Introduction
There is significant controversy regarding target
region delineation by esophageal cancer radiotherapy in different
regions. In the RTOG85-01 randomized trial (1), irradiation of the entire esophagus for
esophageal cancer was recommended. In the RTOG94-05 trial (2), an area with a margin of 2–5 cm
surrounding the gross tumor volume (GTV) was defined as the
clinical target volume (CTV). The supraclavicular nodes were
included only when the tumor was located in the cervical esophageal
area. However, the results OF these two trials were not
satisfactory, as the survival and local control rates did not
improvE significantly. In the RTOG01-33 trial (3), three-dimensional conformal radiation
therapy (3D-CRT) technology was applied. The CTV included a 3-cm
margin around the GTV, while the planning target volume (PTV)
included A margin OF ≤2 cm around the CTV. Over the last few years,
an increasing number of studies have been conducted on elective
nodal irradiation (ENI) for esophageal cancer; however, their
conclusions have been inconsistent. It was previously reported
that, compared with involved-field irradiation (IFI), ENI does not
improve the local control rate OR long-term survival of patients
with esophageal cancer (4). Previous
studies have indicated that ENI may prevent regional lymph node
metastasis (5,6), whereas Other studies (7–9) reported
that the isolate out-of-field nodal failure rate was low and
overall survival did not decrease when IFI was used (6,7). Ji et
al (10) reported the results of
a prospective study ON 3D-CRT in 39 patients with esophageal cancer
without distant metastases, and revealed that lymph node stations
in close proximity to esophageal malignant tumors receive
considerable incidental radiation doses with IFI, which may
contribute to the elimination of subclinical lesions. The number of
studies quantifying Radiation dose to the corresponding lymph
drainage area using 3D-CRT with IFI for esophageal cancer is
currently limited. The present study aimed to determine the amount
of radiation delivered to the corresponding lymph drainage area in
patients with esophageal cancer treated at the fourth affiliated
hospital of hebei medical university using 3D-CRT with IFI.
Patients and methods
Clinical data
A retrospective analysis was conducted on 81
patients with pathologically confirmed esophageal squamous cell
carcinoma (ESCC). All the patients received 3D-CRT and IFI AT the
Department of radiation oncology of The Fourth Affiliated Hospital
of Hebei Medical University (Shijiazhuang, China) between October,
2000 and May, 2004. All the patients were admitted for initial
treatment and had complete clinical data, esophageal barium meal,
chest computed tomography (CT) scans and abdominal B ultrasound or
CT. For patients receiving radiotherapy, the doctors in our
department and the radiologists re-examined archived esophageal
barium meal films and chest CT scans. Based on case history and
radiographic data, all the cases were analyzed for reclassification
and staging, which was conducted according to the standardS of the
2002 Union for International Cancer Control esophageal cancer
staging (11), as shown in Table I.
 | Table I.Clinical data of esophageal cancer
patients (N=81). |
Table I.
Clinical data of esophageal cancer
patients (N=81).
| Observational
indices | ValueS |
|---|
| Gender (NO.) |
|
| Male | 59 |
|
Female | 22 |
| Age (years) |
|
|
Range | 37–81 |
|
Median | 63 |
| Location of lesion
(NO.) |
|
| Upper
thoracic segment | 25 |
| Middle
thoracic segment | 44 |
| Lower
thoracic segment | 12 |
| T stage (NO.) |
|
| T1 | 6 |
| T2 | 44 |
| T3 | 25 |
| T4 | 6 |
| N stage (NO.) |
|
| N0 | 55 |
| N1 | 26 |
| TNM stage (NO.) |
|
| I | 6 |
| IIa | 45 |
| IIb | 10 |
| IV | 20 |
| Length of lesion ON
X-ray (cm) |
|
|
Range | 2.0–16.0 |
|
Median | 6.0 |
| Length of lesion ON
CT (cm) |
|
|
Range | 2.7–16.0 |
|
Median | 7.2 |
| Maximal depth of
invasion ON CT (cm) |
|
|
Range | 1.4–5.5 |
|
Median | 2.8 |
| Planning target
volume (cm3) |
|
|
Range | 49.05–430.43 |
|
Median | 182.0 |
| Prescribed dose
(Gy) |
|
|
Range | 54–70 |
|
Median | 64 |
| IFI (GY) |
|
|
Range | 3–7 |
|
Median | 3 |
This study's protocol conformed to the principles of
the Declaration of Helsinki and was approved by the Ethics
Committee of Hebei Medical University. Written informed consent was
obtained from all the participants.
