Introduction
Pancreatic cancer is the fifth leading cause of
cancer-related mortality in Europe, with a variable mortality rate
of 6.6–8.2/100,000 males and 4.0–5.7/100,000 females. Lung cancer
incidence and mortality increases in females, while pancreatic
cancer mortality rises in the general population. At the same time,
for the majority of other cancer types, the mortality rate is
decreasing (1,2). Pancreatic cancer is associated with a
number of genetic factors, including germline mutations in
BRCA2, p16/CDKN2A, PRSS1,
STK11/LKB1 and DNA mismatch repair genes, as well as
allergies, long-term pancreatitis and cigarette smoking (3). Furthermore, chlorinated hydrocarbons and
polycyclic aromatic hydrocarbons are reported to be occupational
risk factors (4). A recent
meta-analysis determined that multidetector-computed tomography
(CT) and magnetic resonance imaging/cholangiopancreatography have
comparable sensitivity and specificity rates for the diagnosis and
staging of pancreatic cancer. Endoscopic ultrasound exhibits the
most favorable sensitivity and specificity rates for lesions
measuring <2 cm, and improved staging has been observed when
positron emission tomography (PET)-CT scans are included in the
evaluation (5). PET/CT detects
distant metastases not documented by CT, thus affecting the
treatment strategy. PET/CT is also able to monitor the treatment
efficacy, thus identifying metabolic responses to treatment that
are not detected by CT (6). Due to
poor chemotherapy outcomes, surgical resection remains the primary
treatment strategy for pancreatic cancer. Recently, an improved
understanding of pancreatic tumor biology allowed the development
of the novel chemotherapeutic agent, erlotinib. Erlotinib, an
inhibitor of epidermal growth factor receptor (EGFR; ERBB1; HER1),
passed clinical trials and with gemcitabine, is now approved for
the first-line treatment of advanced-stage pancreatic cancer
(7). The current study presents the
case of a 40-year-old male who was diagnosed with medically
inoperable pancreatic adenocarcinoma and was treated with
palliative gemcitabine and erlotinib chemotherapy. The patient
provided written informed consent.
