Introduction
Neurothekeoma is a rare and typically benign
cutaneous tumor. Neurothekeoma possesses a distinctive histological
appearance and characteristic clinical features, and is thought to
be a variant of a peripheral nerve sheath myxoma (1–3).
Neurothekeomas are generally slow-growing and manifest as a
solitary papule or nodule, which is typically located on the head,
neck or upper extremities (4–10). In a small number of cases, the
solitary papule or nodule may be located in the oral cavity,
breast, tongue, maxilla (11–14), cranial cavity (10) or spinal intradural space (15). Though the shoulder has been described
as a common site of neurothekeoma development (3–6), there
have been few cases of humeral neurothekeoma reported in detail in
the relevant literature (6,16). Tumors such as that reported in the
present case study remain a rare occurrence. The current study
aimed to introduce a rare giant neurothekeoma that developed in the
left should blade of an elderly man over >10 years. The present
neurothekeoma originated from the intermuscular space of the left
shoulder blade, and presented with a partially-formed capsule,
scapula erosion and unclear biological behavior. The tumor was
~17×16×10 cm in size, and occurred in an 81-year-old man with a
>10 year medical history of a slow-growing mass.
Initially, the patient was incorrectly diagnosed
with fibromatosis, based on the characteristic clinical symptoms
and imageological diagnosis. Results of computed tomography (CT)
and magnetic resonance imaging (MRI) scans indicated a diagnosis of
fibromatosis. However, immunohistochemical and pathological
examination of the lesion suggested a diagnosis of a neurothekeoma
originating in the peripheral nerve sheath. Clinical examination
and patient history are significant in the diagnosis of disease,
however immunohistochemical staining and pathological sectioning
are the standard methods of diagnosis for neurothekeoma (1–3,17,18). In
order to correctly guide treatment, definitive preoperative
diagnosis of neurothekeoma is of significance. The patient was
treated with a wide local excision, performed by professional bone
tumor surgeons. Written informed consent was obtained from the
patient.
Case report
An 81-year-old man was admitted to the Department of
Orthopedic Oncology of the First Affiliated Hospital of Nanchang
University (Nanchang, China), and presented with a slow-growing,
painless mass that had developed over a period of 10 years, in the
left shoulder blade. In the previous 3 years, the tumor had grown
significantly more rapidly than in the preceding 10 years. The
principal clinical manifestation of the tumor was numbness of the
left hand, which was not mitigated by rest. Physical examination of
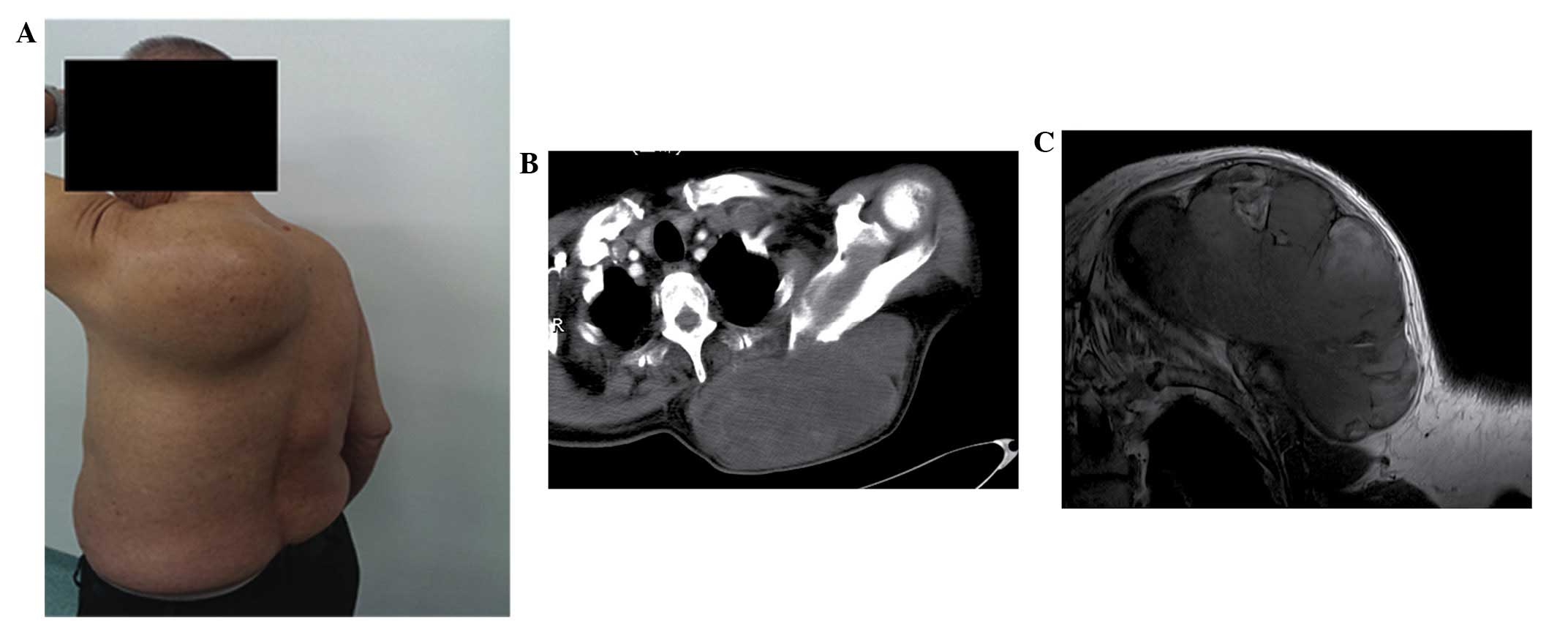
the patient revealed a giant mass on the inner side of the left