Conformal treatment planning
Posture fixation of the thermoplastic phantom was
followed by simulation CT scanning with 3–5 mm slices (CT
Brilliance; Philips Medical Systems, Amsterdam, The Netherlands).
Images were collected for digital transmission and 3D
reconstruction into the 3D-CRT planning system (Focus 3.0; CMS,
Woodlawn, MD, USA). Based on the length OF the lesion ON esophageal
imaging and fiber esophagoscopy, as well as the depth of invasion
shown ON the CT scan, lesions including the mediastinal lymph nodes
were defined as GTV. The CTV was defined as the GTV plus a
0.5–0.8-cm margin in the lateral and anteroposterior directions and
a 2.0–3.0-cm margin in the superoinferior direction of GTV, while
PTV encompassed 0.5–0.8-cm proximal and distal margins and a 1.0-cm
radial margin, based on the CTV. The PTV was also calculated. In
addition, adjacent tissues and organs, including the spinal cord,
trachea, heart and lungs, were delineated. the optimal treatment
was designed according to the isodose curve, such as the
dose-volume histogram (DVH) and two-dimensional image, and
delivered by 6-MV X-rays from a linear accelerator (Elekta Precise
Linear Accelerator; Elekta, Stockholm, Sweden). The prescribed dose
was 56–70 Gy, delivered in 28–35 doses over 6–7 weeks, with a
median dose of 64 Gy.
Delineation of lymph drainage
area
According to the grouping of chest lymph nodes by
the American Thoracic Society (12),
the lymph drainage area in 81 patients with esophageal cancer was
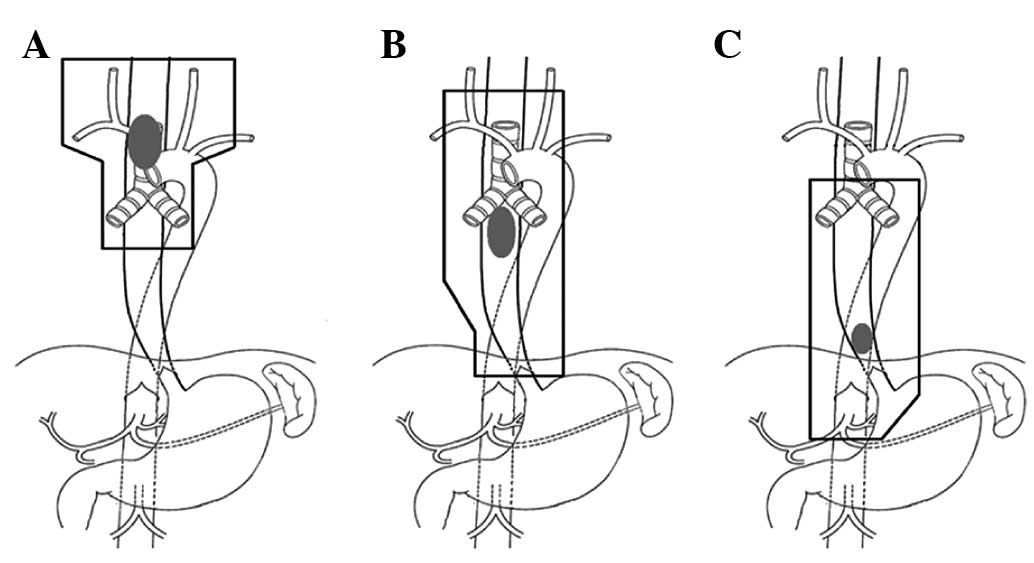
delineated. The upper thoracic lymph node drainage area included
the bilateral supraclavicular regions, as well as regions 2, 4, 5,
6, 7 and 8, and the lower boundary was a 4–5-cm margin around the
carina of the trachea; the lymph drainage area OF the middle
thoracic segment included regions 2, 4, 5, 6, 7 and 8 and the lower
boundary was the cardiac lymph node area; the drainage area OF the
lower thoracic segment included regions 4, 5, 6, 7 and 8, as well
as the lymph nodes of the paracardia, lesser gastric curvature and
left gastric area (Fig. 1). The
delineated lymph drainage area included the PTV, which was defined
as the CTV including clinically positive lymph nodes (CTV-N). PTV-N
was defined as the CTV-N plus a 0.3–0.5-cm margin in the lateral
and anteroposterior directions and a 1.0–1.5-cm margin in the
superoinferior direction. Based on the DVH in the treatment plan,
we calculated the volume percentage when PTV-N received radiation
doses of 30, 35, 40, 45 and 50 Gy, I.E., VPTV-N30,
VPTV-N35, VPTV-N40, VPTV-N45 and
VPTV-N50, respectively.
