Introduction
Lung cancer is the most common cause of
cancer-associated mortality in a number of developed countries
(1), and non-small cell lung cancer
(NSCLC) is the most prevalent form of lung cancer worldwide,
accounting for 85% of all lung cancer cases (2,3). Epidermal
growth factor receptor (EGFR) may play a significant role in NSCLC,
and is thus a potential molecular target for personalized therapy
with tyrosine kinase inhibitors (TKIs) (4).
Somatic activating EGFR mutations, which are
clustered within the tyrosine kinase domain, most commonly occur in
the form of deletions in exon 19 or p.L858R mutations in exon 21.
These somatic activating mutations account for ~85% of all
EGFR mutations, and may indicate the likely sensitivity of
tumors to the effects of small-molecule inhibitors (such as
gefitinib and erlotinib) (4–6). Other, less prevalent EGFR
mutations, including exon 18 p.G719X mutations (3% of all
EGFR mutations) (7) and exon
21 p.L861Q (2% of all EGFR mutations) have been associated
with enhanced efficacy of EGFR-TKIs (8). By contrast, alternative classes of
EGFR mutations may be associated with a lack of response to
TKIs, and this is the case for the majority of exon 20 mutations,
which account for ~5% of all EGFR mutations (9).
EGFR exon 20 mutations occur in patients with
clinicopathological features similar to those of patients with
classical EGFR mutations (women, non-smokers,
adenocarcinomas). Exon 20 mutations encompass the area surrounding
amino acid positions Glu762 to Cys775, located in the N-lobe of the
kinase domain of EGFR following the C-helix. These mutations induce
a pattern of in vitro and in vivo resistance to
EGFR-TKIs (9). A number of mutations
in EGFR exon 20 are thought to increase the affinity of EGFR
for adenosine triphosphate (ATP), thus decreasing the efficacy of
TKI inhibition (10). However, due to
the comparative rarity of EGFR exon 20 mutations, clinical
data concerning the association between EGFR exon 20
mutations and responsiveness to TKIs has, to the best of our
knowledge, been limited so far within the relevant literature,
particularly for certain rare mutations, including p.S768I.
The present study reports the case of a patient with
NSCLC exhibiting p.S768I in the EGFR gene [a substitution at
codon 768 of exon 20 (c.2303G>T, p.S768I)], as well as a
mutation at codon 719, exon 18 (p.G719A), in combination with a
review of the relevant literature regarding this rare EGFR
somatic mutation.
Case report
A 48-year-old Asian male was admitted to Cannizzaro
Hospital (Catania, Italy) in March 2014, presenting with a poor
performance status (PS) and increasing dyspnea. A total body
computed tomography (CT) scan revealed a neoformation at the base
of the left lung, measuring ~4 cm and extending to the visceral
pleura. Furthermore, additional secondary nodules in both lungs,
along with pericardial effusion, were identified. Mediastinal
lymphadenopathy and liver metastases were detected. The patient
underwent a CT-guided biopsy of the left basal pulmonary lesion,
which exhibited the typical histology of an adenocarcinoma,
according to well-established World Health Organization criteria
(11). The neoplasia consisted of
neoplastic glands with focal papillary structures. Immunoreactivity
for thyroid transcription factor-1 and napsin A, and negativity for
thyroglobulin supported the pulmonary origin of the lesion.
Written informed consent was obtained from the
patient for sequencing of the EGFR gene and for publication
of the case report. The PyroMark Q24 system (Qiagen GmbH, Hilden,
Germany) was utilized for pyrosequencing analysis of EGFR
exons 18–21, using 2.5-µm sections of formalin fixed
paraffin-embedded tissue from metastatic supraclavicular lymph
nodes (whole slide) and thinPrep cytological samples from
pericardial effusion. All slides underwent genomic DNA extraction,
using QIAamp MinElute spin columns (Qiagen GmbH), according to the
manufacturer's instructions, and the sequence of interest was
amplified by polymerase chain reaction (PCR; Applied Biosystems
GeneAmp® PCR System 9700; Thermo Fisher Scientific, Inc., Foster
City, CA, USA). Using a therascreen EGFR Pyro kit (Qiagen GmbH),
all hotspot regions (4) of exons of
the EGFR gene were analyzed, and PyroMark Q24 software
(Qiagen, GmbH) was utilized for data analysis.