humeral back, which was immobile, tender and exhibited distinct
borders (Fig. 1A). The large mass
prevented the shoulder moving in all directions and passive
activities were limited. No abnormalities were identified during
the medical examination, with all results of routine laboratory
tests, such as erythrocyte sedimentation rate, within the normal
ranges. The patient exhibited a prior history of hypertension, and
blood pressure had been controlled using antihypertensive drug
treatment (30 mg nifdipine controlled-release tablets, p.o.,
q.d.).
CT scanning revealed a large lobulated and
cystic-solid mass, ~17×16×10 cm in size, exhibiting homogeneous
density and a clear border below the left trapezius muscle
(Fig. 1B). Bone hyperplasia hardening
of the left scapula was also revealed using CT, however, no bone
destruction was observed. Based on the CT scanning results,
borderline or poorly differentiated malignant fibromatosis was
diagnosed by a professional radiologist.
In order to achieve further confirmatory diagnosis,
MRI scanning was performed (Fig. 1C),
which revealed a giant and lobulated lump, measuring ~17×16×10 cm
in size. MRI scanning of the mass revealed miscellaneous signals,
primarily including long intensity for T1 and T2 signals. The tumor
was observed as multiple linear low signal intensity strands, and
the tumor border was well-defined. The soft tissue surrounding the
mass demonstrated normal signal intensity. No obvious destructive
signal intensity in the left scapula was identified. Following MRI
scanning, no enlarged lymph nodes or distant metastases were
identified. MRI scanning also suggested a potential diagnosis of
fibromatosis. However, the tumor exhibited unclear biological
behavior, with no examinations confirming whether the tumor was
benign, borderline or malignant.
The tumor resection was performed by professional
surgeons. Following the administration of general anesthesia (6
mg/kg/h propofol intravenous drip), the operative site was
disinfected with an iodophor three times, and routine sterile
drapes were placed in the right lateral position in order to avoid
contamination and expose the operative field. A spindle-shaped
surgical incision, ~26 cm in length, was made in the center of the
left humeral mass. Subsequently, the surgeons separated the skin,
subcutaneous superficial fascia and left trapezius muscle in order
to isolate the tumor, which was almost entirely surrounded by a
soft tissue capsule. However, the tumor face adjacent to the left
scapula possessed an area of ~3×4 cm without a capsule. Notably,
bone destruction of the left scapula tumor interface was revealed
following removal of the mass. An osteotome and rongeur were
utilized to resect the destroyed bone and residual tumor tissue,
until complete excision was achieved. Pathological examination of
the excised mass was subsequently performed.