Follow-up
All the patients were followed up until December 31,
2012. Patients receiving radiotherapy were followed up for 3–118
months, with a median follow-up time of 20 months. A total of 55,
27 and 18% of the cases were followed up for 1, 3 and 5 years,
respectively, with a follow-up rate of 100%.
Statistical analysis
SPSS 11.5 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The normal distribution
of data required for observation in each group was assessed.
One-way analysis of variance (ANOVA) was used for comparison among
different groups and the Spearman'S correlation test was used for
comparison between groups. The Kaplan-Meier method was applied to
analyse survival and local control rates, and the two-tailed
log-rank method was used TO assess significance. A multivariate
analysis was conducted using the Cox proportional hazards model to
assess the independent prognostic factors. P<0.05 was considered
to indicate statisticalLY significant differences.
Results
Overall survival
At the last date of follow-up, 9 patients remained
alive, with 1-, 3-, 5- and 8-year survival rates of 67.90, 33.33,
20.99 and 11.11%, respectively. The 1-, 3-, 5- and 8-year local
control rates were 76.59, 45.82, 35.81 and 20.14%, respectively.
The univariate analysis revealed that the prognostic factors
affecting patient survival rate included dietary intake and
hoarseness prior to treatment, clinical T, N and TNM stage, length
of esophageal lesions on barium meal X-ray, maximal diameter OF
lesions on CT and immediate curative effect (Table II). The multivariate analysis
revealed that the independent prognostic factors were dietary
intake prior to treatment (χ2=12.062, P=0.001),
hoarseness prior to treatment (χ2=7.656, P=0.006),
clinical TNM stage (χ2=4.462, P=0.031) and X-ray length
of esophageal lesions ON barium meal X-ray (χ2=5.360,
P=0.021) (data not shown).
 | Table II.Univariate analysis results of 81
esophageal cancer patients treated with involved-field
irradiation. |
Table II.
Univariate analysis results of 81
esophageal cancer patients treated with involved-field
irradiation.
