Introduction
Non-small-cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality worldwide. At the time of
diagnosis, the majority of patients are already in the advanced
stages of inoperable disease (1,2). Disease
prevention, early diagnosis and cure rate have essentially remained
unchanged during the past couple of decades, with a five-year
survival rate for non-small cell lung cancer of 9–14% (3). Although platinum-based chemotherapy is
the first-line treatment for advanced NSCLC, the response rate is
only 20-35%, with a median survival time of ~10 months (4), indicating that a high proportion of
advanced NSCLC patients are resistant to platinum-based
chemotherapy. Accumulating evidence suggests that molecularly
targeted therapies can individualize the treatment of NSCLC
patients; however, these therapies depend upon the identification
and validation of potent predictive biomarkers (2). Recent pharmacogenetic studies have
demonstrated that individual variation in genetic background
strongly influences the response of cancer patients to chemotherapy
and radiotherapy (5,6).
BCL2-associated athanogene-1 (BAG-1) is
multifunctional protein that interacts with a wide range of
cellular targets to regulate growth control pathways important for
normal and malignant cells, including apoptosis, signaling,
proliferation, transcription and cell motility. Of particular
relevance to tumor cells, BAG-1 interacts with the anti-apoptotic
BCL-2 protein, various nuclear hormone receptors and the 70 kDa
heat shock proteins, Hsc70 and Hsp70. Interaction with chaperones
may account for many of the pleiotropic effects associated with
BAG-1 overexpression (7). BAG-1 has
also been identified as a multifunctional pro-survival protein
(8,9)
that represses endoplasmic reticulum (ER) stress-induced apoptosis
(10). BAG-1 can repress apoptosis
induced by the stimulation of Fas and TRAIL death-receptors, kinase
inhibitors, withdrawal of serum and cytokines from sensitive cells,
heat shock, dexamethasone, radiation and anti-cancer agents, such
as cisplatin and etopiside. Analysis of BAG-1 isoforms revealed
that they all possess pro-survival activity. For example, previous
studies have reported that inhibition of BAG1 expression prolongs
the lifespan of NSCLC patients (11,12).
Although the activity of BAG-1 is predominantly cytoprotective,
negative effects of BAG-1 on cell survival have also been described
(9). Similarly, in a rodent model of
NSCLC, reduced expression of BAG1 specifically promoted the
apoptosis of tumor cells and delayed tumorigenesis (13).
The present study assessed the association between
BAG1 expression levels and sensitivity to platinum-based
chemotherapy in NSCLC patients. In addition, the involvement of
BAG1-mediated ER stress in cisplatin-induced cell death was
investigated using in vitro cultured human lung
adenocarcinoma A549 cells.
Patients and methods
Patients
Between January 2009 and May 2010, 108 untreated
patients with NSCLC [American Joint Committee on Cancer stages
I–IIIA (14)] were recruited at the
Department of Oncology of the First Affiliated Hospital of Liaoning
Medical University (Jinzhou, China). All patients had a Karnofsky
Performance Status score (15) of
≥70. The median age of the patients was 62 years (range, 43–81
years). Tumors were identified by cytological and histological
examinations, with the tumor mass observed and measured by computed
tomography or magnetic resonance imaging. Prior to chemotherapy,
blood, liver and renal functions, and electrocardiogram results
were within the normal range. The Ethics Committee of the First
Affiliated Hospital of Liaoning Medical University approved the
study protocol, and written informed consent was obtained from each
participant.
Reagents
Tunicamycin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Methyl thiazolyl tetrazolium (MTT) assay was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Cisplatin
was provided by Dezhou Deyao Pharmaceutical, Co., Ltd. (Dezhou,
China). Cisplatin was obtained from China Otsuka Pharmaceutical
Co., Ltd. (Tianjin, China). Vinorelbine (NVB) was obtained from
Jiangsu Hansen Pharmaceutical Co., Ltd. (Lianyungang, China).
RPMI-1640 culture medium was obtained from Gibco Life Technologies
(Carlsbad, CA, USA). A polyclonal rabbit anti-mouse antibody
against 3 isoforms of BAG1 [p50 (BAG1L), p46 (BAG1M) and p33
(BAG1S)] (cat. no. sc-939) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against
procaspase-12 (rabbit anti-mouse polyclonal; cat. no. sc-5627),
glucose-regulated protein 78 (GRP78; rabbit anti-human polyclonal;
cat. no. sc-13968) and β-actin (mouse anti-avian monoclonal; cat.
no. sc-47778) were purchased from Santa Cruz Biotechnology, Inc.
The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) staining kit was obtained from Biouniquer Technology (Nanjing,
China).
Chemotherapy and response
evaluation
Day 1 was defined as the first day on which the
chemotherapeutic agents were administered. Patients received a
regular NVB+cisplatin regimen, with intravenous infusion of
cisplatin (30 mg/m2) on days 2, 3, and 4 and NVB (25
mg/m2) on days 1 and 8; this was repeated every 3 weeks.
