Introduction
The cancer-specific survival rates for patients with
prostate cancer remain high, despite the fact that this type of
cancer is the most common non-cutaneous malignancy, and the second
leading cause of cancer-associated mortality in men in USA
(1). According to Siegel et al
(1), the 5-year relative survival
rate for prostate cancer in the USA between 2003 and 2009 was 99%
for all stages. There were ~233,000 new cases of prostate cancer
diagnosed in the USA in 2014 (1).
Close surveillance of survivors of prostate cancer is important,
with physical examination and prostate specific antigen (PSA)
testing being currently considered the standard of care to detect
potential recurrences. For patients with prostate cancer that
present an average risk of recurrence, the National Comprehensive
Cancer Network recommends conducting a digital rectal examination
every year, and measuring the levels of PSA every three-six months
for the first five years following treatment, and annually
thereafter. More intense monitoring every three months may be
indicated for patients with high risk of recurrence, nodal
involvement or distant metastasis at presentation (2). In the present study, a case of
symptomatic solitary recurrence of prostate cancer that occurred
while the patient was receiving androgen deprivation therapy (ADT),
and presented with low/undetectable serum levels of PSA, is
reported.
Case report
A 74-year-old Caucasian male with a history of
prostate cancer presented to the University of Texas, Southwestern
Medical Center (Dallas, USA) with worsening back pain. The patient
had undergone radical prostatectomy (RP) with pelvic lymph node
dissection four years earlier. Pathological analysis demonstrated
Gleason score 8 disease (4+4 with tertiary grade 5) at stage pT3b,
with involvement of seminal vesicles, perineural invasion,
extracapsular extension and negative margins. None of the pelvic
lymph nodes (0/2 nodes on the right side and 0/4 nodes on the left
side) were involved. Six months following surgery, the levels of
PSA of the patient increased to 1.0 ng/ml, with a doubling time of
1.7 months. Work-up was not conclusive for metastatic or regional
disease recurrence.
The patient underwent salvage radiation therapy (RT)
with 6,662 cGy to the prostatic fossa and 4,500 cGy to the pelvic
lymph nodes, in addition to short-term neoadjuvant therapy and
concurrent ADT, consisting of leuprolide acetate depot every three
months and daily bicalutamide. One month subsequently to the
completion of RT, the levels of PSA of the patient reduced to 0.2
ng/ml. However, five months later, his levels of PSA increased to
2.84 ng/ml, and his levels of testosterone were 455 ng/dl. Thus,
the patient was considered to present biochemical failure, and
salvage ADT was consequently initiated. Following treatment, the