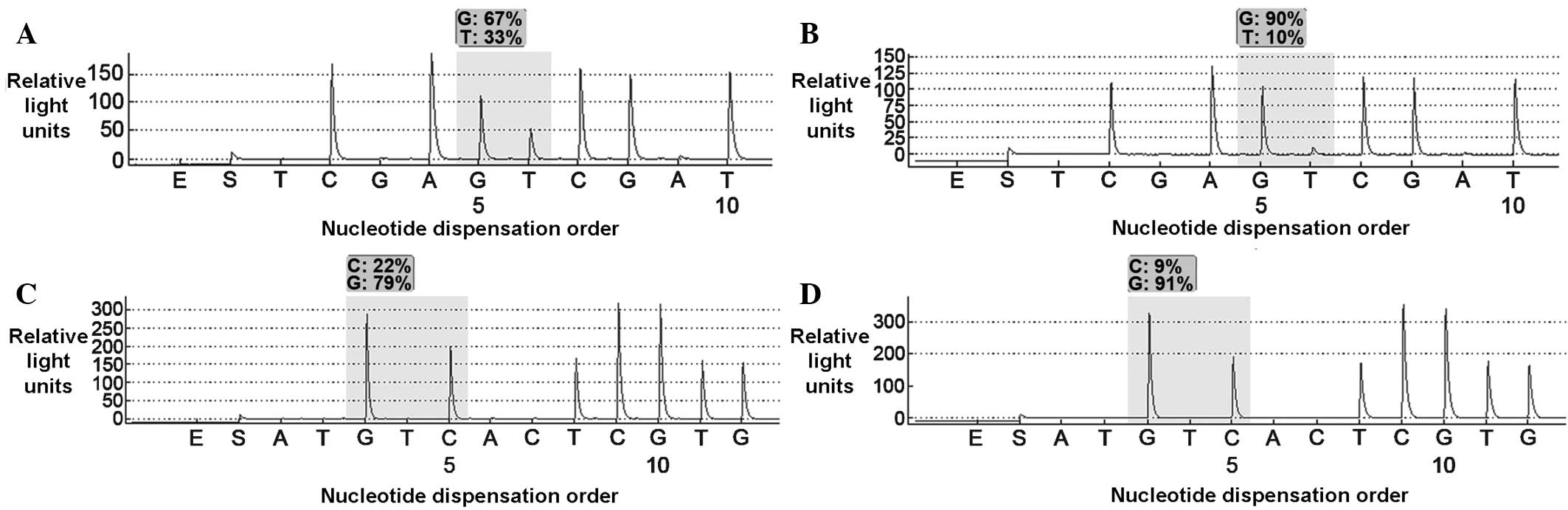
Pyrosequencing analysis of the full exome of
EGFR from each sample type, revealed the presence of a rare
mutation at codon 768, exon 20 (p.S768I; Fig. 1A and B), as well as a mutation at
codon 719, exon 18 (p.G719A; Fig. 1C and
D).
