Introduction
Gastric cancer is one of the most aggressive tumors,
with 951,594 cases diagnosed worldwide in 2012. Furthermore,
gastric cancer was the third leading cause of cancer-associated
mortality worldwide in 2012, accounting for 723,027 mortalities
(1,2).
Notably, the age-standardized incidence rates for gastric cancer
are approximately six times higher in Eastern Asia when compared
with the USA (3). The 5-year survival
rate for gastric cancer is <20% (4). Angiogenesis is the formation of novel
blood vessels from existing vessels and is required for the growth
of solid tumors (5). Angiogenesis
occurs at various stages during the malignant progression of the
tumor and is a key step in tumor invasion and metastasis (6,7). Notably,
angiogenesis has been found to closely correlate with prognosis and
hematogenous metastasis of gastric cancer (8). A balance between pro-angiogenic and
anti-angiogenic factors in the local environment is important for
the development of angiogenesis (5–7,9–11).
Numerous pro-angiogenic factors, including factors that act
directly and indirectly, are involved in the complex regulation of
angiogenesis (5–7,9,12,13).
Interleukin (IL)-8 is a pro-inflammatory chemokine
that belongs to the CXC subfamily and has been revealed to function
as a significant regulatory factor within the tumor
microenvironment (14). IL-8 is
likely to be produced by a variety of human cancer cells, including
gastric cancer cells (15). As a
directly acting angiogenic factor, IL-8 promotes angiogenic
responses in in vivo models (14,16,17), and
is markedly associated with tumor angiogenesis, including
hepatocellular carcinoma (18,19),
cervical cancer (20), malignant
melanoma (21) and nasopharyngeal
carcinoma (22). However, the role of
IL-8 in the activation of angiogenesis in gastric cancer remains
unclear. Vascular endothelial growth factor (VEGF)-A interacts with
VEGF receptor (VEGFR)-1 and VEGFR-2. As a key mediator of blood
vessel growth, VEGF-A has been demonstrated to be a critical
regulatory protein during angiogenesis and pathological
neovascularization (7,23,24).
The aim of the present study was to investigate the
role of IL-8 in the process of angiogenesis in gastric cancer. The
present study evaluated the effects of IL-8 in angiogenesis and
additionally investigated the expression of selected angiogenesis
markers, consisting of VEGF-A, VEGFR-1 and VEGFR-2, using a
co-culture model of human gastric cancer SGC-7901 cells and human
umbilical vein endothelial cells (HUVECs).
Materials and methods
Cell culture
Human gastric cancer SGC-7901 cells and HUVECs were
obtained from the cell bank of the Chinese Academy of Sciences
(Beijing, China). All cells were propagated in endothelial cell
medium (ECM; ScienCell, Carlsbad, CA, USA) and supplemented with 5%
fetal bovine serum (FBS; Zhejiang Tianhang Biotechnology Co., Ltd.,
Hangzhou, Zhejiang, China), 1% endothelial cell growth supplement
(ScienCell), 1% penicillin and streptomycin (Biological Industries,
Beit Haemek, Israel) and 1% L-glutamine (Biological Industries) for
all experiments, with the exception of the tube formation assay.
For the tube formation assay, SGC-7901 cells and HUVECs were
propagated in Gibco Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and supplemented
with 10% FBS, 1% L-glutamine, 1% penicillin and streptomycin and 1%
L-glutamine. All cells were maintained at 37°C in a humidified
chamber containing 5% CO2.
Co-culture model, cell grouping and
IL-8 treatment
SGC-7901 cells were seeded in 24-well plates
(5.5×104 cells/well) and cultured for 24 h with
predetermined concentrations of IL-8 stock solution (Sigma-Aldrich,
St. Louis, MO, USA). Subsequently, the SGC-7901 cell culture media
was collected and added to HUVECs for additional incubation. In
total, 6 groups were established according to various
specificities, as follows: Control group, ECM/DMEM without SGC7901
cell culture medium; 0.0 ng/ml IL-8 with SGC-7901 cell culture
medium; 0.2 ng/ml IL-8 with SGC-7901 cell culture medium; 0.5 ng/ml
IL-8 with SGC-7901 cell culture medium; 0.8 ng/ml IL-8 with
SGC-7901 cell culture medium; and 1.0 ng/ml IL-8 with SGC-7901 cell
culture medium.
Transwell chamber-induced migration
assay
HUVEC cell migration was evaluated using Corning®
Costar® Transwell chambers (Corning Life Sciences, Tewksbury, MA,
USA), according to the manufacturer's protocol. Briefly, HUVECs
(4×104cells) were seeded in the top chamber of the
Transwell plate, while 600 µl cell culture medium and various
concentrations of IL-8 were placed in the lower chamber. Subsequent
to 12 h incubation, the cells remaining on the upper surface of the
polycarbonate membrane (non-migrated cells) were removed with
blunt-ended cotton swabs. The cells that had attached to the
opposite side of the membrane (migrated cells) were fixed with 4%
paraformaldehyde (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 15 min and stained for 20 min using a crystal violet cell
colony staining kit (Shanghai Sunred Biological Technology Co.,
Ltd, Shanghai, China). Following washing 3 times with PBS, images
of the cells on the membrane were captured using an Olympus®
CK40-F200 inverted microscope (Olympus, Tokyo, Japan). The results
were expressed as the mean number of cells in 4 randomly selected
microscopic fields at ×10 magnification.