Case report
A 40-year-old male with a medical history of chronic
pancreatitis and hypertension was admitted to the Deaprtment of
Surgery, Military Institute of Medicine (Warsaw, Poland) in July
2011 due to cholestasis. The patient underwent an endoscopic
retrograde cholangiopancreatography, with the insertion of stents
into the pancreatic and common bile ducts. With the exception of a
marginally dilated Wirsung's duct (diameter, 5 mm), CT of the
abdomen revealed no abnormalities; however, an endoscopic
ultrasound procedure performed in August 2011 identified a
poorly-defined hypoechogenic mass with irregular borders in the
head of the pancreas. The patient underwent palliative open
surgery, including a cholecystectomy, a Roux-en-Y
hepaticojejunostomy bypass and Braun's enteroanastomosis due to
cancer infiltration of the superior mesenteric vein. A subsequent
histological analysis of the lesion, which revealed a ductal
adenocarcinoma: the tumor formed a firm, poorly defined yellowish
mass. In the tumor glandular structures imitating pancreatic ducts
were embedded in the abundant desmoplastic stroma. The tumor
demonstrated a mixture of medium-sized duct-like and tubular
structures of variable shape and incompletely formed glands. Cells
with an eosinophilic cytoplasm with a variation in nuclear size,
chromatin structure and nucleoli were observed and multiple mitotic
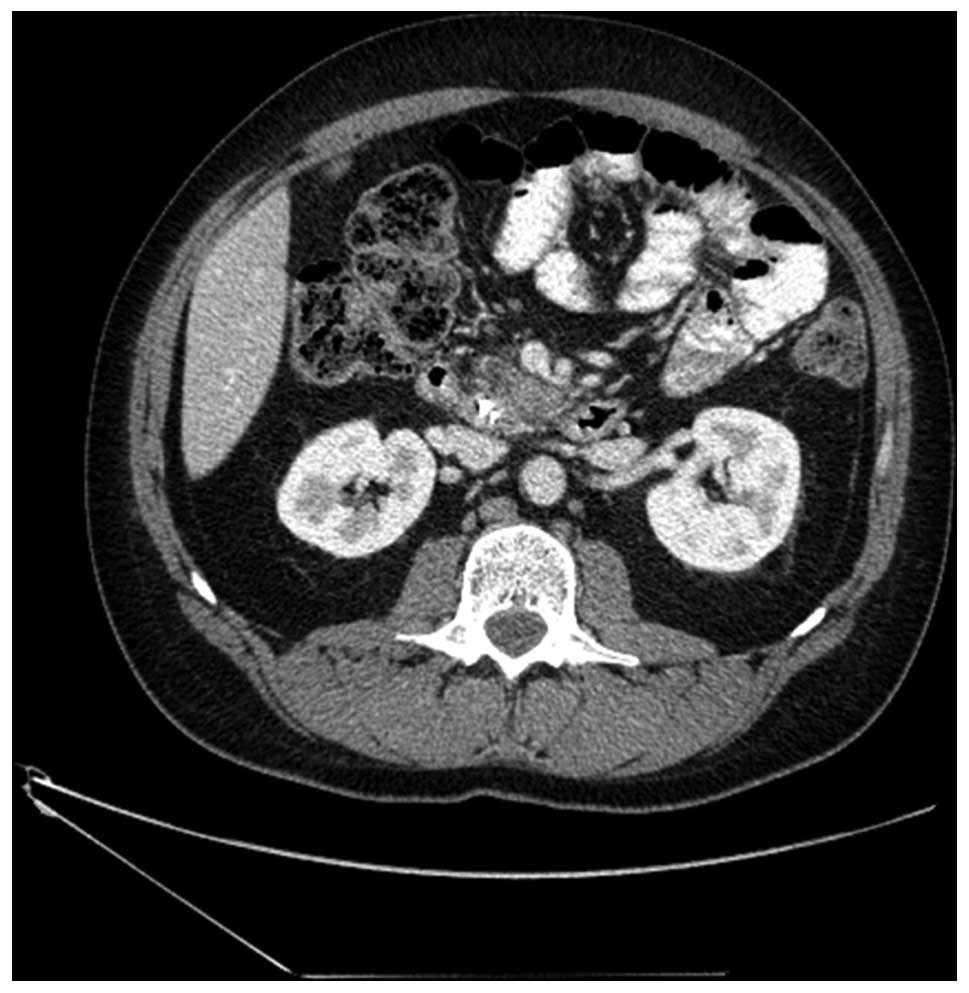
figures were reported. Furthermore, a CT scan performed in
September 2011 identified ascites, a 26-mm lesion in the pancreas,
enlarged duodenal lymph nodes measuring ≤12 mm, and splenic and
superior mesenteric vein thrombosis (Fig.
1). Therefore, the patient was transferred to the Department of
Oncology, Military Institute of Medicine to commence 26 courses of
palliative combined chemotherapy with 1,000 mg/m2
intravenous gemcitabine every week and 100 mg erlotinib every day.
According to the Common Toxicity Criteria (CTC) (8), grade II neutropenia and grade III
leukopenia occurred during treatment, causing courses 4, 8, 13 and
23 to be delayed; these adverse hematological effects were treated
with pegfilgrastim at a dose of 6 mg (one dose per cycle).