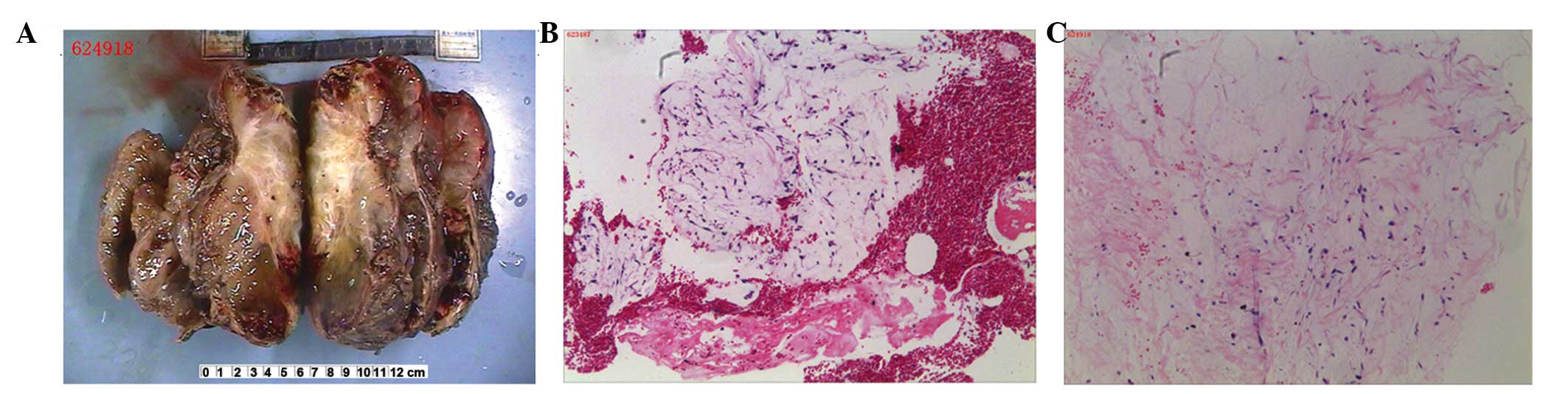
The excised specimen was off-white in color, and was
measured to be ~17×16×10 cm (Fig.
2A). The tumor surface was smooth and clear, however there was
an area of ~3×4 cm on the scapula-tumor interface from which
capsule was absent. Microscopic evaluation of hematoxylin and eosin
(H&E) stained slides (Fig. 2B and
C) revealed that the tumor possessed myxoid lobulated lesions.