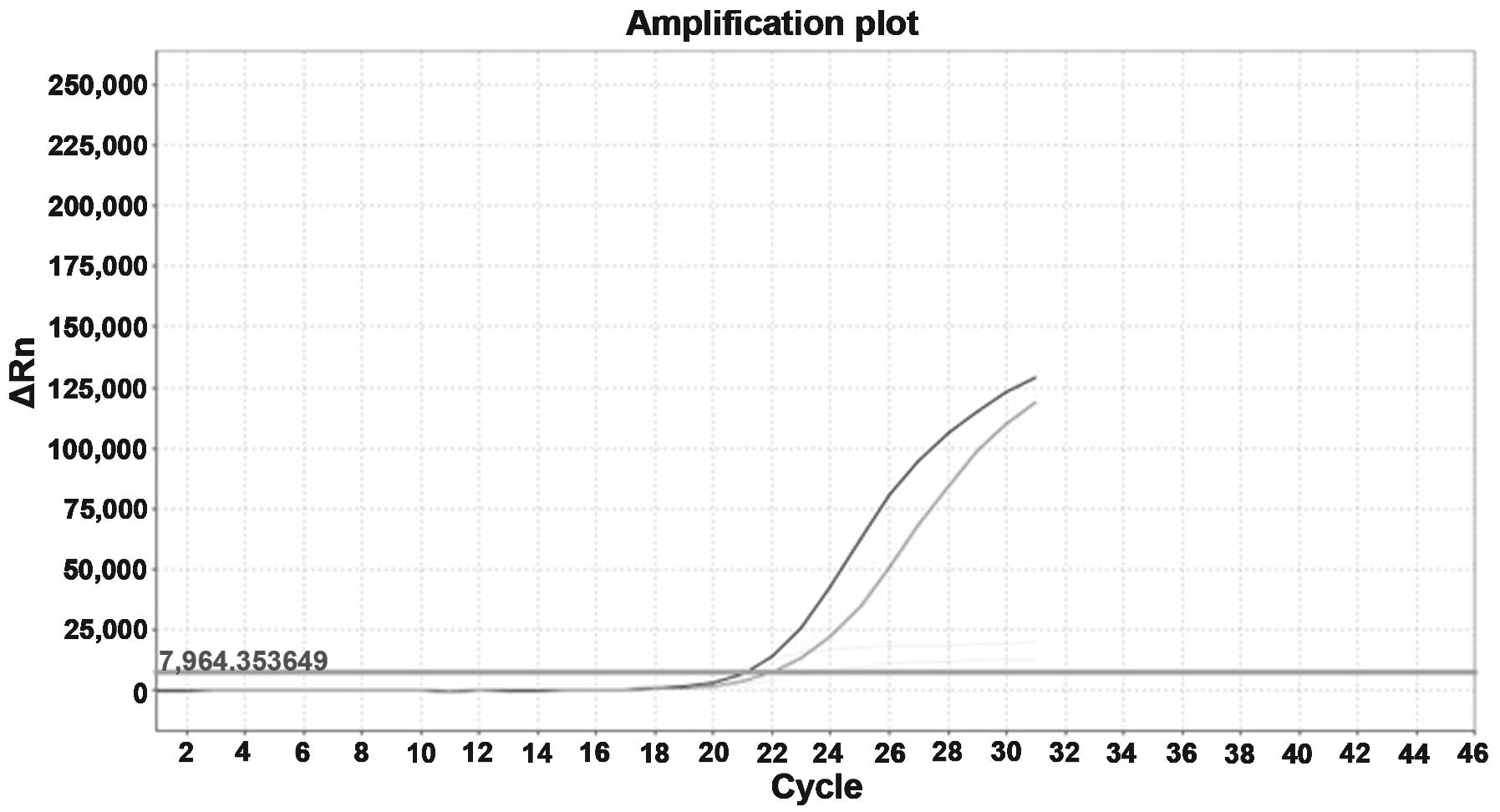
Patient DNA was subsequently retested for the
presence of a p.S768I mutation in exon 20 of the EGFR gene,
and its association with a mutation at codon 719, exon 18
(p.G719A). Molecular results were confirmed using the AmoyDx EGFR
Mutation Test kit (Amoy Diagnostics Co. Ltd., Xiamen, China) for
the detection of somatic mutations in the EGFR gene, using
the principle of amplified refractory mutation. The assay was
performed according to the manufacturer's instructions, using the
Applied Biosystems StepOnePlus™ Real-Time PCR system (Thermo Fisher
Scientific, Inc.; Fig. 2).