Furthermore, the patient developed a grade II skin rash, according
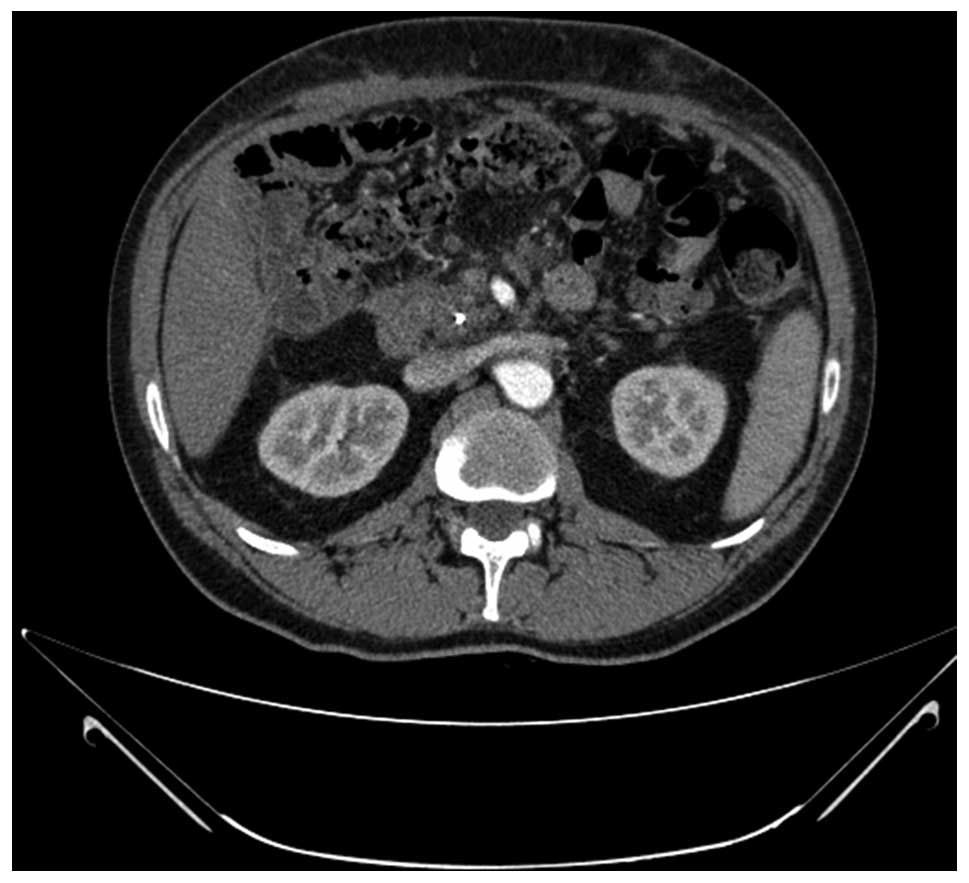
to the CTC. After three months of treatment, in December 2011, a CT
scan demonstrated reduced ascites with the absence of any
measurable lesions (Fig. 2). In
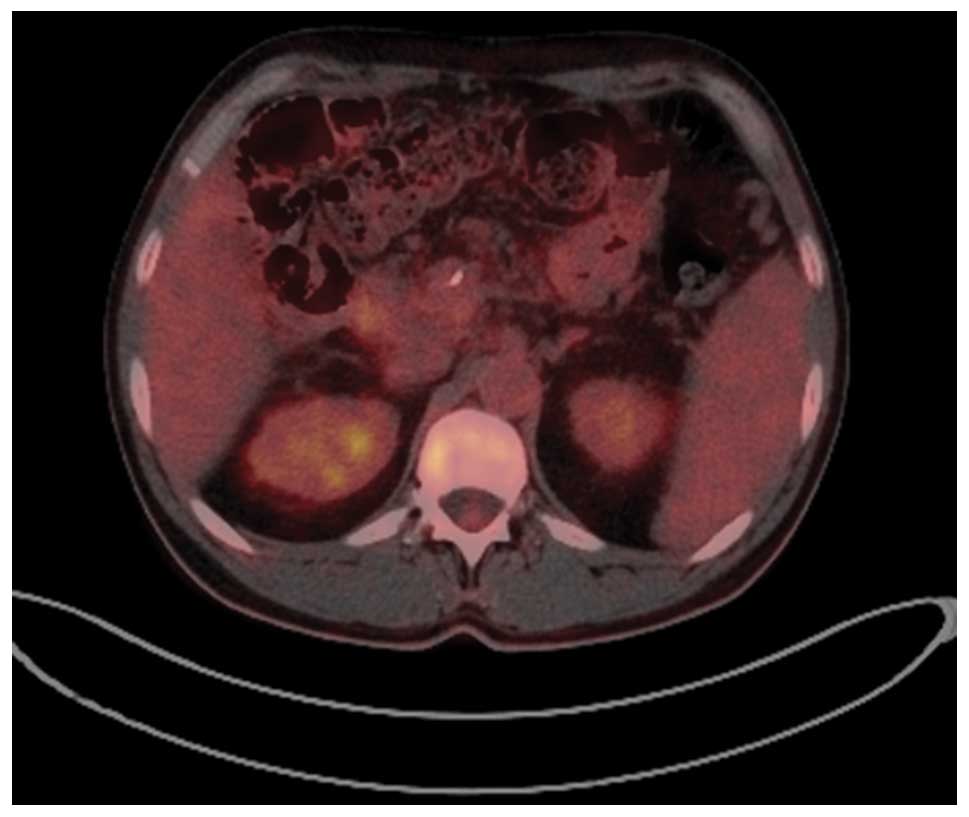
addition, the patient underwent laparotomy one month after CT,
which revealed no visible malignant processes, and a PET-CT
performed in April 2012 confirmed this favorable treatment outcome
(Fig. 3). However, towards the end of
April 2012, the patient experienced severe hematemesis caused by
esophageal and gastric fundal varices, which had arisen as a result
of portal hypertension from a vein thrombosis that had developed as
a complication of gemcitabine treatment. Therefore, gemcitabine
infusions were rescheduled and now administered every second week.