The tumor was encapsulated by a thin fibrous connective tissue and
was composed of ovoid lobules, separated by fibrous septae and
arranged in well-formed micronodules. The lobules were formed of
loosely arranged stellate and spindle-shaped cells. Necrosis and
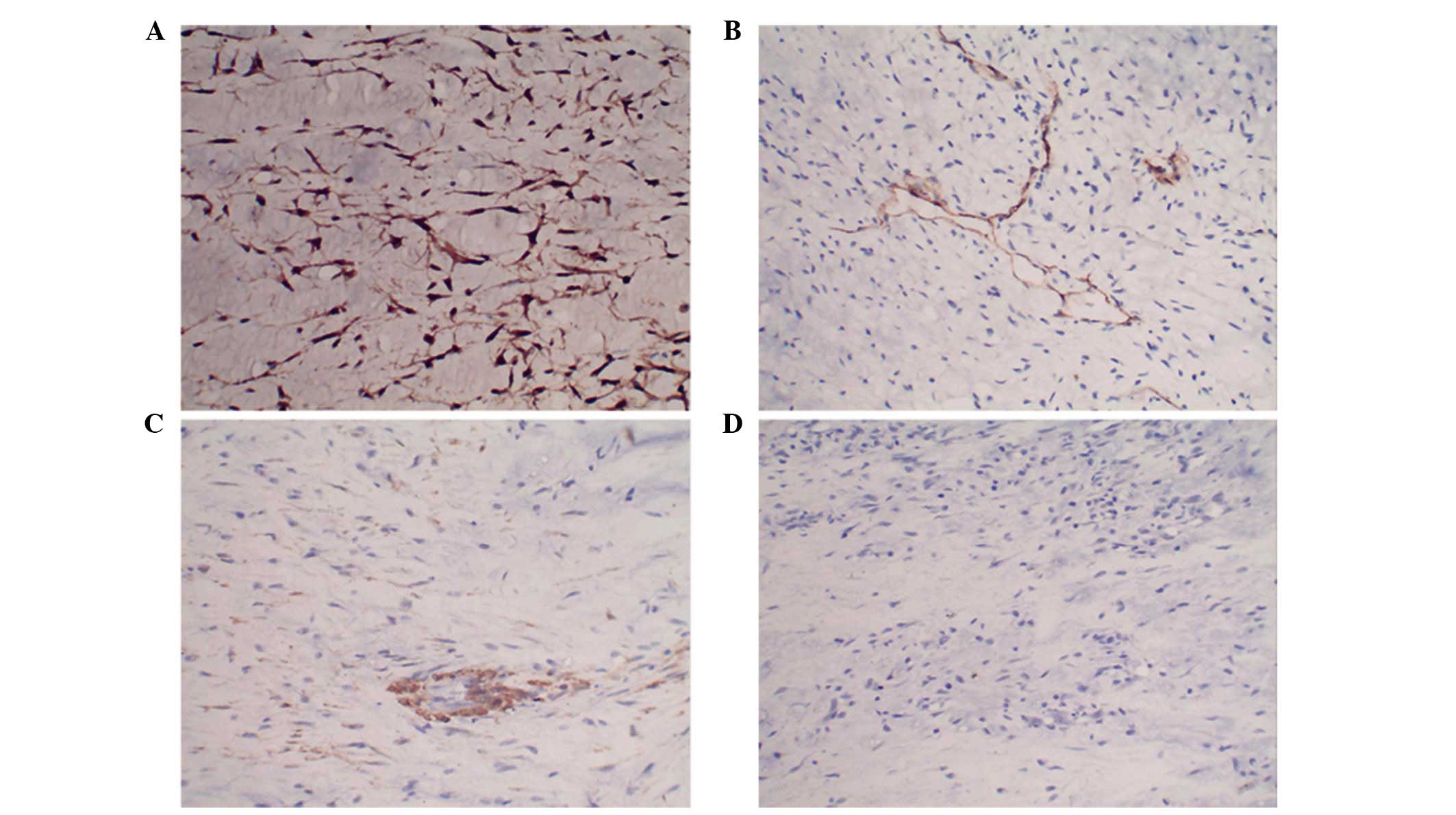
mitosis were almost absent. Immunohistochemical staining revealed
that the tumor cells were positive for S100 and negative for
desmin, cluster of differentiation 34 and smooth muscle actin
(Fig. 3). Based on these
histopathological results, the present case was diagnosed as
neurothekeoma. The patient demonstrated no evidence of tumor
recurrence for 3 years subsequent to the performance of
surgery.
Discussion
To the best of our knowledge, neurothekeoma is an
uncommon and benign dermal tumor, originating from the sheath of
peripheral nerves (19). Harkin and
Reed (20) initially reported
neurothekeoma in 1969, as a rare neoplasm arising in the
endoneurium of peripheral nerves, characterized by an abundant
mucoid matrix; and classed it as a myxoma of the nerve sheath.