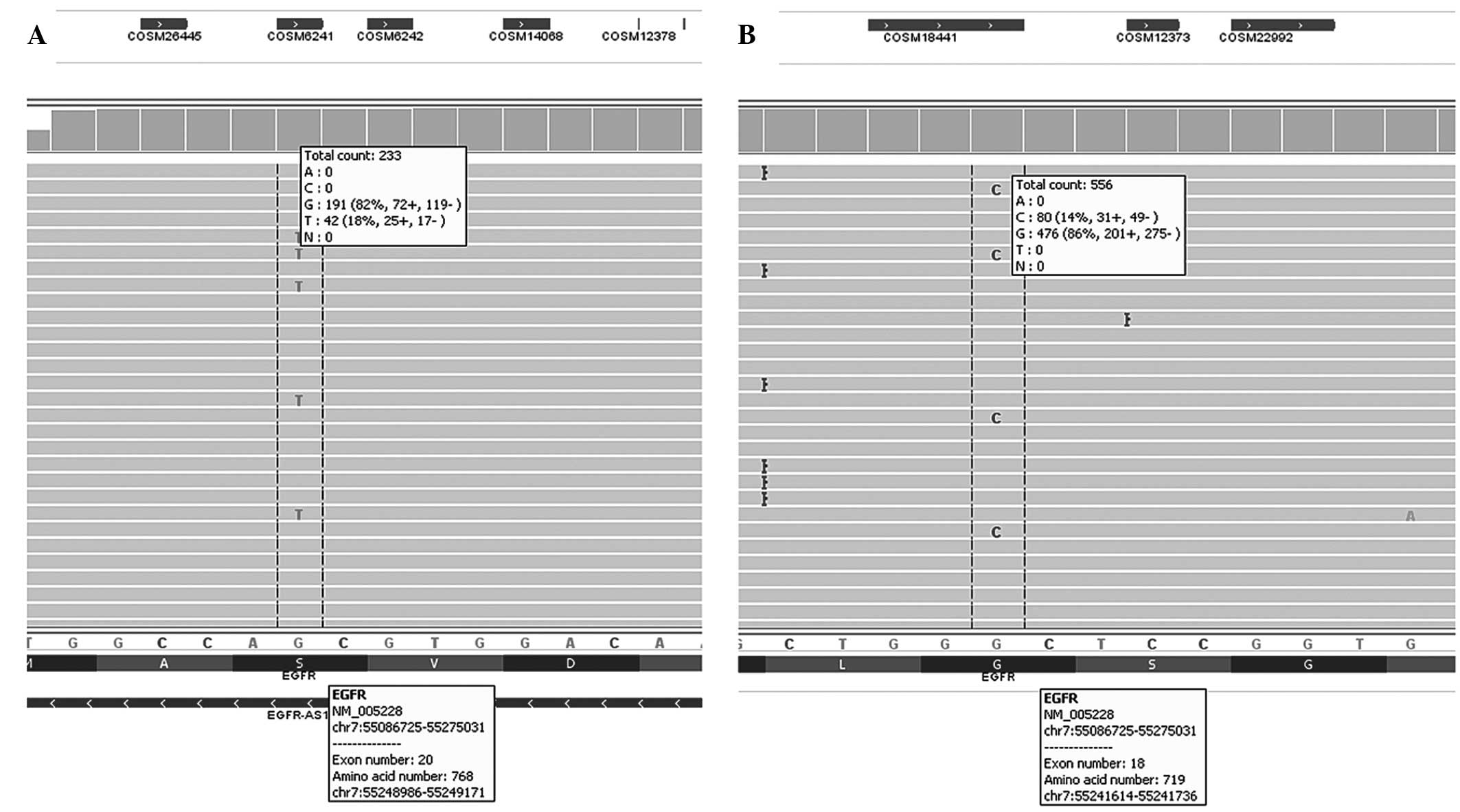
Patient DNA was additionally analyzed using next
generation sequencing on an Ion Torrent™ with Applied Biosystems
Colon-Lung v2 panel (Thermo Fisher Scientific, Inc.). This analysis
identified two mutations: EGFR (c.2303G>T, p.S768I) with
a target coverage depth of 233 in exon 20, and EGFR
(c.2156G>C, p.G719A) in exon 18 with a target coverage depth of
556 (Fig. 3).
Due to conflicting data in the existing literature
regarding the effectiveness of EGFR-TKIs in the presence of a
p.S768I mutation, and due to the poor PS of the patient, a decision
was reached to administer the patient with supportive care only.
The patient succumbed to the disease 6 weeks subsequent to
diagnosis.