|
|
| Survival rate
(%) |
|
|
|---|
| Variables | NO. | 1-year | 3-year | 5-year | 8-year | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
1.07 |
0.2999 |
|
Male | 59 | 71.19 | 33.90 | 22.03 | 13.56 |
|
|
|
Female | 22 | 59.09 | 31.82 | 18.18 |
4.55 |
|
|
| Age (years) |
|
|
|
|
|
0.21 |
0.6438 |
|
≤60 | 37 | 62.16 | 27.03 | 24.32 | 10.81 |
|
|
|
>60 | 44 | 72.73 | 38.64 | 18.18 | 11.36 |
|
|
| Dietary intake
prior to treatment |
|
|
|
|
|
9.28 |
0.0023 |
|
Normal | 24 | 79.17 | 54.17 | 41.67 | 25.00 |
|
|
|
Abnormal | 57 | 63.16 | 24.56 | 12.28 |
5.26 |
|
|
| Chest and back
pain |
|
|
|
|
|
2.85 |
0.0913 |
| No | 60 | 71.67 | 36.67 | 23.33 | 13.33 |
|
|
|
Yes | 21 | 57.14 | 23.81 | 14.29 |
4.76 |
|
|
| Hoarseness prior to
treatment |
|
|
|
|
|
5.76 |
0.0164 |
| No | 72 | 69.44 | 37.50 | 23.61 | 12.50 |
|
|
|
Yes | 9 | 55.56 |
0.00 |
0.00 |
0.00 |
|
|
| Location of
lesion |
|
|
|
|
|
1.47 |
0.4792 |
| Upper
thoracic segment | 25 | 64.00 | 32.00 | 24.00 |
8.00 |
|
|
| Middle
thoracic segment | 44 | 72.73 | 38.64 | 20.45 | 13.64 |
|
|
| Lower
thoracic segment | 12 | 58.33 | 16.67 |
8.33 |
8.33 |
|
|
| T stage |
|
|
|
|
| 21.55 |
0.0001 |
| T1 | 6 | 83.33 | 83.33 | 33.33 | 33.33 |
|
|
| T2 | 44 | 79.55 | 40.91 | 29.55 | 11.36 |
|
|
| T3 | 25 | 52.00 | 16.00 |
8.00 |
8.00 |
|
|
| T4 | 6 | 33.00 |
0.00 |
0.00 |
0.00 |
|
|
| N stage |
|
|
|
|
| 14.80 |
0.0001 |
| N0 | 55 | 76.36 | 43.64 | 29.09 | 16.36 |
|
|
| N1 | 26 | 50.00 | 11.54 |
3.85 |
0.00 |
|
|
| TNM stage |
|
|
|
|
| 23.29 | <0.0001 |
| I | 6 | 83.33 | 83.33 | 33.33 | 33.33 |
|
|
|
IIa | 45 | 77.78 | 40.00 | 28.89 | 13.33 |
|
|
|
IIb | 10 | 70.00 | 30.00 | 20.00 | 10.00 |
|
|
|
III | 20 | 40.00 |
5.00 |
0.00 |
0.00 |
|
|
| Length of lesion on
esophageal barium meal X-ray (cm) |
|
|
|
|
| 21.15 | <0.0001 |
|
≤5.0 | 24 | 83.33 | 70.83 | 37.50 | 20.83 |
|
|
|
5.0–9.0 | 47 | 63.96 | 21.28 | 17.02 |
8.51 |
|
|
|
>9.0 | 10 | 40.00 |
0.00 |
0.00 |
0.00 |
|
|
| Max diameter of
lesion ON CT (cm) |
|
|
|
|
|
5.31 |
0.0213 |
|
≤4.0 | 54 | 72.22 | 42.59 | 27.78 | 12.96 |
|
|
|
>4.0 | 27 | 59.26 | 14.81 |
7.41 |
7.41 |
|
|
| Prescribed dose
(Gy) |
|
|
|
|
|
0.06 |
0.8095 |
|
≤64 | 38 | 65.79 | 26.32 | 21.05 | 15.79 |
|
|
|
>64 | 43 | 69.77 | 39.53 | 20.93 |
6.98 |
|
|
| Immediate curative
effect |
|
|
|
|
| 13.09 |
0.0114 |
|
Complete response | 34 | 76.47 | 52.94 | 41.18 | 25.53 |
|
|
| Partial
response | 35 | 57.14 | 22.86 |
8.57 |
2.86 |
|
|
| No
response | 12 | 75.00 |
8.33 |
0.00 |
0.00 |
|
|
DVH analysis of PTV-N
The corresponding lymph drainage area in the 81
patients was delineated without altering the originally delineated
GTV, CTV, PTV and prescribed dose, thereby obtaining CTV-N and
PTV-N. In the absence of additional treatment, available
radiotherapy planning and DVH were used to calculate the minimal,
maximal and median valueS, as well as the mean ± standard deviation
of VPTV-N30, VPTV-N35, VPTV-N40,
VPTV-N45 and VPTV-N50 (Table III).
 | Table III.Dose-volume histogram analysis
results of PTV-N. |
Table III.