The outcomes of chemotherapy were assessed according to the
Response Evaluation Criteria in Solid Tumors (RECIST) (16) after four cycles. The overall responses
to chemotherapy were classified as complete response, partial
response, stable disease or progressive disease. Chemotherapy was
considered efficacious if responses were complete or partial,
whilst stable or progressive disease was defined as
ineffective.
Immunohistochemistry
The expression of BAG1 in tumor tissues from
patients was detected by immunohistochemistry. The 10%
formalin-fixed and paraffin-embedded tissue sections were stained
with anti-BAG1 antibodies at a dilution of 1:150. The
immunoreactivity was visualized using an SP immunohistochemistry
staining kit, in accordance with the manufacturer's instructions
(Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing,
China), and an IX70 inverted microscope (Olympus Corporation,
Tokyo, Japan).
Therapeutic evaluation and survival
analysis
Therapeutic effect was evaluated at two or three
weeks following chemotherapy based on RECIST. The overall survival
(OS) time of individual patients was defined from the day of
surgery up until the final follow-up examination (July 31, 2012);
median progression-free survival (PFS) and OS were plotted.
Cell culture
Human lung adenocarcinoma A549 cells were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Cells were maintained in RPMI-1640 medium containing 10%
fetal bovine serum (NQBB International Biological Corporation,
Guangzhou, China), and incubated in an atmosphere of 5%
CO2 in a humidified incubator (Sheldon Manufacturing,
Inc., Cornelius, OR, USA) at 37°C. Cells in logarithmic growth
phase were used for experiments.
Evaluation of cell proliferation
To evaluate cell proliferation, an MTT assay was
performed. A549 cells were seeded into a 96-well plate at a density
of 6×103 cells/well. At 24 h after seeding, cells were
treated with various concentrations of tunicamycin (1.25–10 µg/ml)
for 8 h. For combined treatment, cells were treated with 1.25 µg/ml
tunicamycin for 8 h followed by 24 h of cisplatin treatment
(1.25–40 µg/ml). Cells without drug treatment were used as the
negative control. Five wells were examined for each group.
Subsequent to the chemotherapeutic treatments, 20 µl
MTT solution (5 mg/ml) was added to each well. Following 4 h of
incubation at 37°C, the medium was removed and 200 µl dimethyl
sulfoxide (MP Biomedicals, LCC, Santa Ana, CA, USA) was added to
each well to resuspend the MTT metabolic product. The absorbance of
the dissolved formazan was measured at 492 nm (A492) using a
microplate spectrophotometer (Model 500, Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The rate of growth inhibition was
determined using the following formula: Growth inhibition rate (%)
= (1 - [A492Sample/A492Control] × 100%. The
half maximal inhibitory concentration (IC50) was
calculated using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA).
Determination of cell apoptosis
Cell apoptosis was determined via flow cytometric
analysis. Briefly, cells were treated with 1.25 µg/ml tunicamycin
for 8 h followed by a 24-h incubation with cisplatin (1.25, 2.5, or
40 µg/ml). Cells were collected by centrifugation (100 × g for 5
min at 4°C) and (1–5)×105 cells were resuspended in
Annexin V Binding Buffer. Subsequently, 5 µl Annexin V-FITC and 5
µl PI were added to the cell suspension and incubated at room
temperature for 10 min in the dark. Apoptosis was analyzed by flow
cytometry (BD FACSVerse™; BD Biosciences).
Western blot analysis
Following the drug treatments, cells were washed
with ice-cold phosphate-buffered saline and incubated with
radioimmunoprecipitation assay buffer (Beijing BLKW Biotechnology
Co., Ltd., Beijing, China). The protein was collected and the
protein concentration was determined by Lowry assay (17). The total cell protein was resolved by
15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a nitrocellulose membrane (eBioscience, Inc., San
Diego, CA, USA). Membranes were blocked with 5% non-fat milk for 1
h at room temperature, followed by incubation with primary
antibodies against BAG1 (BAG1L, BAG1M and BAG1S; dilution, 1:500),
GRP78 (dilution, 1:500), procaspase-12 (dilution, 1:500) or β-actin
(dilution, 1:200) overnight at 4°C. After washing three times with
Tris-buffered saline containing 0.1% Tween, membranes were probed
with horseradish peroxidase-conjugated secondary antibody
(monoclonal mouse anti-rabbit; cat. no. sc-51625; dilution, 1:800;
Santa Cruz Biotechnology, Inc.) at 37°C for 30 min. Proteins were
detected using an electrochemiluminescence assay (Shanghai Rong Wei
Industrial Co., Ltd.,Shanghai, China).