Discussion
EGFR mutations are considered to be a robust
predictive biomarker of clinical response to EGFR-TKIs in clinical
practice (4). Gefitinib, an
EGFR-targeting agent, is an orally active small molecule drug,
which has been demonstrated to exhibit antitumor activity in NSCLC.
The response of NSCLC to gefitinib has been closely associated with
EGFR mutations in the kinase domain (4,5); Lynch
et al (4) suggested that
repositioning of critical residues due to such mutations may act to
stabilize their interaction with ATP and with gefitinib (its
competitive inhibitor), and gefitinib-induced inhibition may thus
be enhanced by certain mutations. However, as EGFR mutations
may occur at varying positions within the kinase domain, the
biochemical properties of these mutations and the sensitivity to
gefitinib of tumors possessing rare mutations may not be identical
(4). Therefore, the association
between EGFR mutations and sensitivity to EGFR-TKIs in
NSCLCs remains controversial, particularly for rare mutations
(4–6,7,12–17).
The p.S768I mutation in exon 20 of the EGFR
gene is a rare mutation that has been identified sporadically in
previous studies and is reported to confer reduced sensitivity to
gefitinib in vitro compared with the two most commonly
observed types of mutations: Exon 19 deletions and p.L858R
mutations (18,19). Due to the relative rarity of
EGFR exon 20 mutations, clinical data concerning their
associations with drug responsiveness are limited, and conflicting
data exist regarding the sensitivity to EGFR-TKIs of tumors
harboring p.S768I mutations (20,21). The
literature review conducted for the present report revealed a
limited number of cases involving p.S768I mutations (Table I), and conflicting data with regard to
its clinical association with EGFR-TKI efficacy. A notable
observation, which was confirmed by the results of the present
study, is the association between p.S768I in exon 20 and other
EGFR mutations, identified frequently in exon 18 and 21
(22). The significance of this
molecular/mutational association remains to be elucidated, and may
require further investigation. In previous studies where this
molecular/mutational association was not observed (23–26), there
may have been a lack of utilization of sensitive detection
techniques such as next-generation sequencing approaches.
 | Table I.Summary of review of the literature
concerning the p.S768I mutation, as well as its associated
mutations at alternative EGFR codons |
Table I.
Summary of review of the literature
concerning the p.S768I mutation, as well as its associated
mutations at alternative EGFR codons
| Author (reference
no.) | Year | Nationality | Patients, n | EGFR
mutants, n | p.S768I mutants,
n | Reported
mutations | RECIST |
|---|
| Huang et al
(13) | 2004 | Taiwanese | 101 | 39 | 1 | S768I+G719C |
|
|
|
|
|
|
| 1 | S768I+G719S |
|
| Kosaka et al
(12) | 2004 | Japanese | 277 | 111 | 1 | S768I+V769L |
|
| Shigematsu et
al (7) | 2005 | Japanese,
Taiwanese, Australian | 617 | 134 | 1 | S768I+G719S |
|
| Takano et al
(31) | 2005 | Japanese | 66 | 43 | 1 | S768I+L858R |
|
|
|
|
|
|
| 1 | S768I+G719C |
|
| Asahina et
al (27) | 2006 | Japanese | 1 | 1 | 1 | S768I+V769L | PD |
| Pugh et al
(23) | 2007 | Japanese | 349 | 102 | 2 | S768I |
|
|
|
|
|
|
| 1 | S768I+V769L |
|
|
|
|
|
|
| 1 | S768I+V774M |
|
|
|
| Asian | 39 | 8 | 1 | S768I | PR |
| Wu et al
(20) | 2008 | Taiwanese | 515 | 253 | 1 | S768I+G719A | PD |
|
|
|
|
|
| 1 | S768I+L858R | PR |
| Masago et al
(25) | 2010 | Japanese | 1 | 1 | 1 | S768I | PR |
| Wu et al
(8) | 2011 | Taiwanese | 1,261 | 627 | 2 | S768I+L858R | PR |
| Szumera-Ciećkiewicz
et al (26) | 2013 | Polish | 273 | 29 | 1 | S768I |
|
| Kobayashi et
al (29) | 2013 | Japanese | 79 | 11 | 2 | S768I+G719A | PR |
|
|
|
|
|
| 1 | S768I+V769L | PR |
| Weber et al
(24) | 2014 | Danish | 462 | 57 | 1 | S768I | PD |
| Pallan et al
(28) | 2014 | British | 2 | 2 | 2 | S768I | PD |
As shown in Table I,
Asahina et al (27) reported