Dose-volume histogram analysis
results of PTV-N.
| Values | VPTV-N30
(%) | VPTV-N35
(%) | VPTV-N40
(%) | VPTV-N45
(%) | VPTV-N50
(%) |
|---|
| Minimum | 16 | 13 | 10 | 6 | 0 |
| Maximum | 99 | 98 | 98 | 98 | 97 |
| Median | 73 | 70 | 67 | 64 | 58 |
| Mean ± standard
deviation | 71.35±1.89 | 68.70±1.87 | 65.84±1.84 | 62.40±1.97 | 56.83±2.00 |
Correlation analysis of
VPTV-NX and associated factors
The results demonstrated that the prescribed dose
size exhibited no correlation with VPTV-N30-35, but WAS
significantLY correlatED with VPTV-N40-50; the
irradiation field exhibited no correlation with
VPTV-N30-45, but was significantly correlated with
VPTV-N50; the length of the lesion on esophageal barium
meal X-ray and PTV were significantly correlated with
VPTV-N30–50 (Table
IV).
 | Table IV.Correlation analysis of
VPTV-NX and associated factors |
Table IV.
Correlation analysis of
VPTV-NX and associated factors
|
| Prescribed
dose | Irradiation
field | Length of lesion on
esophageal barium meal X-ray | PTV volume |
|---|
|
|
|
|
|
|
|---|
|
VPTV-NX | r | P-value | r | P-value | r | P-value | r | P-value |
|---|
|
VPTV-N30 | 0.183 | 0.215 | 0.870 | 0.438 | 0.258 | 0.020 | 0.679 | 0.013 |
|
VPTV-N35 | 0.101 | 0.054 | 0.070 | 0.535 | 0.288 | 0.009 | 0.745 | 0.008 |
|
VPTV-N40 | 0.246 | 0.027 | 0.090 | 0.425 | 0.322 | 0.003 | 0.730 | 0.007 |
|
VPTV-N45 | 0.336 | 0.002 | 0.114 | 0.313 | 0.339 | 0.002 | 0.851 | 0.001 |
|
VPTV-N50 | 0.438 | 0.000 | 0.223 | 0.045 | 0.389 | 0.000 | 0.937 | 0.000 |
Association between VPTV-NX
and lesion location
The anova demonstrated that the values of
VPTV-N30 and VPTV-N35 were significantLY
differenT due to the different locations of the lesions; however,
no correlation was observed with VPTV-N40,
VPTV-N45 OR VPTV-N50 (Table V). Further stratification analysis
suggested that the values of VPTV-N30 and
VPTV-N35 OF the upper and middle thoracic segments
exhibited significant differences compared with those OF the lower
thoracic segment (P-values OF 0.023, 0.021 and 0.029, 0.038,
respectively); the values of VPTV-N40 OF the
upper/middle and the lower thoracic segmentS were similar,
approaching statistical significanCE (P-values OF 0.050 and 0.056,
respectively), while the values of VPTV-N45 and
VPTV-N50 OF the upper/middle and the lower thoracic
segmentS did not differ significantLY (P-values OF 0.094, 0.131 and
0.427, 0.525, respectively). No statistical difference was observed
in VPTV-N30–50 between the middle and lower thoracic
segments (P-values OF 0.449, 0.572, 0.580, 0.732 and 0.941,
respectively).
 | Table V.One-way analysis of variance results
of VPTV-NX and different location of the lesions. |
Table V.
One-way analysis of variance results
of VPTV-NX and different location of the lesions.