A follow-up CT scan performed in August 2012 demonstrated
maintained ascites due to portal hypertension as a side-effect of
the therapy. The patient maintained a CR for >12 months,
however, cancer cells were present in the ascitic fluid in November
2012 and new lesions were identified in a CT scan performed in
August 2012. The patient demonstrated disease progression
manifested as novel lesions in the peritoneal cavity and was
transferred to a folinic acid, 5-fluorouracil (5-FU), irinotecan
and oxaliplatin (FOLFIRINOX) chemotherapy regimen in January 2013.
The patient progressed rapidly and died in July 2013.
Discussion
Pancreatic cancer remains one of the most lethal
types of tumor and is typically associated with a poor prognosis.
The majority of pancreatic cancer patients are diagnosed with
advanced-stage disease, therefore, the median survival period is
<6 months. Until 1997, the management of pancreatic cancer was
based on a 5-FU regimen; in 1997, Burris et al reported that
gemcitabine-treated patients demonstrated significant clinical
benefits compared with 5-FU-treated patients, including higher
response rates (23.8 vs. 4.8%; P=0.0022), longer median survival
times (5.65 vs. 4.41 months; P=0.0025) and higher one-year survival
rates (18 vs. 2%; P=0.0025) (9).
Trials based on combination therapy with oxiplatin, 5-FU,
irinotecan, pemetrexed, capacetabine and biological agents, such as
the farnesyl transferase inhibitor, tipifarnib, and the matrix
metalloproteinases, revealed inferior outcomes compared with
gemcitabine treatment alone (10).
Furthermore, combined therapeutic strategies have exhibited
superior outcomes compared with single agent-based
molecular-targeted therapies (for example, those targeted to
KRAS, VEGF, VEGF-C, NF-κB, HER-2
and HER-3 mutations) (11–14). In
2005, a novel chemotherapeutic agent regimen involving erlotinib in
combination with gemcitabine was approved for the first-line
treatment of advanced-stage pancreatic cancer. A placebo-controlled
phase III trial demonstrated that administration of the EGFR
inhibitor in combination with gemcitabine is particularly efficient
in preventing pancreatic ductal adenocarcinoma patients from
developing skin toxicity. However, the EGFR expression levels of
tumor cells were not predictive of response in this phase III trial
and markers to characterize an erlotinib-responding subgroup are
currently unavailable (15,16). Furthermore, a hypothetical managed
care plan determined that the addition of erlotinib to the
gemcitabine regimen resulted in a low budget impact, estimated at
$0.02 per member per month (17).