that p.S768I and p.V769L mutations were associated with
insensitivity to EGFR-TKIs in the patient cohort investigated. In a
Danish patient cohort investigated by Weber et al (24), one patient possessed a p.S768I point
mutation in exon 20. This patient exhibited no response to
treatment with the EGFR-TKI erlotinib, and succumbed to progressive
disease 4 weeks subsequent to the start of treatment. An additional
case concerning a Taiwanese patient with progressive disease and
harboring two distinct mutations (p.S768I and p.G719A), was
identified by Wu et al (20),
and an a further two cases were reported by Pallan et al
(28). By contrast, a positive
clinical response to gefitinib in an NSCLC patient harboring the
rare mutation p.S768I was observed by Masago et al (25). Additional previous studies have also
reported partial responses to EGFR-TKIs in patients exhibiting
p.S768I and other mutations (8,20,23,29,30). In
addition, a number of retrospective analyses of EGFR
mutations (Table I) have investigated
the p.S768I mutation; however, the clinical responsiveness to
EGFR-TKIs has not been reported (7,12,13,26,31).
In certain in vitro studies, a number of
mutations have been shown to exhibit distinctive phosphorylation
patterns in several C-terminal tyrosine (Tyr) residues of the
EGFR gene, and have demonstrated varying sensitivities to
gefitinib when stably transfected into NSCLC cell lines (19,32). A
number of these mutants, including p.S768I, are hyperphosphorylated
on the Tyr 1045 residue, which is normally involved in the
recruitment of Casitas B-lineage lymphoma (Cbl) to EGFR and the
initiation of Cbl-mediated receptor multi-ubiquitination; mutations
at this site are refractory to EGF-induced ubiquitination and
degradation (33–35). Gefitinib treatment exerts reduced
growth-suppressive effects on cells expressing exon 20 mutations
compared with cells expressing exon 19 deletions or L858R
mutations, or those expressing the wild-type counterpart (19).
Kancha et al (18) identified four sets of EGFR
mutations based on their drug sensitivity profiles in vitro:
i) mutations sensitive to all three drugs investigated (gefitinib,
erlotinib and AEE788) with half maximal inhibitory concentration
(IC50) values in the low nanomolar range (L858R and Del
747–753 insS mutations); ii) mutations exhibiting reduced
sensitivity to gefitinib (IC50 >100 nmol/l), but
sensitivity (IC50<100 nmol/l) to both erlotinib and
AEE788 (G719S, V742A and R776C mutations); iii) mutations
exhibiting reduced sensitivity to both gefitinib and erlotinib, but
sensitivity to AEE788 (D761N, S768I, S748F, L838V and L861Q
mutations); and iv) mutations resistant to all three drugs
investigated (N826S and T790M mutations).
However, despite the in vitro results
reported by Kancha et al (18), data regarding the clinical
significance of all EGFR mutations in the literature are
unavailable at present. This includes p.S768I and other relatively
rare mutations, whose association with EGFR-TKIs remains to be
elucidated. Although a number of mutations in exons 18–21 have been
identified to be associated with EGFR-TKI resistance, only p.T790M
is known for its clinical significance to primary TKI drug
resistance. This resistance is caused by a conformational change in
the ATP-binding pocket, which increases the affinity of EGFR for
its natural substrate, and reduces its affinity for EGFR-TKIs
(7,36).
Kancha et al (18) categorized p.S768I in exon 20 as a
mutation that confers reduced sensitivity to the in vitro
activity of gefitinib. The relevant literature indicates that this
type of mutation is rare, and is associated with insensitivity to
EGFR-TKIs in vitro and in vivo, as previously
described by Asahina et al (27). However, conflicting results have also
been reported regarding the in vivo sensitivity of p.S768I
mutants to TKIs; Masago et al (25), for example, reported a case of a
patient with NSCLC harboring the p.S768I mutation who demonstrated
a good clinical response to gefitinib.