Statistical analyses
Statistical analyses were performed using SPSS 17.0
software. Comparisons between two groups of subjects were made
using the χ2 test. Survival rate was calculated through
Kaplan-Meier analysis. Differences between factors were evaluated
by log-rank test and a Cox regression analysis was used for
determining prognostic factors. Data calculated from three
independent experiments were presented as the mean ± standard
deviation and a Student's t-test was performed to compare two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
BAG1 expression is associated with
differentiation stage
The potential correlation between BAG1 expression
and clinicopathological characteristics of NSCLC patients was
evaluated. The results indicated that expression of BAG1 was
closely associated with differentiation stage, as BAG1 expression
was higher in patients with moderate/high differentiation compared
with that of patients with poorly differentiated tumors (P=0.041;
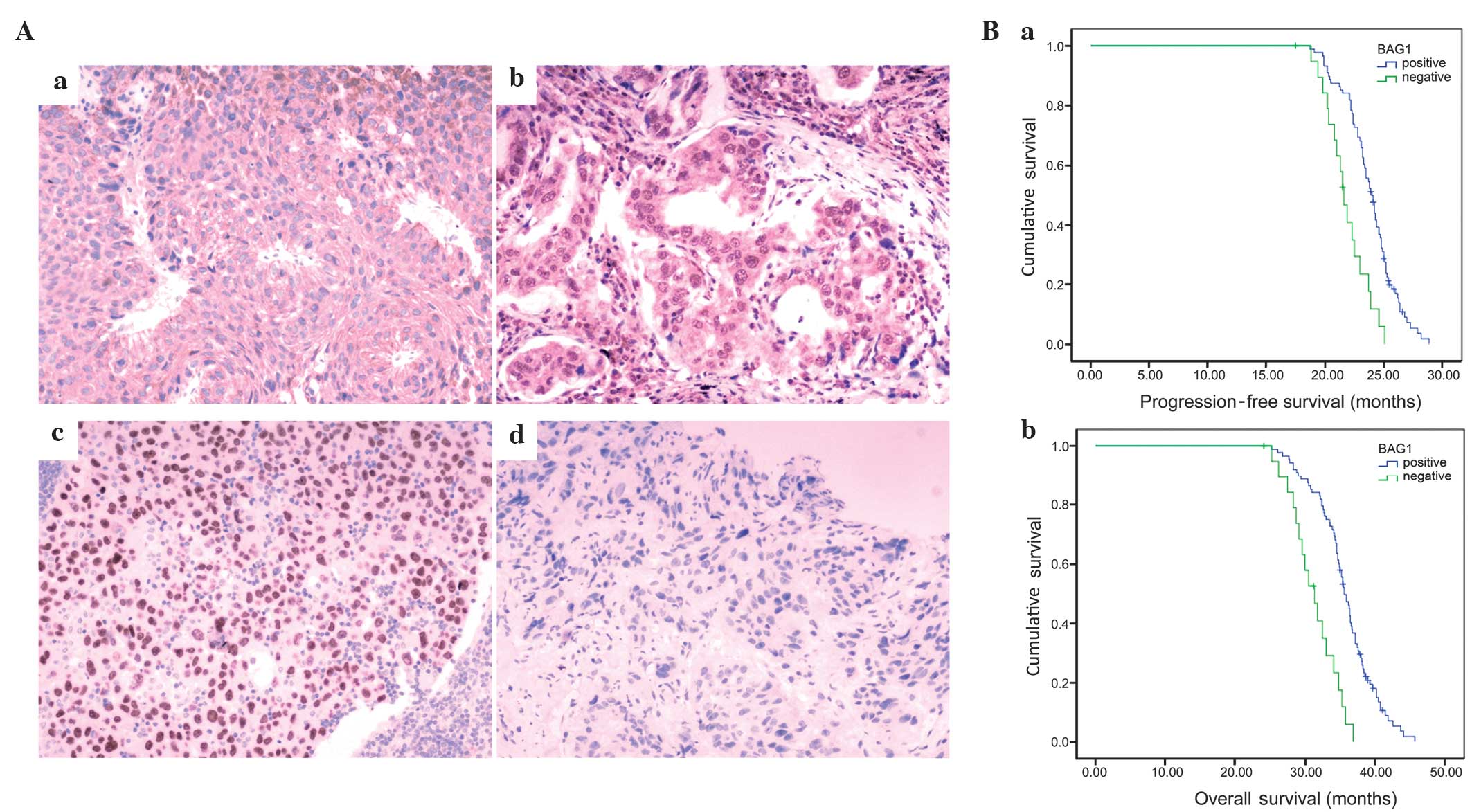
Table I, Fig. 1A). However, no significant difference
in BAG1 expression was identified based on gender, age,
pathological type, clinical stage or lymph node metastasis status
(P>0.05, Table I).
 | Table I.Correlation between BAG1
chemosensitivity and clinicopathological features of non-small-cell
lung cancer patients (n=108). |
Table I.