| VPTV-NX
(%) | Thoracic
segment | Mean ± SD | F | P-value | 95% confidence
interval |
|---|
|
VPTV-N30 |
Upper | 78.56±11.48 | 3.762 | 0.028 | 73.82–83.30 |
|
| Middle | 69.00±18.14 |
|
| 63.18–74.52 |
|
|
Lower | 64.92±18.65 |
|
| 53.07–76.77 |
|
VPTV-N35 |
Upper | 75.48±12.05 | 3.227 | 0.045 | 70.51–80.45 |
|
| Middle | 66.36±17.74 |
|
| 60.97–71.76 |
|
|
Lower | 63.33±18.96 |
|
| 51.29–75.38 |
|
VPTV-N40 |
Upper | 71.88±12.05 | 2.659 | 0.076 | 66.90–76.86 |
|
|
Middle | 63.77±17.70 |
|
| 58.39–69.16 |
|
|
Lower | 60.83±18.05 |
|
| 49.37–72.30 |
|
VPTV-N45 |
Upper | 67.84±12.91 | 1.795 | 0.173 | 62.51–73.17 |
|
| Middle | 60.39±19.63 |
|
| 54.42–66.35 |
|
|
Lower | 58.42±17.89 |
|
| 47.05–69.78 |
|
VPTV-N50 |
Upper | 59.40±13.88 | 0.366 | 0.695 | 63.67–65.13 |
|
| Middle | 55.77±20.25 |
|
| 49.61–61.93 |
|
|
Lower | 55.33±17.66 |
|
| 44.12–66.55 |
Analysis resultS of VPTV-NX
and survival rate
Based on previous studies, grouping was performed
according to the different values of VPTV-NX, in order
to compare survival rates. The results revealed no significant
effect on long-term patient survival (Table VI).
 | Table VI.Analysis results of
VPTV-NX and survival rate. |
Table VI.
Analysis results of
VPTV-NX and survival rate.
|
|
|
| Survival rate
(%) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Observational
indices | Grouping (%) | n | 1-year | 3-year | 5-year | 8-year | χ2 | P-value |
|---|
|
VPTV-N30 | ≥83.3 | 24 | 66.67 | 29.17 | 20.83 |
4.17 | 0.86 | 0.3534 |
|
| <83.3 | 57 | 68.42 | 35.09 | 21.05 | 17.22 |
|
|
|
VPTV-N35 | ≥71.5 | 40 | 70.00 | 35.00 | 22.50 |
8.57 | 0.05 | 0.8222 |
|
| ≥71.5 | 41 | 65.85 | 31.71 | 19.51 | 17.07 |
|
|
|
VPTV-N40 | ≥62.5 | 51 | 68.63 | 35.29 | 25.49 | 12.83 | 0.17 | 0.6798 |
|
| <62.5 | 30 | 66.67 | 30.00 | 13.33 | 13.33 |
|
|
|
VPTV-N45 | ≥55.6 | 59 | 66.10 | 35.59 | 23.73 | 12.85 | 0.02 | 0.9002 |
|
| <55.6 | 22 | 72.73 | 27.27 | 13.64 | 13.63 |
|
|
|
VPTV-N50 | ≥50.0 | 57 | 70.18 | 36.84 | 26.32 | 15.11 | 1.37 | 0.2412 |
|
| <50.0 | 24 | 62.50 | 25.00 |
8.33 |
8.33 |
|
|
Discussion
The main causes of radiotherapy treatment failure of
esophageal cancer are local recurrence and distant metastasis, with
the former mainly characterized by lymph node and esophageal
recurrence. Therefore, radiotherapists face the challenge of
identifying methods to effectively reduce the local recurrence
rate, thereby improving their long-term survival rate. In recent
years, A number OF studies on radiotherapy combined with ENI have
been conducted. ENI aims to reduce the local recurrence rate,
particularly that in regional lymph nodes (5,6). However,
certain studies demonstrated that, in patients who underwent
radiotherapy rather than ENI, the lymph node recurrence rate
outside the field was 2–8% (7,8). A number
of studies in China and abroad also demonstrated that, compared
with ENI, the toxicity of which was considered acceptable for the
majority of the patients, the recurrence rate and distant
metastasis in patients treated with IFI was not notably increaseD
(4,13,14).
Therefore, regarding patients treated with IFI, it remains unclear
how they should be irradiated in order to control the recurrence of
regional lymph nodes. It was previously demonstrated that, when the
corresponding lymph drainage area of esophageal cancer is exposed
to a lower dose of Radiation, the local metastasis rate decreases
and that an irradiation dose of 24 Gy may help reduce the
metastasis rate by 30–50% (13,14). Ji
et al (10) investigated 39
patients with stage T0–4N0M0 ESCC
who were treated with IFI and demonstrated that, when ESCC patients
were treated exclusively by 3D-CRT with IFI, adjacent lymph
drainage areas were also exposed to a high dose of Radiation, which
may play a role in controlling subclinical metastasis.