levels of PSA of the patient declined, and were maintained at a
nadir of 0.07 ng/ml. However, one year later, the patient presented
with worsening back pain, numbness and burning sensation radiating
to his left anterior thigh and knee, which was consistent with
radiculopathy in the lumbar levels 3 and 4. The patient rated the
level of pain as 9/10, which was unresponsive to pregabalin and
hydrocodone, although the addition of tramadol reduced the pain to
a level of 4/10. Magnetic resonance imaging (MRI) of the lumbar
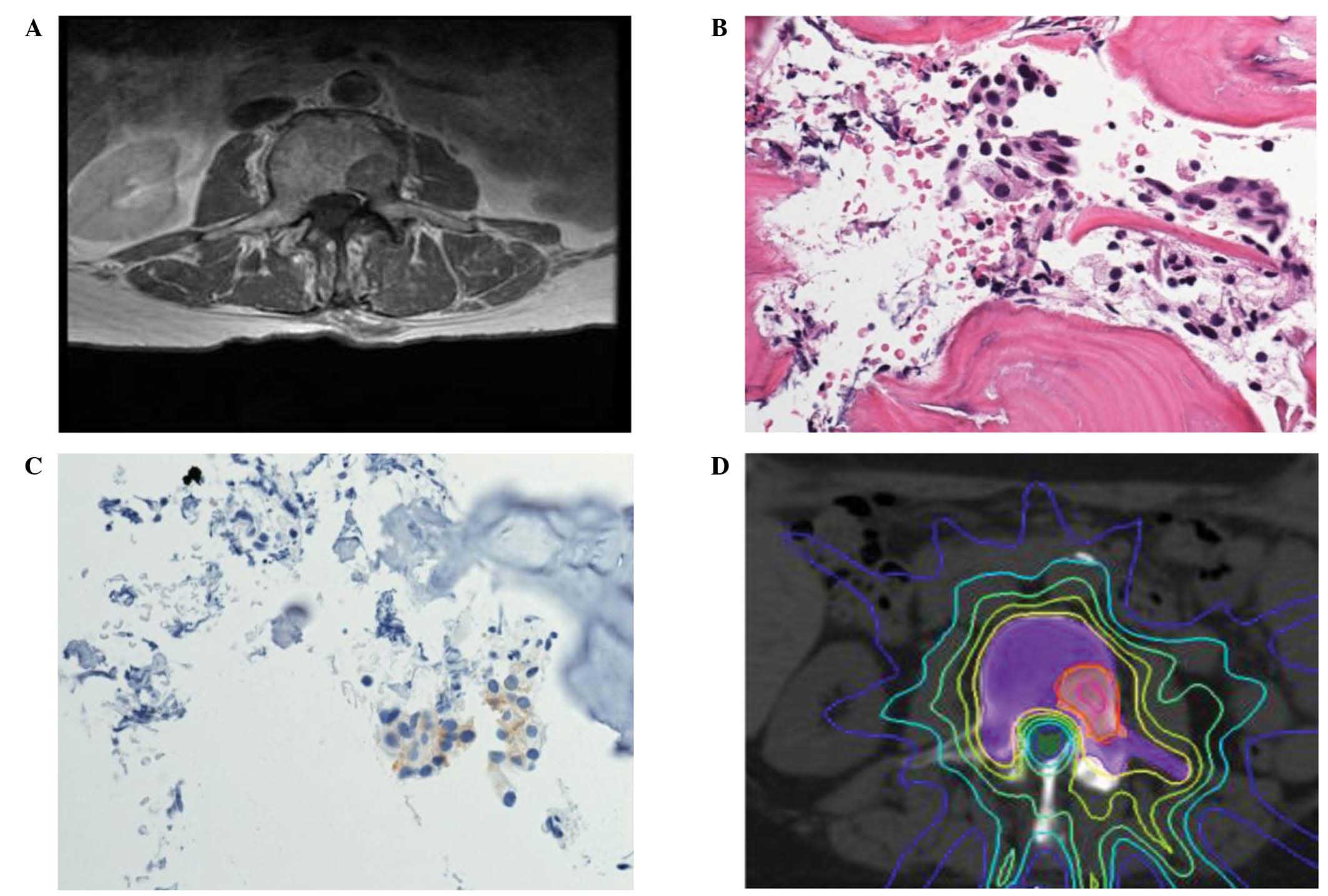
spine identified a 2.1×2.8 cm sclerotic lesion in the left L3
vertebral body that extended to the pedicle (Fig. 1A), which was suggestive of metastatic
disease, and possibly responsible for the symptoms experienced by
the patient. However, bone scan did not reveal any lesion. PSA
analysis was then repeated, and the levels of PSA detected were
0.05 ng/ml, while the levels of testosterone were 2.5 ng/dl. These
findings would have been considered as undetectable PSA prior to
the era of ultrasensitive PSA assay, particularly due to the nadir
levels of PSA exhibited by the patient while receiving ADT.
Based on this presentation, concerns about the
etiology of the L3 lesion were raised, and a biopsy of the L3
lesion was then performed. Routine pathological and
immunohistochemical analysis was performed using a Ventana
BenchMark detection system and the following antibodies: Polyclonal
rabbit anti-human PSA (#760–2506) and monoclonal mouse anti-human
PSA (ER-PR8) (#760–4271; all from Ventana Medical Systems, Inc.,
Tucson, AZ, USA). Pathological analysis of the specimen
demonstrated it to be a well-differentiated adenocarcinoma of the
prostate, with focally positive PSA staining, thus confirming
recurrent prostate cancer (Fig. 1B and
C). Neuroendocrine features were not observed. With the
confirmation of symptomatic solitary metastatic disease, the L3
lesion was then treated using stereotactic body RT (SBRT), with
2,000 cGy to 86% gross tumor volume and 1,400 cGy to 97% planning
treatment volume (PTV) (Fig. 1D). The
pain experienced by the patient subsequently improved to a level of
2/10, without requiring an increase in pain medications, and the
levels of PSA immediately reduced to undetectable levels (<0.05
ng/ml) two weeks following SBRT, and remained undetectable during
the subsequent three measurements conducted at six months
post-therapy. Subsequent clinical examination and imaging studies,
including lumbar MRI performed at eight months post-SBRT, did not
provide any radiographical evidence of disease progression in the
site subjected to SBRT treatment or novel metastatic disease.
Following completion of SBRT, no systemic therapy additional to
leuprolide acetate has been administered to the patient to
date.