Correlation between BAG1
chemosensitivity and clinicopathological features of non-small-cell
lung cancer patients (n=108).
|
| BAG1, n |
|
|
|---|
|
|
|
|
|
|---|
| Features | Positive | Negative | χ2 | P-value |
|---|
| Gender |
|
| 0.462 | 0.497 |
| Male | 47 | 9 |
|
|
|
Female | 41 | 11 |
|
|
| Age, years |
|
| 2.681 | 0.102 |
|
<60 | 57 | 9 |
|
|
| ≥60 | 31 | 11 |
|
|
| Smoking history |
|
| 3.377 | 0.066 |
| Yes | 32 | 10 |
|
|
| No | 56 | 10 |
|
|
| Pathology |
|
| 0.022 | 0.882 |
|
Squamous-cell carcinoma | 38 | 9 |
|
|
|
Adenocarcinoma | 50 | 11 |
|
|
| Tumor
differentiation |
|
| 6.376 | 0.041 |
|
High | 38 | 4 |
|
|
|
Medium | 23 | 4 |
|
|
|
Low | 27 | 2 |
|
|
| Tumor stage |
|
| 0.700 | 0.705 |
| I | 35 | 6 |
|
|
| II | 28 | 7 |
|
|
|
IIIa | 25 | 7 |
|
|
| Lymph node
metastasis |
|
| 1.608 | 0.205 |
|
Present | 39 | 12 |
|
|
|
Absent | 49 | 8 |
|
|
BAG1-positive expression is associated
with prolonged survival
A multivariate analysis (Cox regression model) was
used to quantify the associations between prognostic factors and
survival time in NSCLC patients. Differentiation stage, clinical
stage and positive BAG1 expression were identified as independent
prognostic factors for survival in patients with NSCLC (P<0.05;
Table II). A life-table analysis
revealed that the median PFS of NSCLC patients with BAG1-negative
expression was 21.6 months. By contrast, the median PFS of NSCLC
patients with BAG1-positive expression was markedly increased (PFS,
24.0 months; χ2=18.018, P<0.001; Fig. 1Ba). In addition, OS times (calculated
from the day of surgery to the final follow-up examination) and the
results of the life-table analysis revealed that the median OS time
of NSCLC patients with BAG1-negative expression (20 cases) was 31.4
months and the 3-year survival rate was 8.69%. By contrast, the
median OS time and rate of NSCLC patients with BAG1-positive
expression (88 cases) were markedly increased (OS, 35.6 months;
χ2=24.057, P<0.001; Fig.
1Bb) and the 3-year survival rate was 14.73%. Collectively,
these results suggest that the positive expression of BAG1 is
associated with prolonged survival.
 | Table II.Survival analysis in non-small-cell
lung cancer patients by Cox proportional hazards model. |
Table II.
Survival analysis in non-small-cell
lung cancer patients by Cox proportional hazards model.
| Features | F | Hazard ratio | Wald | P-value |
|---|
| Gender | 1 | 0.214 |
1.008 | 0.315 |
| Pathological
types | 1 | 0.391 |
2.865 | 0.090 |
| Differentiation
stage | 1 | 1.603 | 27.449 | <0.001 |
| Clinical stage | 1 | 1.834 | 28.550 | <0.001 |
| Node
metastasis | 1 | 0.517 |
1.833 | 1.677 |
| BAG1-positive | 1 | 2.359 | 41.663 | <0.001 |
Tunicamycin induces ER stress and
upregulation of BAG1L in A549 cells
Clinical data from the present study revealed that
NSCLC patients with BAG1-positive expression responded more
favorably to platinum-based chemotherapies. BAG1 is involved in ER
stress (10), and tunicamycin has
been demonstrated to induce ER stress (18). To elucidate the molecular mechanism by
which NSCLC patients with decreased expression of BAG1 become
resistant to platinum-based chemotherapies, tunicamycin was used to
induce ER stress and the effects of tunicamycin and cisplatin in
cultured human lung adenocarcinoma A549 cells were investigated.
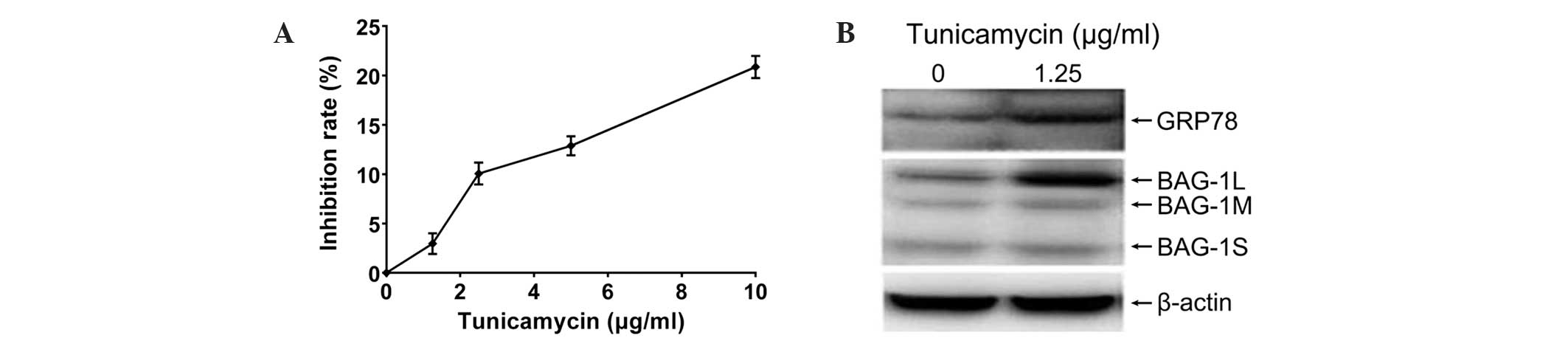
Incubation with tunicamycin for 8 h inhibited the proliferation of
A549 cells in a dose-dependent manner (Fig. 2A). As 1.25 µg/ml tunicamycin induced
~3% growth inhibition, this dose of tunicamycin was used for
studying ER stress. The ER molecular chaperone, GRP78, was
upregulated after 8 h of treatment with 1.25 µg/ml tunicamycin,
indicating that tunicamycin evoked ER stress in A549 cells
(Fig. 2B). Furthermore, protein
levels of the BAG1L (p50) subtype were markedly elevated, whilst
marginal increases in the BAG1M (p46) and BAG1S (p33) subtypes were
observed following tunicamycin treatment. Thus, tunicamycin induces
ER stress and upregulates BAG1L in A549 cells.