Gallager and Helwig initially suggested the terminology of
neurothekeoma in 1980 (3). Based on
Papadopoulos et al (19), who
performed a study of the largest group of neurothekeoma cases
(n=292) to the best of our knowledge, it may be concluded that the
most common site of neurothekeoma occurrence is the upper
extremities (33.6%), followed by the head and neck (29.4%), trunk
(17.2%), lower extremities (9.7%) and mucosal membranes (9.3%). A
markedly lower number of neurothekeomas (~0.8%) were located in the
spinal marrow (19). Alexandru et
al (10) reported the case of a
neurothekeoma identified in the posterior fossa. Neurothekeomas
have been identified in patients ranging in age from 15 months to
84 years, with a mean age of 28 years, and the lesions are most
commonly identified in patients aged between 10 and 30 years old
(19,21). Neurothekeomas remain uncommon in
patients >80 years of age (19,21). The
incidence of neurothekeomas is 2-fold greater in women, compared
with that of men (19). Papadopoulos
et al (19) additionally
reported that the average diameter of a neurothekeoma was 1.2
cm.
In the present case, an 81-year-old man with a
>10 year clinical history of a slow-growing mass, was diagnosed
with neurothekeoma via immunohistochemical and pathological
examination. CT and MRI scanning identified the neoplasm between
the left trapezius muscle and scapula. Neurothekeomas are typically
asymptomatic and slow growing (19,22).
However, in the present case, the mass had grown considerably more
rapidly in the most recent 3 years, compared with the preceding 10
years, and the patient experienced numbness in the left hand, which
was not mitigated by rest. Based on the unique clinical
characteristics and large size of the tumor, to the best of our
knowledge, the present case report is the first description of a
giant neurothekeoma with unclear biological behavior, which
originated in the intermuscular space.
Previous studies have reported that neurothekeomas
possessing spindle or stellate cells, embedded in an abundant
myxoid background, may be classified as classical, cellular and
mixed types, based on their cellularity, mucin content and growth
pattern, or the quantity of myxoid matrix (5,23,24). However, certain scientists do not
support the current classification of neurothekeoma, due to the
lack of immunohistochemical and ultrastructural evidence to support
a nerve sheath origin (9,25). Fetsch et al (5) reported that the term neurothekeoma was
used to describe cellular and mixed tumor variants, and that the
term nerve sheath myxoma was used for lesions. According to
previous studies, immunohistochemical markers, including S100
protein, glial fibrillary acidic protein, nerve growth factor
receptor, cluster of differentiation 57, NKI/C3, Ki-M1p, and
cluster of differentiation 68, may be used in order to distinguish
between the 3 subtypes of neurothekeoma (4,5,11,19,26–28).
In addition, Sheth et al (29)
initially used the molecular technique of gene expression profiling
to evaluate the hypothesis that dermal nerve sheath myxomas are of
peripheral nerve sheath origin, and suggested that neurothekeomas
may be a variant of fibrous histiocytomas. There was no definite
somatotype of neurothekeoma or nerve sheath myxoma identified in
the present study. To clarify the somatotype, immunohistochemical
marker testing and gene expression profiling technology may be
useful.
The clinical differential diagnosis of neurothekeoma
is multitudinous, including schwannoma, true neuroma, myxoid
neurofibroma, ossifying fibromyxoid tumor, myxoid malignant fibrous
histiocytoma, melanocytic lesions and epithelioid hemangioma
(19,24,30). In
order to perform a definitive diagnosis of neurothekeoma, a full
review, including clinical manifestation, careful physical and
imageological (CT and MRI) examination, immunohistochemical
staining and pathological sectioning, should be taken into
consideration. Furthermore, immunohistochemical marker testing and
gene expression profiling technology may be utilized in order to
clarify the tumor somatotype.