The present retrospective case study was approved by
the Ethics Committee of the University of Texas, Southwestern
Medical Center (#STU 052012-019).
Discussion
PSA is generally a reliable biomarker with a high
negative predictive value for detecting recurrence of prostate
cancer in patients that had been previously subjected to RP
(3). Ultrasensitive PSA testing
methods enable the prediction of biochemical recurrence-free
survival following prostatectomy (4).
The threshold for biochemical failure subsequent to RT has evolved
over time (5). However, recurrence of
prostate cancer with low or undetectable levels of PSA has been
previously reported (6–9), and therefore, low levels of PSA alone
should not discard the possibility of recurrence of prostate cancer
in a suspicious clinical setting.
Possible explanations for low or undetectable levels
of PSA in the context of metastatic disease include technical
limitations (10), effectiveness of
the treatment (11,12) and biological characteristics of the
tumor (7,8,13). The
sensitivity of serum PSA assays has remarkably improved over the
years, and false negative errors have become less likely with the
modern detection methods currently available (4). A clinically detectable lesion suggestive
of metastasis generally implies sufficient tumor burden possible to
detect by serology. However, the patient of the present case report
was on ADT at the time of recurrence, which may have reduced the
levels of PSA and masked the detection of an increase in the levels
of this marker. Furthermore, previous pathological reviews of
prostatectomy-derived specimens demonstrated an inverse correlation
between the Gleason score and the PSA content in prostate cancer,
suggesting reduced PSA production in cases of high grade,
de-differentiated prostate cancer (13). Previous retrospective studies have
demonstrated that de-differentiated prostate cancer, which exhibits
neuroendocrine features, may progress without increased levels of
PSA in <3% of all cases of recurrent prostate cancer, which
often appear to be more aggressive (7,8). However,
in the present case, the pathological analysis did not detect these
features, but demonstrated a well-differentiated adenocarcinoma
with focally positive PSA. Intralesional heterogeneity, resulting
in certain tumor tissues not expressing PSA, may have contributed
to the low levels of PSA detected in the patient of the present
study. This tumor heterogeneity may be due to the effect of the ADT
treatment or the biology of the tumor. Previous literature reviews
demonstrated that the majority of cases of recurrent prostate
cancer without detectable levels of PSA displayed pathological
confirmation of PSA on immunohistochemical stain (14). In the present case, it is conceivable
that the recurrent cancer cells may have acquired novel mutations
that prevented the secretion of PSA, resulting in false-negative
PSA serology.
The current standard of care for recurrent prostate
cancer following salvage RT is ADT, which is considered to be a
non-curative treatment (15,16). Furthermore, PSA− prostate
cancer may be less sensitive to hormones, and responds unfavorably
to salvage hormonal therapy (17).
Since hormonal therapy markedly affects the quality of life of
patients, limited ADT is often recommended for biochemical failure
following definitive therapy (18).
The majority of salvage treatments for castration-resistant
prostate cancer provide a marginal improvement on survival
(19), highlighting the importance of
contemplating the quality of life of the patients while treating
prostate cancer (20). In the present
case, the lack of requirement for additional second line hormonal
therapies following local ablative therapy was beneficial for the
patient.
Traditionally, local therapy such as RT is generally
reserved for symptomatic palliation (21,22). SBRT
enables precise delivery of ablative radiation to the target, with
minimum dose to the surrounding normal tissue, using image guidance
and immobilization devices (23).
SBRT has been previously used to treat spine metastasis in a
cooperative group setting (Radiation Therapy Oncology Group #0631)
(24). In order to account for
microscopic extension of the tumor cells when treating spine
metastasis by SBRT, the clinical target volume (CTV) must include
the gross tumor volume defined by imaging, and the contiguous bone
marrow cavity, including the two pedicles. The posterior element of
the vertebra should be included in the CTV only if it is directly
involved by the gross tumor. Due to the minimum motion of the spine
and the use of daily image guidance, there is no margin for set-up
errors in the PTV in spine SBRT.