Tunicamycin enhances cisplatin-induced
growth inhibition and apoptosis in A549 cells
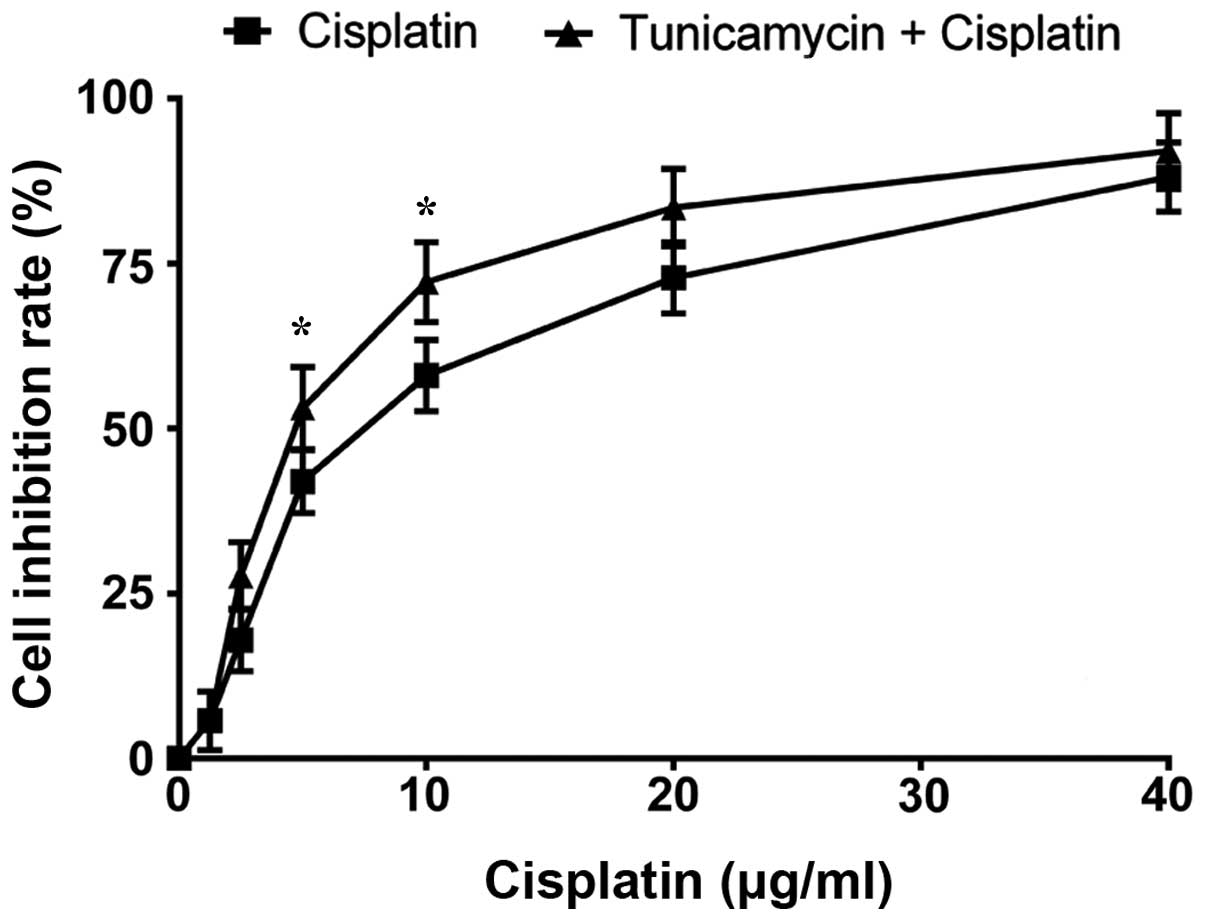
The viability of A549 cells following treatment with
tunicamycin plus cisplatin were assessed. Specifically, incubation
with 1.25 µg/ml tunicamycin for 8 h followed by 24 h with cisplatin
significantly enhanced the growth inhibition rate in A549 cells
(Fig. 3): The combined treatment
markedly decreased the IC50 value compared with that of
cisplatin alone (6.11±1.27 µg/ml vs. 8.53±1.68 µg/ml; P<0.05).