The postoperative pathologicAL findings of
esophageal cancer play a decisive role in delineating the
prophylactic irradiation range ensured by ENI. In recent years,
following the analysis of the lymph node metastasis in ESCC,
several studies have suggested that the level of lymph node
metastasis is associated with primary tumor location. Nakamura
et al (15) investigated 95
patients with stage T1–3N0M0
esophageal cancer based on preoperative clinical staging,
recommending corresponding lymph node irradiation based on primary
tumor location (no prophylactic irradiation of abdominal lymph
nodes in patients with upper and middle thoracic esophageal cancer,
and no prophylactic irradiation of the supraclavicular area in
patients with middle and lower thoracic esophageal cancer). Huang
et al (5) analyzed 1,077 cases
with lymph node metastasis following surgery for ESCC. The results
demonstrated that the lymph node metastasis rate OF upper thoracic
esophageal cancer to the neck, upper, middle and lower mediastinum
and abdominal area were 16.7, 38.9, 11.1, 5.6 and 5.6%,
respectively; for ESCC of the middle thoracic segment, the
respective rates were 4.0, 3.8, 32.9, 7.1 and 17.1%; for the lower
thoracic segment, the respective rates were 1.0, 3.0, 22.7, 37.0
and 3%. That study recommended that, in the delineated lymph node
drainage area, the upper boundary of the upper thoracic segment
should be the cervical esophageal area and the bilateral
supraclavicular area lymph nodes, whereas the lower boundary should
involve the lymph nodes under the tracheal carina; the lymph nodes
under the carina should be the upper boundary of the lower thoracic
segment, with the stomach, hepatic artery and adjacent lymph nodes
as the lower boundary. Prophylactic irradiation of the
corresponding lymph node area in patients with middle thoracic
esophageal cancer should be determined by tumor location, with the
mediastinal lymph drainage area being included in patients in a
good overall condition. In the present study, the irradiation dose
to the corresponding lymph drainage area was investigated in 81
patients who received 3D-CRT with IFI. The results demonstrated
that the corresponding lymph drainage areas were exposed to 30–50
Gy of radiation in varying degrees, referred TO AS incidental
irradiation dose (IID). The results of the study also indicated
that the median IID of VPTV-N35, VPTV-N40,
VPTV-N45 and VPTV-N50 was >24 Gy.
Compared with patients with middle and lower
thoracic esophageal cancer, patients with upper thoracic esophageal
cancer exhibit a relatively early onset of clinical symptoms.
Therefore, the majority of those patients seek treatment when the
tumor body is small, while the majority of patients with middle or
lower thoracic esophageal cancer have unresectable tumors at
presentation and receive radiation therapy. As regardS earlier
esophageal cancer, higher clinically prescribed doses are generally
administered for radical treatment. To avoid damage to normal
tissues and organs, the prescribed dose is generally lower for
larger tumors compared with that for smaller tumors. Furthermore,
due to the anatomical structure of the lungs, the prescribed doses
may be higher in patients with upper thoracic esophageal cancer
compared with those with middle or lower thoracic cancer, thus
preventing increased radiation dose to the lungs. The present
results suggest that the mean IID in patients with upper thoracic
esophageal cancer was higher compared with that in patients with
middle or lower thoracic esophageal cancer, with statistically
significant differences between the radiation dose of
VPTV-N30 and VPTV-N35, which may be
associated with the higher dose received by patients with upper
thoracic esophageal cancer. The present findings also indicate that
VPTV-NX was significantly correlated with the prescribed
dose. When the esophageal lesions are longer, the PTV length and
volume are accordingly larger. The present study demonstrated that
VPTV-NX was significantly correlated with the length of
esophageal lesions AS determined by X-ray and the PTV. This was
consistent with the results reported by Ji et al (10), suggesting that PTV and length of
esophageal lesions exhibited a linear correlation with the
equivalent uniform dose.