The current patient was diagnosed with a
neurothekeoma, following discussion by the pathologists in the
First Affiliated Hospital of Nanchang University. Due to the fact
that incomplete excision of the lesion may lead to local recurrence
(5,17), surgical resection was performed in
order to minimize the risk of tumor relapse. Neurothekeoma is a
rare benign tumor and, to the best of our knowledge, there have
been no reported cases of malignant transformation (31), therefore treatment with chemotherapy
and radiotherapy is not required. However, the neoplasm presented
with a partially-formed capsule, scapula erosion and unclear
biological behavior, which indicated the potential for malignant
transformation. A comprehensive follow-up strategy was conceived by
the professional bone tumor surgeons, and used to confirm that the
patient recovered well following surgical resection of the
tumor.
In conclusion, the present case study described a
rare giant neurothekeoma, which was identified in the intermuscular
space of the left shoulder blade. The mass was painless and had
been slowly growing for >10 years. Immunohistochemical and
pathological observations allowed the achievement of a definitive
diagnosis, whereas initial imageological examinations resulted in a
false diagnosis. Therefore, diagnostic pathology and immunostaining
is necessary for the diagnosis of a neurothekeoma. Due to the
possibility of malignant transformation, complete excision is
recommended for the treatment of neurothekeomas of this size.
Follow-up was accomplished and the patient has recovered, and
demonstrated no evidence of tumor recurrence for 3 years subsequent
to surgery.
References
|
1
|
Isoda M and Katayama M: Neurothekeoma.
Cutis. 41:255–256. 1988.PubMed/NCBI
|
|
2
|
Aronson PJ, Fretzin DF and Potter BS:
Neurothekeoma of Gallager and Helwig (dermal nerve sheath myxoma
variant): Report of a case with electron microscopic and
immunohistochemical studies. J Cutan Pathol. 12:506–519. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallager RL and Helwig EB: Neurothekeoma -
a benign cutaneous tumor of neural origin. Am J Clin Pathol.
74:759–764. 1980.PubMed/NCBI
|
|
4
|
Yun SJ, Park HS, Lee JB, Kim SJ, Lee SC
and Won YH: Myxoid cellular neurothekeoma, A new entity of
S100-negative, CD68-positive myxoid neurothekeoma. Ann Dermatol.
26:510–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fetsch JF, Laskin WB, Hallman JR, Lupton
GP and Miettinen M: Neurothekeoma An analysis of 178 tumors with
detailed immunohistochemical data and long-term patient follow-up
information. Am J Surg Pathol. 31:1103–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang YW, Shih IH, Huang YH, Kuo TT and
Hong HS: Mixed-type neurothekeoma presenting with an unusual
clinical appearance of multiple satellite lesions on the back.
Dermatol Surg. 31:720–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oh SH, Lee HJ, Chang SE, Lee MW, Choi JH,
Moon KC and Koh JK: A case of cellular neurothekeoma. Korean J
Dermatol. 44:1126–1129. 2006.
|
|
8
|
Ryu DJ, Kim HJ, Jung JY, Kwon YS and Lee
JH: A case of myxoid neurothekeoma on the hand. Korean J Dermatol.
47:982–985. 2009.
|
|
9
|
Fetsch JF, Laskin WB and Miettinen M:
Nerve sheath myxoma, A clinicopathologic and immunohistochemical
analysis of 57 morphologically distinctive, S-100 protein- and
GFAP-positive, myxoid peripheral nerve sheath tumors with a
predilection for the extremities and a high local recurrence rate.
Am J Surg Pathol. 29:1615–1624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexandru D, Satyadev R and So W:
Neurothekeoma in the posterior fossa, Case report and literature
review. Perm J. 16:63–64. 2012.PubMed/NCBI
|
|
11
|
Vered M, Fridman E, Carpenter WM and
Buchner A: Classic neurothekeoma (nerve sheath myxoma) and cellular
neurothekeoma of the oral mucosa, Immunohistochemical profiles. J
Oral Pathol Med. 40:174–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wee A, Tan CE and Raju GC: Nerve sheath
myxoma of the breast. Histopathology. Virchows Arch A Pathol Anat
Histopathol. 416:163–167. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makino T, Utsunomiya T, Kamino Y,
Kobayashi R, Fukumoto M, Yamamoto H and Nagura H: Nerve sheath
myxoma of the tongue in a child. Int J Oral Maxillofac Surg.