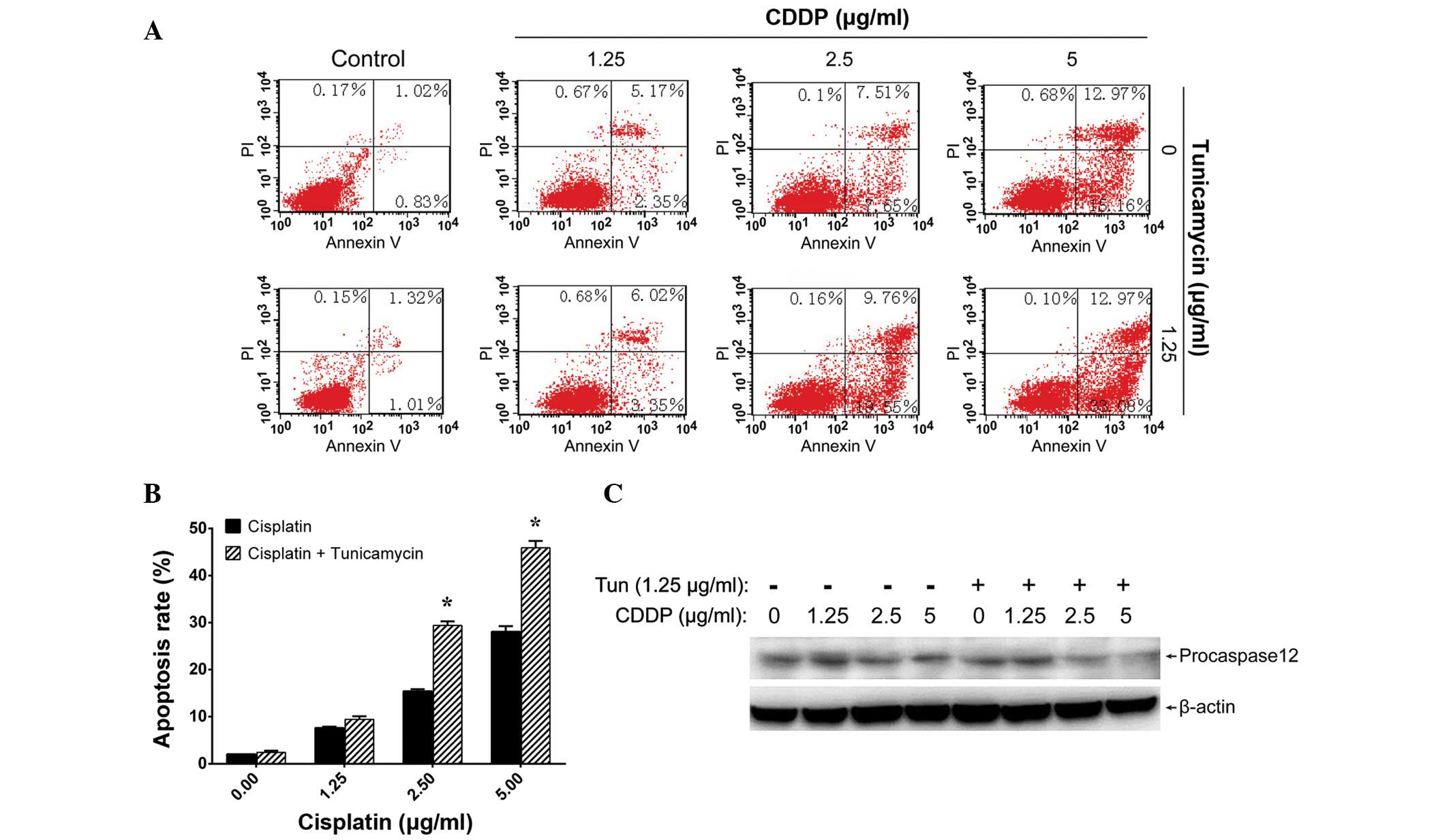
In addition, Annexin V-FITC/PI staining and flow cytometric
analysis revealed that tunicamycin enhanced the cisplatin-induced
apoptosis of A549 cells (Fig. 4A and
B).
As ER stress may trigger apoptosis through the
activation of caspase-12 (19),
procaspase-12 levels were also monitored in A549 cells following
different treatments. Treatment with 1.25 µg/ml tunicamycin for 8 h
plus an additional 24-h incubation with 2.5 or 5 µg/ml cisplatin
significantly downregulated procaspase-12 levels (Fig. 4C), indicating the activation of
caspase-12. These results suggest that tunicamycin may enhance the
effects of cisplatin in cultured lung adenocarcinoma cells.