Excluding the present study, thus far only two
patients receiving 24 cycles of gemcitabine combined with erlotinib
for the first-line treatment of pancreatic cancer exhibited a
transient CR (18). However, one
patient also achieved a CR in a trial using a regimen that
consisted of 28-day cycles of gemcitabine treatment (1,200
mg/m2 in 120-min infusions on days 1, 8 and 15) plus
erlotinib (100 mg, orally once daily) (19). In addition, erlotinib single-agent
therapy appeared to be an effective treatment strategy for
gemcitabine-refractory advanced pancreatic cancer patients,
exhibiting a benefit rate of 22% (20). Recently, the
FOLFIRINOX regimen, based on an infusion of 5-FU/folic acid plus
irinotecan and oxaliplatin, was reported to be superior to
gemcitabine, but only in patients with a good performance status of
90–100%, according to the Karnofsky scale. Compared with
gemcitabine treatment alone, the FOLFIRINOX regimen demonstrated
improved objective response rates (31.6 vs. 9.4%; P<0.001),
progression-free survival [PFS; 6.4 vs. 3.3 months; hazard ratio
(HR), 0.47; P<0.001], overall survival (OS; 11.1 vs. 6.8 months;
HR, 0.57; P<0.001) and one-year survival (48.4 vs. 20.6%);
however, a higher toxicity rate with grade III–IV neutropenia (45.7
vs. 21%; P<0.001), febrile neutropenia (5.4 vs. 1.2%; P=0.03),
grade III–IV thrombocytopenia, diarrhea and sensory neuropathy were
also observed during treatment (21). Although a
partial response during gemcitabine-erlotinib combination therapy
was observed, cases with a CR were rarely reported. According to
Carbonell et al (22), studies
conducted over the last decade describe 29 patients who achieved a
CR during treatment with only one of the agents (gemcitabine or
erlotinib) following chemotherapy alone. In 2014, a phase II study
was published to evaluate the combination of treatment with daily
erlotinib (100 mg, orally) and weekly gemcitabine (1,000
mg/m2, infused at a rate of 10 mg/m2/min) in
a cohort of 46 previously untreated patients with locally advanced,
inoperable or metastatic pancreatic cancer (23). The median PFS time was 14 weeks, the
one-year OS rate was 20.2% and the median OS time was 26 weeks,
with only five patients (10.9%) achieving an objective response.
Furthermore, the overall disease control rate was 56.5% and no
patients achieved a CR (23). To the
best of our knowledge, the current study presents the first
reported case of an extended CR in pancreatic cancer achieved using
combination therapy of low-dose gemcitabine (1,000
mg/m2) and erlotinib.
EGFR belongs to the ErbB family of tyrosine kinase
receptors and contains four members: ERBB1 (EGFR or HER1), ERBB2
(HER2/neu), ERBB3 (HER3) and ERBB4 (HER4). In pancreatic cancer,
ErbB overexpression occurs at a frequency of 30–60%, promoting
angiogenesis and cell proliferation, and inhibiting apoptosis
(11,14). The EGFR kinase inhibitors, erlotinib
and gefitinib, have demonstrated clinical efficacy against
pancreatic and non-small cell lung cancer (NSCLC), however, EGFR
status in the two diseases does not appear to be associated with
disease response or stability (10).
In NSCLC, mutations in exons 19 and 21 of the EGFR gene have been
associated with improved outcomes (24); however, the presence of the same
mutations (exons 19 and 21) in pancreatic cancer is rare, with an
incidence of 2%, and has demonstrated no effect on erlotinib
activity in the clinical setting (25). Furthermore, the presence of skin
toxicity, improved performance status and lower pain intensity
scores have been associated with greater OS in patients with
pancreatic cancer, whereas age and comorbidity have not (15,26).