The present study reported a case of NSCLC harboring
a rare EGFR somatic mutation, along with the conflicting
data from the literature regarding the clinical significance of
this mutation. In vitro results reported by Kancha et
al (18) do not consider the
‘impact and the influence’ of the tumor microenvironment; it is not
necessarily notable that the sensitivity to certain drugs in
vitro differs from that observed in vivo. Thus, it may
be speculated that the p.S768I mutation is drug sensitive.
In conclusion, further examination of the
sensitivity of EGFR-TKIs in a more representative cohort of NSCLC
patients harboring a range of rare mutations may be required in
order to optimize the individual treatment of patients with such
mutations.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY
and Yang PC: Effectiveness of tyrosine kinase inhibitors on
‘uncommon’ epidermal growth factor receptor mutations of unknown
clinical significance in non-small cell lung cancer. Clin Cancer
Res. 17:3812–3821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda H, Kobayashi S and Costa DB: EGFR
exon 20 insertion mutations in non-small-cell lung cancer:
Preclinical data and clinical implications. Lancet Oncol.
13:e23–e31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasuda H, Park E, Yun CH, Sng NJ,
Lucena-Araujo AR, Yeo WL, Huberman MS, Cohen DW, Nakayama S,
Ishioka K, et al: Structural, biochemical, and clinical
characterization of epidermal growth factor receptor (EGFR) exon 20
insertion mutations in lung cancer. Sci Transl Med. 5:216ra1772013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Adenocarcinoma. WHO Classification of Tumours of
the Lung, Pleura, Thymus and Heart. 7:(4th). (Lyon). IARC Press.
26–37. 2015.
|
|
12
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: Biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang SF, Liu HP, Li LH, Ku YC, Fu YN,
Tsai HY, Chen YT, Lin YF, Chang WC, Kuo HP, et al: High frequency
of epidermal growth factor receptor mutations with complex patterns
in non-small cell lung cancers related to gefitinib responsiveness
in Taiwan. Clin Cancer Res. 10:8195–8203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokumo M, Toyooka S, Kiura K, Shigematsu
H, Tomii K, Aoe M, Ichimura K, Tsuda T, Yano M, Tsukuda K, et al:
The relationship between epidermal growth factor receptor mutations
and clinicopathologic features in non-small cell lung cancers. Clin
Cancer Res. 11:1167–1173. 2005.PubMed/NCBI
|
|
15
|
Han SW, Kim TY, Hwang PG, Jeong S, Kim J,
Choi IS, Oh DY, Kim JH, Kim DW, Chung DH, et al: Predictive and
prognostic impact of epidermal growth factor receptor mutation in
non-small-cell lung cancer patients treated with gefitinib. J Clin
Oncol. 23:2493–2501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitsudomi T, Kosaka T, Endoh H, Horio Y,
Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T and Yatabe Y:
Mutations of the epidermal growth factor receptor gene predict
prolonged survival after gefitinib treatment in patients with
non-small-cell lung cancer with postoperative recurrence. J Clin
Oncol. 23:2513–2520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsao MS, Sakurada A, Cutz JC, Zhu CQ,
Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, et
al: Erlotinib in lung cancer - molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kancha RK, von Bubnoff N, Peschel C and
Duyster J: Functional analysis of epidermal growth factor receptor
(EGFR) mutations and potential implications for EGFR targeted
therapy. Clin Cancer Res. 15:460–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YR, Fu YN, Lin CH, Yang ST, Hu SF,
Chen YT, Tsai SF and Huang SF: Distinctive activation patterns in
constitutively active and gefitinib-sensitive EGFR mutants.
Oncogene. 25:1205–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu JY, Wu SG, Yang CH, Gow CH, Chang YL,
Yu CJ, Shih JY and Yang PC: Lung cancer with epidermal growth
factor receptor exon 20 mutations is associated with poor gefitinib
treatment response. Clin Cancer Res. 4:4877–4882. 2008. View Article : Google Scholar
|
|
21
|
Beau-Faller M, Prim N, Ruppert AM,
Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet
JL, Rouquette I, et al: Rare EGFR exon 18 and exon 20 mutations in
non-small-cell lung cancer on 10 117 patients: A multicentre
observational study by the French ERMETIC-IFCT network. Ann Oncol.