31:451–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen NA, Samadi DS, Pawel BR and Kazahaya
K: Cellular neurothekeoma of the maxilla. Ann Otol Rhinol Laryngol.
113:384–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paulus W, Jellinger K and Perneczky G:
Intraspinal neurothekeoma (nerve sheath myxoma). Histopathology. Am
J Clin Pathol. 95:511–516. 1991.PubMed/NCBI
|
|
16
|
Woo EK, Lim TK and Tan SH: Neurothekeomas
of the upper limb - case series and clinicopathological review.
Hand Surg. 10:311–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiemeyer S and Hafer G: Neurothekeoma of
the toe. Foot Ankle Spec. 6:479–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Cepeda LD, Navarrete-Franco G,
Novales-Santacoloma J and Enriquez-Merino J: Scar-like lesion on
dorsal nose (cellular neurothekeoma). Head Face Med. 3:392007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Papadopoulos EJ, Cohen PR and Hebert AA:
Neurothekeoma Report of a case in an infant and review of the
literature. J Am Acad Dermatol. 50:129–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harkin JC and Reed RJ: Solitary benign
nerve sheath tumors myxoma of the nerve sheath. Tumors of the
Peripheral Nervous System, Atlas of Tumor Pathology, Second Series,
Fascicle 3. Harkin JC and Reed RJ: (Washington, DC). Armed Forces
Institute of Pathology. 60–64. 1969.
|
|
21
|
Seo BF, Kang HW, Lee JY, Kwon H and Jung
SN: Ankle neurothekeoma, A case report. J Foot Ankle Surg.
52:678–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rawal YB, Mustiful-Martin D, Rosebush MS,
Anderson KM and Mincer HH: Slow-growing gingival mass. Oral Surg
Oral Med Oral Pathol Oral Radiol. 113:161–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Argenyi ZB, LeBoit PE, Cruz Santa D,
Swanson PE and Kutzner H: Nerve sheath myxoma (neurothekeoma) of
the skin, Light microscopic and immunohistochemical reappraisal of
the cellular variant. J Cutan Pathol. 20:294–303. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishioka M, Aguirre RL, Ishikawa A, Nagumo
K, Wang LH and Okada N: Nerve sheath myxoma (neurothekeoma) arising
in the oral cavity, Histological and immunohistochemical features
of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
107:e28–e33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laskin WB, Fetsch JF and Miettinen M: The
‘neurothekeoma’: Immunohistochemical analysis distinguishes the
true nerve sheath myxoma from its mimics. Hum Pathol. 31:1230–1241.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang SE, Lee TJ, Ro JY, Choi JH, Sung KJ,
Moon KC and Koh JK: Cellular neurothekeoma with possible
neuroendocrine differentiation. J Dermatol. 26:363–367. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Husain S, Silvers DN, Halperin AJ and
McNutt NS: Histologic spectrum of neurothekeoma and the value of
immunoperoxidase staining for S-100 protein in distinguishing it
from melanoma. Am J Dermatopathol. 16:496–503. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahalingam M, Alter JN and Bhawan J:
Multiple cellular neurothekeomas - a case report and review on the
role of immunohistochemistry as a histologic adjunct. J Cutan
Pathol. 33:51–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheth S, Li X, Binder S and Dry SM:
Differential gene expression profiles of neurothekeomas and nerve
sheath myxomas by microarray analysis. Mod Pathol. 24:343–354.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tiffee JC and Pulitzer DR: Nerve sheath
myxoma of the oral cavity: Case report and review. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 82:423–425. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kah TA, Yong KC and Annuar FH:
Neurothekeoma palpebrae in association with multiple superficial
angiomyxomas, Tegumental angiomyxoma-neurothekeoma syndrome (TAN
syndrome). Clin Pract. 1:e672011. View Article : Google Scholar : PubMed/NCBI
|