Additional studies are required to understand the underlying
mechanism that leads to skin rashes benefitting OS. In addition,
patients with elevated cancer antigen (CA)19-9 levels prior to
surgical resection exhibited worse outcomes than patients with
CA19-9 levels within the normal range, and CA19-9 levels decreased
or normalized by ≥20–50% of the baseline pretreatment levels were
associated with a survival benefit (27). Another factor involved in pancreatic
treatment outcome is KRAS mutation status, which was
determined not to be predictive of the objective response to
anti-EGFR treatment with erlotinib. In a post-hoc analysis study of
AIO-PK0104, a phase III trial comparing gemcitabine/erlotinib
followed by capecitabine with capecitabine/erlotinib followed by
gemcitabine for the treatment of advanced pancreatic cancer,
KRAS mutation status was verified and no association was
identified with objective response (P=0.40; however, wild-type
KRAS patients did exhibit improved OS (HR, 1.68; P=0.005)
(28). AIO-PK0104 patients were also
evaluated for KRAS exon 2 mutations, EGFR expression,
phosphatase and tensin homolog (PTEN) expression, and R497 K
polymorphisms (PMs) of EGFR intron 1 and exon 13. It was reported
that wild-type KRAS status is associated with improved OS
(HR, 1.68; P=0.005), however, no significant OS correlation was
identified for EGFR (HR, 0.96) or PTEN (HR, 0.77) overexpression,
EGFR amplification (HR, 1.22), or EGFR intron 1 (HR, 0.91) or exon
13 (HR, 0.83) R497K PMs. In addition, the expression of none of the
six biomarkers investigated correlated with the occurrence of a
skin rash (29). Furthermore, a trial
conducted by the National Cancer Institute of Canada Clinical
Trials Group PA.3 clarified that no correlation exists between
PFS-OS data and KRAS mutation status (16).
In conclusion, the present case report indicated
that a combination of erlotinib and gemcitabine may be an effective
treatment strategy for patients with local advanced pancreatic
cancer. However, additional studies are required to identify
clinically important molecular markers that may facilitate the
prediction of the treatment response, as well as improve our
understanding of the biology of this disease, with incorporation of
this knowledge into clinical trials. Furthermore, future studies
should attempt to identify predictive and prognostic markers to aid
in the development and application of therapeutic agents. Finally,
control of pancreatic cancer will require a combination of targeted
agents and individualized therapies based on tumor genetics,
therefore, research such as the recent investigation (30,31) into
the mechanisms of drug resistance and the combined targeted agents
required in the clinic must continue.
Acknowledgements
The present study was supported by statutory funding
from the Military Institute of Medicine (Warsaw, Poland; grant no.
WIM/1/1744).
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malvezzi M, Bertuccio P and Levi F: LaV
ecchia C and Negri E: European cancer mortality predictions for the
year 2012. Ann Oncol. 23:1044–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koorstra JB, Hustinx SR, Offerhaus GJ and
Maitra A: Pancreatic carcinogenesis. Pancreatology. 8:110–125.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andreotti G and Silverman DT: Occupational
risk factors and pancreatic cancer: a review of recent findings.
Mol Carcinog. 51:98–108. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shrikhande SV, Barreto SG, Goel M and Arya
S: Multimodality imaging of pancreatic ductal adenocarcinoma, a
review of the literature. HPB (Oxford). 14:658–668. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Picchio M, Giovannini E, Passoni P, et al:
Role of PET/CT in the clinical management of locally advanced
pancreatic cancer. Tumori. 98:643–651. 2012.PubMed/NCBI
|
|
7
|
Moore MJ, Goldstein D, Hamm J, et al:
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Institutes of Health: Common
Terminology Criteria for Adverse Events (CTCAE). Version 4.0 May
28. 2009.http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
|
|
9
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD and Von Hoff DD:
Improvements in survival and clinical benefit with gemcitabine as
first-line therapy for patients with advanced pancreas cancer. a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
10
|
Burris HA III: Recent updates on the role
of chemotherapy in pancreatic cancer. Semin Oncol. 32(4 Suppl 6):
S1–S3. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borja-Cacho D, Jensen EH, Saluja AK,
Buchsbaum DJ and Vickers SM: Molecular targeted therapies for
pancreatic cancer. Am J Surg. 196:430–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleespies A, Jauch KW and Bruns CJ:
Tyrosine kinase inhibitors and gemcitabine, new treatment options
in pancreatic cancer? Drug Resist Updat. 9:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Longo R, Cacciamani F, Naso G and
Gasparini G: Pancreatic cancer, from molecular signature to target
therapy. Crit Rev Oncol Hematol. 68:197–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Starling N, Neoptolemos J and Cunningham
D: Role of erlotinib in the management of pancreatic cancer. Ther
Clin Risk Manag. 2:435–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vickers MM, Powell ED, Asmis TR, et al:
Comorbidity, age and overall survival in patients with advanced
pancreatic cancer - results from NCIC CTG PA.3: a phase III trial
of gemcitabine plus erlotinib or placebo. Eur J Cancer.