SBRT has also been previously used to treat
oligometastasis or disease that is non-responsive to systemic
therapy (25–27). A recent phase II SBRT trial combining
erlotinib in patients with oligometastatic non-small cell lung
cancer who had failed first time treatment with chemotherapy,
demonstrated an alteration in the pattern of failure experienced by
the patients, and a marked delay in disease progression when
subjected to SBRT, compared with historic controls
(progression-free survival, 2–4 vs. 14.7 months; overall survival,
9 vs. 20.4 months) (28). A previous
preliminary report from the Eastern Cooperative Oncology Group
E3805 study indicated that the addition of docetaxel to ADT
improves survival in patients with metastatic prostate cancer
(29). While the benefits of local
treatment of oligometastatic recurrence in castrate-resistant
prostate cancer are unclear, consideration for such treatment has
been suggested in the hormone-sensitive setting (30), and it has been proposed that local
treatment may complement the effects of systemic therapy. Prostate
cancer is generally a slow-growing tumor, and long-term survival
may be achieved by early detection, effective surgery and
advancements in systemic therapy (31). Nonetheless, disease progression in
prostate cancer tends to occur at sites of tumor bulk, and
castration-resistant clones may be present at the early stages of
the disease (15,32). Application of local therapy such as
SBRT may enable longer disease control, by complementing the
effects of systemic therapy. Therefore, SBRT may possess a
potential curative role in certain subgroups of patients with
metastatic prostate cancer, and should be considered in cases of
oligometastasis.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology - Prostate Cancer
(Version 1.2015). PROS–6. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdfAccessed.
10 01–2015
|
|
3
|
Pound CR, Christens-Barry OW, Gurganus RT,
Partin AW and Walsh PC: Digital rectal examination and imaging
studies are unnecessary in men with undetectable prostate specific
antigen following radical prostatectomy. J Urol. 162:1337–1340.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong SK, Park HZ, Lee WK, Kim DS, Lee JS,
Doo SH, Jeong SJ, Yoon CY, Byun SS and Lee SE: Prognostic
significance of undetectable ultrasensitive prostate-specific
antigen nadir after radical prostatectomy. Urology. 76:723–727.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American society for therapeutic radiology
and oncology consensus panel: Consensus statement: Guidelines for
PSA following radiation therapy. Int J Radiat Oncol Biol Phys.
37:1035–1041. 1997.PubMed/NCBI
|
|
6
|
Leibman BD, Dillioglugil O, Wheeler TM and
Scardino PT: Distant metastasis after radical prostatectomy in
patients without an elevated serum prostate specific antigen level.
Cancer. 76:2530–2534. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leibovici D, Spiess PE, Agarwal PK, Tu SM,
Pettaway CA, Hitzhusen K, Millikan RE and Pisters LL: Prostate
cancer progression in the presence of undetectable or low serum
prostate-specific antigen level. Cancer. 109:198–204. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oefelein MG, Smith N, Carter M, Dalton D
and Schaeffer A: The incidence of prostate cancer progression with
undetectable serum prostate specific antigen in a series of 394
radical prostatectomies. J Urol. 154:2128–2131. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schriefer P, Steurer S, Huland H and
Graefen M: Is undetectable prostate-specific antigen always
reliable to rule out prostate cancer recurrence after radical
prostatectomy? J Clin Oncol. 30:e341–e344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jansen FH, Roobol M, Bangma CH and van
Schaik RH: Clinical impact of new prostate-specific antigen WHO
standardization on biopsy rates and cancer detection. Clin Chem.
54:1999–2006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moul JW, Wu H, Sun L, McLeod DG, Amling C,
Donahue T, Kusuda L, Sexton W, O'Reilly K, Hernandez J, Chung A and
Soderdahl D: Early versus delayed hormonal therapy for prostate
specific antigen only recurrence of prostate cancer after radical
prostatectomy. J Urol. 171:1141–1147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aihara M, Lebovitz RM, Wheeler TM, Kinner
BM, Ohori M and Scardino PT: Prostate specific antigen and gleason
grade: An immunohistochemical study of prostate cancer. J Urol.
151:1558–1564. 1994.PubMed/NCBI
|
|
14
|
Safa AA, Reese DM, Carter DM, Phillipson
J, Smith R and Dougherty S: Undetectable serum prostate-specific
antigen associated with metastatic prostate cancer: A case report
and review of the literature. Am J Clin Oncol. 21:323–326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Studer UE, Whelan P, Wimpissinger F,
Casselman J, de Reijke TM, Knönagel H, Loidl W, Isorna S, Sundaram
SK and Collette L: EORTC Genitourinary Cancer Group: Differences in
time to disease progression do not predict for cancer-specific
survival in patients receiving immediate or deferred
androgen-deprivation therapy for prostate cancer: Final results of
EORTC randomized trial 30891 with 12 years of follow-up. Eur Urol.