Impacts of ER stress on BAG1 induced
by cisplatin treatment
The protein levels of BAG1 were assessed by western
blot analysis following treatment of A549 cells with the
chemotherapeutic drugs cisplatin, tunicamycin or a combination of
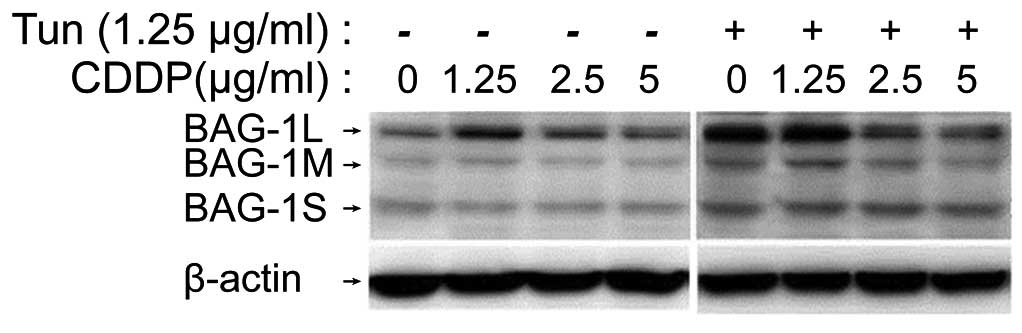
the two. Administration of cisplatin alone (1.25, 2.5 or 5 µg/ml)
markedly elevated the level of BAG1L compared with that of the
control group; a dose of 1.25 µg/ml was found to exert the maximal
effect on BAG1L levels (Fig. 5).
Treatment with 1.25 µg/ml tunicamycin for 8 h also enhanced BAG1L
protein levels in A549 cells. Furthermore, incubation with
tunicamycin (1.25 µg/ml) for 8 h plus 24 h of cisplatin treatment
(1.25 µg/ml) further increased BAG1L expression compared with that
observed in cells treated with tunicamycin alone. Similar results
were observed for BAG1M. With regard to BAG1S, enhanced expression
was detected after tunicamycin or tunicamycin plus cisplatin
treatment, while marked differences were observed among different
dose groups. These results suggest that both ER stress and DNA
damage responses lead to the upregulation of BAG1 in A549
cells.
Discussion
Although platinum-based chemotherapy is the
first-line treatment option for advanced NSCLC patients (2), patients with clinically similar
characteristics respond variably (20). Inter-individual differences in genetic
background have been demonstrated to affect the response to
chemotherapy and OS rate of NSCLC patients receiving platinum-based
chemotherapy (21–25). BAG1 is important in the development
and progression of NSCLC (11,12);
however, the association between expression levels of BAG1 and the
sensitivity of NSCLC patients to platinum-based chemotherapy
remains to be determined.
The present study revealed that BAG1 expression was
associated with the degree of differentiation of NSCLCs, but not
with other clinicopathological characteristics, such as gender,
age, pathological types, clinical stage or lymph node metastasis
status (Table I). This suggests that
BAG1 may be involved in the progression of NSCLC and contribute to
disease development. The Cox multivariate analysis revealed that
degree of differentiation, clinical stage and BAG1 expression were
independent prognostic factors for survival in patients with NSCLC
(Table II). Compared with patients
with BAG1-negative expression, the median PFS time and survival
rate of NSCLC patients with BAG1-positive expression were
significantly higher (PFS, 21.6 vs. 24.0 months;
χ2=18.018, P<0.001). These results indicate that BAG1
may sensitize NSCLC cells to chemotherapy and could serve as an
independent prognostic factor in NSCLC. However, the association
between BAG1 expression and survival of patients with lung cancer
is controversial: A recent study reported that the survival curves
of the BAG1 low-expression group were favorable compared with those
of the BAG1 high-expression group in lung cancer patients of TNM
stage I (26). This discrepancy may
be due to the different types of lung cancer studied.
It has been reported that BAG1 contributes to the
induction of ER stress in chondrocytes (10). Consistent with this observation, the
present study revealed that tunicamycin induced-ER stress was
accompanied by an upregulation of BAG1 and GRP78 in A549 cells. In
addition, the enhancement of ER stress induced by tunicamycin
significantly increased cell growth inhibition and apoptosis in
cisplatin-treated A549 cells. Activation of caspase-12 (i.e.,
reduced procaspase-12), a component of an ER stress-specific
caspase cascade in apoptosis (27,28), was
detected following tunicamycin and cisplatin administration.
However, the underlying mechanism of BAG1-regulated ER stress and
apoptosis remains to be clarified.
It should be noted that, in the present study,
low-dose cisplatin treatment (1.25 µg/ml) dramatically upregulated
the level of BAG1 protein, while at higher concentrations (2.5 or 5
µg/ml) BAG1 levels increased only marginally. This suggests that
low concentrations of cisplatin may promote BAG1 levels and thus
enhance its antiapoptotic activity (8). Accordingly, the percentage of apoptotic
cells gradually increased with the increase of cisplatin dose.
Collectively, the results demonstrate that BAG1 is
involved in the response to platinum-based chemotherapy in NSCLC
patients and in cultured human lung adenocarcinoma A549 cells.
These findings may provide valuable insights into how genetic
variations influence sensitivity to chemotherapy in NSCLC patients,
as well as evidence of the involvement of ER stress in
cisplatin-induced tumor cell death.
In conclusion, the results indicate that BAG1 plays
a positive role in cisplatin-induced cell death in lung
adenocarcinoma, suggesting that ER stress may promote sensitivity
to chemotherapy in NSCLC patients.
