal adhesion
kinase/Src signal transduction pathway corassociate with increased
migratory capacity of prostate carcinoma cells. Oncogene.
20:1152–1163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
MacCorkle RA and Tan TH: Mitogen-activated
protein kinases in cell-cycle control. Cell Biochem Biophys.
43:451–461. 2005. View Article : Google Scholar : PubMed/NCBI
|