66:829–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warde P, Mason M, Ding K, Kirkbride P,
Brundage M, Cowan R, Gospodarowicz M, Sanders K, Kostashuk E,
Swanson G, et al: NCIC CTG PR.3/MRC UK PR07 investigators: Combined
androgen deprivation therapy and radiation therapy for locally
advanced prostate cancer: A randomised, phase 3 trial. Lancet.
378:2104–2111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Birtle AJ, Freeman A, Masters JR, Payne HA
and Harland SJ: BAUS Section of Oncology Cancer Registry: Clinical
features of patients who present with metastatic prostate carcinoma
and serum prostate-specific antigen (PSA) levels <10 ng/ml: The
PSA negative patients. Cancer. 98:2362–2367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crook JM, OCallaghan CJ, Duncan G,
Dearnaley DP, Higano CS, Horwitz EM, Frymire E, Malone S, Chin J,
Nabid A, et al: Intermittent androgen suppression for rising PSA
level after RT. N Engl J Med. 367:895–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzman DL and Antonarakis ES:
Castration-resistant prostate cancer: Latest evidence and
therapeutic implications. Ther Adv Med Oncol. 6:167–179. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moul JW and Dawson N: Quality of life
associated with treatment of castration-resistant prostate cancer:
A review of the literature. Cancer Invest. 30:1–12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartsell WF, Scott CB, Bruner DW,
Scarantino CW, Ivker RA, Roach M 3rd, Suh JH, Demas WF, Movsas B,
Petersen IA, et al: Randomized trial of short-versus long-course
radiotherapy for palliation of painful bone metastases. J Natl
Cancer Inst. 97:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chow E, van der Linden YM, Roos D,
Hartsell WF, Hoskin P, Wu JS, Brundage MD, Nabid A, Tissing-Tan CJ,
Oei B, et al: Single versus multiple fractions of repeat radiation
for painful bone metastases: A randomised, controlled,
non-inferiority trial. Lancet Oncol. 15:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timmerman RD, Herman J and Cho LC:
Emergence of stereotactic body radiation therapy and its impact on
current and future clinical practice. J Clin Oncol. 32:2847–2854.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Radiation Therapy Oncology Group: Phase
II/III Study of Image-Guided Radiosurgery/SBRT for Localized Spine
Metastasis - RTOG CCOP Study. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0631
|
|
25
|
Milano MT, Katz AW, Zhang H and Okunieff
P: Oligometastases treated with stereotactic body RT: Long-term
follow-up of prospective study. Int J Radiat Oncol Biol Phys.
83:878–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salama JK, Kirkpatrick JP and Yin FF:
Stereotactic body RT treatment of extracranial metastases. Nat Rev
Clin Oncol. 9:654–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tree AC, Khoo VS, Eeles RA, Ahmed M,
Dearnaley DP, Hawkins MA, Huddart RA, Nutting CM, Ostler PJ and van
As NJ: Stereotactic body RT for oligometastases. Lancet Oncol.
14:e28–e37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iyengar P, Kavanagh BD, Wardak Z, Smith I,
Ahn C, Gerber DE, Dowell J, Hughes R, Abdulrahman R, Camidge DR, et
al: Phase II trial of stereotactic body radiation therapy combined
with erlotinib for patients with limited but progressive metastatic
non-small-cell lung cancer. J Clin Oncol. 32:3824–3830. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sweeney C, Chen YH, Carducci MA, Liu G,
Jarrard DF, Eisenberger MA, Wong YN, Hahn NM, Hohli M, Vogelzang
NJ, et al: Impact on overall survival (OS) with chemohormonal
therapy versus hormonal therapy for hormone-sensitive newly
metastatic prostate cancer (mPrCa): An ECOG-led phase III
randomized trial. J Clin Oncol. 32Suppl; 2014 ASCO Annual Meeting
Abstracts; abstr LBA2. (5s)2014.
|
|
30
|
Berkovic P, De Meerleer G, Delrue L,
Lambert B, Fonteyne V, Lumen N, Decaestecker K, Villeirs G, Vuye P
and Ost P: Salvage stereotactic body RT for patients with limited
prostate cancer metastases: Deferring androgen deprivation therapy.
Clin Genitourin Cancer. 11:27–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schroder FH, Hugosson J, Roobol MJ,
Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L,
Lilja H, et al: ERSPC Investigators: Screening and prostate cancer
mortality: results of the European Randomised Study of Screening
for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet.
384:2027–2035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tombal B: Castration-resistant prostate
cancer: Adaptation or clonal selection? Insight from the EORTC
30891 trial. Eur Urol. 66:839–840. 2014. View Article : Google Scholar : PubMed/NCBI
|