The results OF the present study indicate that it is
not beneficial for patients to expose the lymph drainage area to
higher IID in terms of survival. This may be explained as follows:
i) Higher IID received by patients may compromise immunity to some
extent, which may be detrimental to prognosis in long-term
surviving patients; ii) esophageal cancer recurrence beyond the
irradiatED field is not a major cause of treatment failure. Higher
recurrence rate within the field and distant metastasis may be
offset by the survival benefit achieved by higher IID; iii) the
irradiated field is larger in patients receiving higher IID,
compared with those receiving lower IID. It is likely that the
larger field will result in more extensive radiation injury, which
may reduce the survival benefit achieved by higher IID.
In conclusion, the curative effect of IFI on
esophageal cancer was not significantly reduced. AS regardS
dosimetry, this may be associated with the hypothesis that the
corresponding lymph drainage area in patients treated with IFI may
be exposed to different radiation doseS, which may play a
preventive role. In the clinical setting, A proportion of patients
may present with local recurrence or distant metastasis prior to
regional lymph node recurrence, thereby masking the regional lymph
node recurrence. Therefore, further confirmation is required as to
whether IID in esophageal cancer using 3D-CRT with IFI may play a
preventive role in regional lymph node metastasis.
References
|
1
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: Long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajani JA, Winter K, Komaki R, et al: Phase
II randomized trial of two nonoperative regimens of induction
chemotherapy followed by chemoradiation in patients with localized
carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 26:4551–4556.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma JB, Song YP, Yu JM, Zhou W, Cheng EC,
Zhang XQ and Kong L: Feasibility of involved-field conformal
radiotherapy for cervical and upper-thoracic esophageal cancer.
Onkologie. 34:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang W, Li B, Gong H, et al: Pattern of
lymph node metastases and its implication in radiotherapeutic
clinical target volume in patients with thoracic esophageal
squamous cell carcinoma: A report of 1077 cases. Radiother Oncol.
95:229–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Liu S, Pan J, et al: The pattern
and prevalence of lymphatic spread in thoracic oesophageal squamous
cell carcinoma. Eur J Cardiothorac Surg. 36:480–486. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao KL, Ma JB, Liu G, Wu KL, Shi XH and
Jiang GL: Three-dimensional conformal radiation therapy for
esophageal squamous cell carcinoma: is elective nodal irradiation
necessary? Int J Radiat Oncol Biol Phys. 76:446–451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Button MR, Morgan CA, Croydon ES, Roberts
SA and Crosby TD: Study to determine adequate margins in
radiotherapy planning for esophageal carcinoma by detailing
patterns of recurrence after definitive chemoradiotherapy. Int J
Radiat Oncol Biol Phys. 73:818–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawaguchi Y, Nishiyama K, Miyagi K, Suzuki
O, Ito Y and Nakamura S: Patterns of failure associated with
involved field radiotherapy in patients with clinical stage I
thoracic esophageal cancer. Jpn J Clin Oncol. 41:1007–1012. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji K, Zhao LJ, Yang CW, Meng M and Wang P:
Three-dimensional conformal radiation for esophageal squamous cell
carcinoma with involved-field irradiation may deliver considerable
doses of incidental nodal irradiation. Radiat Oncol. 7:2002012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours (6th). Hoboken, NJ: John Wiley
& Sons. 2002.
|
|
12
|
Korst RJ, Rusch VW, Venkatraman E, Bains
MS, Burt ME, Downey RJ and Ginsberg RJ: Proposed revision of the
staging classification for esophageal cancer. J Thorac Cardiovasc
Surg. 115:660–669; discussion, 669–670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Withers HR, Peters LJ and Taylor JM:
Dose-response relationship for radiation therapy of subclinical
disease. Int J Radiat Oncol Biol Phys. 31:353–359. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Withers HR, Peters LJ and Taylor JM:
Dose-response for subclinical diseas E - In response to Dr.
Ben-Josef. Int J Radiat Oncol Biol Phys. 32:1267–1268. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura T, Hatooka S, Kodaira T, et al:
Determination of the irradiation field for clinical T1-T3N0M0
thoracic/abdominal esophageal cancer based on the postoperative
pathological results. Jpn J Clin Oncol. 39:86–91. 2009. View Article : Google Scholar : PubMed/NCBI
|