25:126–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shih JY, Gow CH and Yang PC: EGFR mutation
conferring primary resistance to gefitinib in non-small-cell lung
cancer. N Engl J Med. 353:207–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pugh TJ, Bebb G, Barclay L, et al:
Correlations of EGFR mutations and increases in EGFR and HER2 copy
number to gefitinib response in a retrospective analysis of lung
cancer patients. BMC Cancer. 7:1282007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weber B, Hager H, Sorensen BS, McCulloch
T, Mellemgaard A, Khalil AA, Nexo E and Meldgaard P: EGFR mutation
frequency and effectiveness of erlotinib: A prospective
observational study in Danish patients with non-small cell lung
cancer. Lung Cancer. 83:224–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masago K, Fujita S, Irisa K, Kim YH,
Ichikawa M, Mio T and Mishima M: Good clinical response to
gefitinib in a non-small cell lung cancer patient harboring a rare
somatic epidermal growth factor gene point mutation; codon 768 AGC
> ATC in exon 20 (S768I). Jpn J Clin Oncol. 40:1105–1109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szumera-Ciećkiewicz A, Olszewski WT,
Tysarowski A, et al: EGFR mutation testing on cytological and
histological samples in non-small cell lung cancer: A Polish,
single institution study and systematic review of European
incidence. Int J Clin Exp Pathol. 6:2800–2812. 2013.PubMed/NCBI
|
|
27
|
Asahina H, Yamazaki K, Kinoshita I,
Yokouchi H, Dosaka-Akita H and Nishimura M: Non-responsiveness to
gefitinib in a patient with lung adenocarcinoma having rare EGFR
mutations S768I and V769L. Lung Cancer. 54:419–422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pallan L, Taniere P and Koh P: Rare EGFR
exon 20 S768I mutation predicts resistance to targeted therapy: A
report of two cases. J Thorac Oncol. 9:e752014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi S, Canepa HM, Bailey AS,
Nakayama S, Yamaguchi N, Goldstein MA, Huberman MS and Costa DB:
Compound EGFR mutations and response to EGFR tyrosine kinase
inhibitors. J Thorac Oncol. 8:45–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han SW, Kim TY, Lee KH, et al: Clinical
predictors versus epidermal growth factor receptor mutation in
gefitinib-treated non-small-cell lung cancer patients. Lung Cancer.
54:201–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non-small-cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greulich H, Chen TH, Feng W, Jänne PA,
Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR
and Meyerson M: Oncogenic transformation by inhibitor-sensitive and
-resistant EGFR mutants. PLoS Med. 2:e3132005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levkowitz G, Waterman H, Ettenberg SA,
Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover
A, et al: Ubiquitin ligase activity and tyrosine phosphorylation
underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol
Cell. 4:1029–1040. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haglund K, Sigismund S, Polo S,
Szymkiewicz I, Di Fiore PP and Dikic I: Multiple monoubiquitination
of RTKs is sufficient for their endocytosis and degradation. Nat
Cell Biol. 5:461–466. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mosesson Y, Shtiegman K, Katz M, Zwang Y,
Vereb G, Szollosi J and Yarden Y: Endocytosis of receptor tyrosine
kinases is driven by monoubiquitylation, not polyubiquitylation. J
Biol Chem. 78:21323–21326. 2003. View Article : Google Scholar
|
|
36
|
Pallis AG, Voutsina A, Kalikaki A,
Souglakos J, Briasoulis E, Murray S, Koutsopoulos A, Tripaki M,
Stathopoulos E, Mavroudis D and Georgoulias V: ‘Classical’ but not
‘other’ mutations of EGFR kinase domain are associated with
clinical outcome in gefitinib-treated patients with non-small cell
lung cancer. Br J Cancer. 97:1560–1566. 2007. View Article : Google Scholar : PubMed/NCBI
|