48:1434–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
da Cunha Santos G, Dhani N, Tu D, et al:
Molecular predictors of outcome in a phase 3 study of gemcitabine
and erlotinib therapy in patients with advanced pancreatic cancer:
National Cancer Institute of Canada Clinical Trials Group Study
PA.3. Cancer. 116:5599–5607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Danese MD, Reyes C, Northridge K, Lubeck
D, Lin CY and O'Connor P: Budget impact model of adding erlotinib
to a regimen of gemcitabine for the treatment of locally advanced
nonresectable or metastatic pancreatic cancer. Clin Ther.
30:775–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klapdor R, Klapdor S and Bahlo M:
Combination therapy with gemcitabine (GEM) and erlotinib (E) in
exocrine pancreatic cancer under special reference to RASH and the
tumour marker CA19-9. Anticancer Res. 32:2191–2197. 2012.PubMed/NCBI
|
|
19
|
Feliu J, Borrega P, León A, et al: Phase
II study of a fixed dose-rate infusion of gemcitabine associated
with erlotinib in advanced pancreatic cancer. Cancer Chemother
Pharmacol. 67:215–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iyer NIKRV, Tan W, Litwin A, Starostik P,
Levea C, Tucker C, Ma W, Fakih M and Adjei AA: A phase II study of
erlotinib in patients (PTS) with advanced pancreatic cancer (APC)
who are refractory to gemcitabine (G). ASCO Gastrointestinal
Cancers Symposium abstract. 258:2010.
|
|
21
|
Heinemann V, Haas M and Boeck S: Systemic
treatment of advanced pancreatic cancer. Cancer Treat Rev.
38:843–853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carbonell S, Espinosa J, Zarco A, et al:
Complete pathological response after chemotherapy alone in a
patient with pancreatic adenocarcinoma. Pancreas. 41:657–659. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renouf DJ, Tang PA, Hedley D, Chen E,
Kamel-Reid S, Tsao MS, Tran-Thanh D, Gill S, Dhani N, Au HJ, Wang L
and Moore MJ: A phase II study of erlotinib in gemcitabine
refractory advanced pancreatic cancer. Eur J Cancer. 50:1909–1915.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdel-Wahed MM, Asaad NY and Aleskandarany
M: Expression of matrix metalloproteinase-2 in renal cell
carcinoma. J Egypt Natl Canc Inst. 16:168–177. 2004.PubMed/NCBI
|
|
25
|
Riely GJ, Politi KA, Miller VA and Pao W:
Update on epidermal growth factor receptor mutations in non-small
cell lung cancer. Clin Cancer Res. 12:7232–7241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiss GA, Rossi MR, Khushalani NI, et al:
Evaluation of phosphatidylinositol-3-kinase catalytic subunit
(PIK3CA) and epidermal growth factor receptor (EGFR) gene mutations
in pancreaticobiliary adenocarcinoma. J Gastrointest Onco. 4:20–29.
2013.
|
|
27
|
Wacker B, Nagrani T, Weinberg J, Witt K,
Clark G and Cagnoni PJ: Correlation between development of rash and
efficacy in patients treated with the epidermal growth factor
receptor tyrosine kinase inhibitor erlotinib in two large phase III
studies. Clin Cancer Res. 13:3913–3921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
29
|
Boeck S, Jung A, Laubender RP, et al: KRAS
mutation status is not predictive for objective response to
anti-EGFR treatment with erlotinib in patients with advanced
pancreatic cancer. J Gastroenterol. 48:544–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boeck S, Jung A, Laubender RP, et al: EGFR
pathway biomarkers in erlotinib-treated patients with advanced
pancreatic cancer: translational results from the randomised,
crossover phase 3 trial AIO-PK0104. Br J Cancer. 108:469–476. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renouf D and Moore M: Evolution of
systemic therapy for advanced pancreatic cancer. Expert Rev
Anticancer Ther. 10:529–540. 2010. View Article : Google Scholar : PubMed/NCBI
|