Acknowledgements
This study was supported by Liaoning Provincial
Health Department Medical Peak Construction.
Glossary
Abbreviations
Abbreviations:
|
BAG1
|
BCL2-associated athanogene
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
KPS
|
karnofsky performance status
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
NSCLC
|
non-small cell lung cancer
|
|
NVB
|
vinorelbine
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bareschino MA, Schettino C, Rossi A,
Maione P, Sacco PC, Zeppa R and Gridelli C: Treatment of advanced
non small cell lung cancer. J Thorac Dis. 3:122–133.
2011.PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003-2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waters JS and O'Brien ME: The case for the
introduction of new chemotherapy agents in the treatment of
advanced non small cell lung cancer in the wake of the findings of
the national institute of clinical excellence (NICE). Br J Cancer.
87:481–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernet M and Hall J: Genetic biomarkers of
therapeutic radiation sensitivity. DNA Repair (Amst). 3:1237–1243.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulrich CM, Robien K and McLeod HL: Cancer
pharmacogenetics, Polymorphisms, pathways and beyond. Nat Rev
Cancer. 3:912–920. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Townsend PA, Cutress RI, Sharp A, Brimmell
M and Packham G: BAG-1: A multifunctional regulator of cell growth
and survival. Biochim Biophys Acta. 1603:83–98. 2003.PubMed/NCBI
|
|
8
|
Takayama S, Sato T, Krajewski S, Kochel K,
Irie S, Millan JA and Reed JC: Cloning and functional analysis of
BAG-1G1: A novel Bcl-2-binding protein with anti-cell death
activity. Cell. 80:279–284. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Townsend PA, Stephanou A, Packham G and
Latchman DS: BAG-1G1: A multi-functional pro-survival molecule. Int
J Biochem Cell Biol. 37:251–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, McBurney D, Tang SC, Carlson SG
and Horton WE Jr: A novel role for Bcl-2 associated-athanogene-1
(Bag-1) in regulation of the endoplasmic reticulum stress response
in mammalian chondrocytes. J Cell Biochem. 102:786–800. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rorke S, Murphy S, Khalifa M, Chernenko G
and Tang SC: Prognostic significance of BAG-1 expression in
nonsmall cell lung cancer. Int J Cancer. 95:317–322. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Bai Y, Liu B, Wang Z, Wang M, Zhou
Q and Chen J: The expression of BAG-1 and its clinical significance
in human lung cancer. Zhongguo Fei Ai Za Zhi. 11:489–494. 2008.(In
Chinese). PubMed/NCBI
|
|
13
|
Gotz R, Kramer BW, Camarero G and Rapp UR:
BAG-1 haplo-insufficiency impairs lung tumorigenesis. BMC Cancer.
4:852004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greene FL, Page DL, Fritz AG, et al: Lung.
AJCC Cancer Staging Manual (6th). (New York, NY). Springer.
170–171. 2001.
|
|
15
|
Karnofsky DA and Burchenal JH: The
clinical evaluation of chemotherapeutic agents in cancer.
Evaluation of Chemotherapeutic Agents. MacLeod CM: (New York, NY).
Columbia University Press. 191–205. 1949.
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. Histopathology.
J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
18
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress, Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group. Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurubhagavatula S, Liu G, Park S, Zhou W,
Su L, Wain JC, Lynch TJ, Neuberg DS and Christiani DC: XPD and
XRCC1 genetic polymorphisms are prognostic factors in advanced
non-small-cell lung cancer patients treated with platinum
chemotherapy. J Clin Oncol. 22:2594–2601. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Booton R, Ward T, Heighway J, Taylor P,
Power F, Ashcroft L, Morris J and Thatcher N: Xeroderma pigmentosum
group D haplotype predicts for response, survival and toxicity
after platinum-based chemotherapy in advanced nonsmall cell lung
cancer. Cancer. 106:2421–2427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann NY Acad Sci. 1095:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matakidou A, El Galta R, Webb EL, Rudd MF,
Bridle H, Gelcaps Consortium, Eisen T and Houlston RS: Genetic
variation in the DNA repair genes is predictive of outcome in lung
cancer. Hum Mol Genet. 16:2333–2340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu X, Lu C, Ye Y, Chang J, Yang H, Lin J,
Gu J, Hong WK, Stewart D and Spitz MR: Germline genetic variations
in drug action pathways predict clinical outcomes in advanced lung
cancer treated with platinum-based chemotherapy. Pharmacogenet
Genomics. 18:955–965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Bai Y, Liu B, Wang Z, Wang M, Zhou
Q and Chen J: The expression of BAG-1 and its clinical significance
in human lung cancer. Zhongguo Fei Ai Za Zhi. 11:489–494.
2008.PubMed/NCBI
|
|
27
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Histopathology. J Biol Chem.
277:34287–34294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan Y, Dourdin N, Wu C, De Veyra T, Elce
JS and Greer PA: Ubiquitous calpains promote caspase-12 and JNK
activation during endoplasmic reticulum stress-induced apoptosis. J
Biol Chem. 281:16016–16024. 2006. View Article : Google Scholar : PubMed/NCBI
|