Matrigel tube formation assay
The formation of HUVECs into capillary-like
structures was assessed using Matrigel (BD Biosciences, San Jose,
CA, USA) in a tube formation assay. Briefly, Matrigel was thawed
overnight, and the pipette and 96-well plates were pre-chilled for
30 min at 4°C. The Matrigel was added to each well of a 96-well
plate (80 µl/well). All plates were maintained at 4°C for 30 min
and 37°C for 30 min, allowing the gel to polymerize. Subsequently,
HUVECs were seeded on the Matrigel (1×104 cells/well)
with 20 µl DMEM and various concentrations of IL-8. Following
additional 8, 12 and 16 h incubations, the formation of
capillary-like structures was observed using a Zeiss laser confocal
scanning microscope (model no., LSM710; Carl Zeiss AG, Oberkochen,
Germany) at ×40 magnification. The tube length was analyzed by the
AxioVision Rel software, version 4.8 (Carl Zeiss AG). The results
were expressed as the mean length of 4 randomly selected tubes.
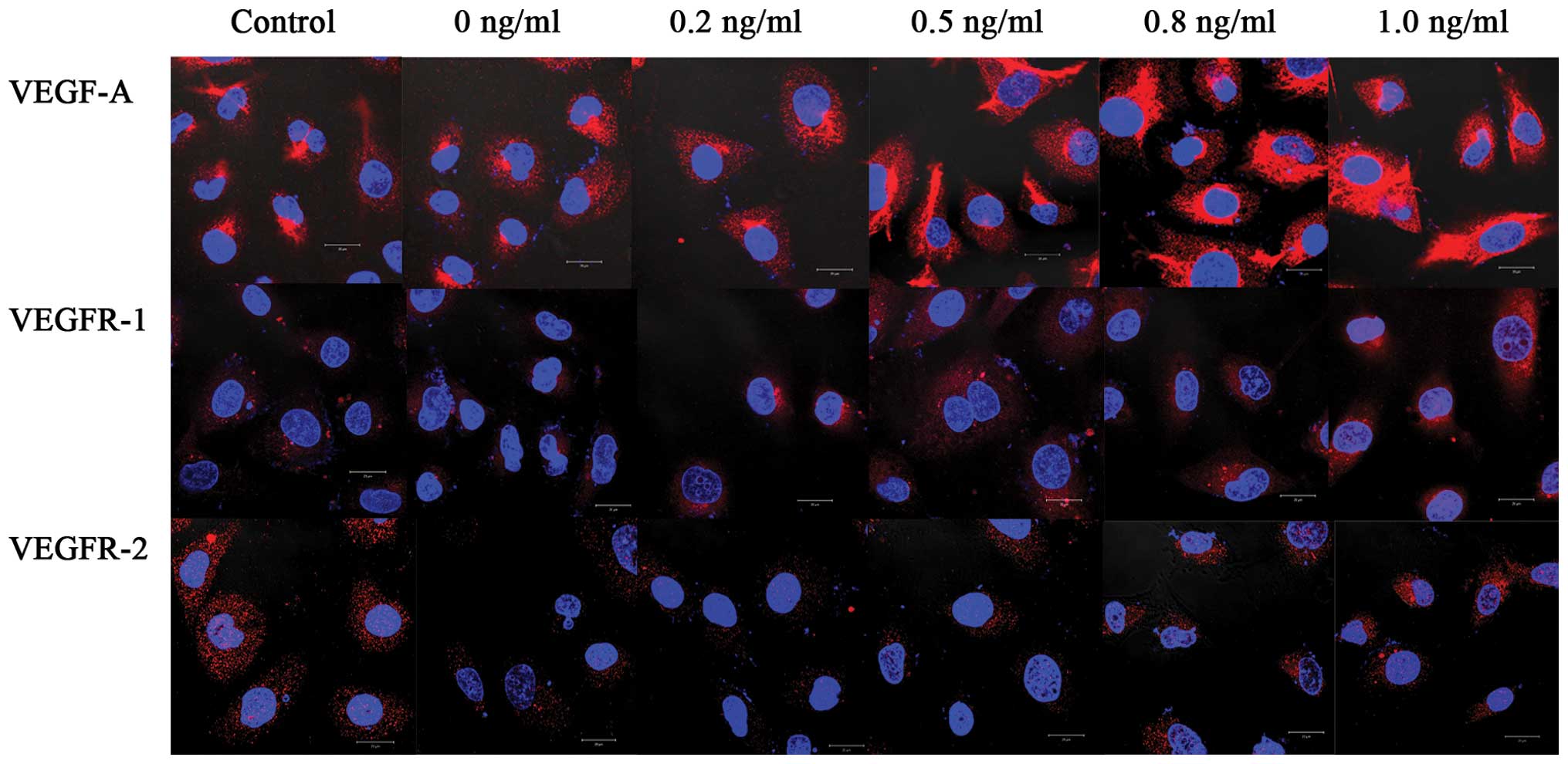
Immunofluorescence staining
HUVECs were seeded onto coverslips on 24-well plates
(5×104 cells/well) and cultured with various
concentrations of IL-8 for 24 h. Subsequently, the cells were fixed
in 4% paraformaldehyde for 15 min, permeabilized with 0.5%
Triton-100 (Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai,
China) for 10 min, incubated in 4% bovine serum albumin (Wisent
Inc., St Bruno, QC, Canada), and cultured with rabbit anti-human
VEGF-A (cat. no. 1909-1; dilution, 1:300; Epitomics, Burlingame,
CA, USA) and VEGFR-1 monoclonal antibodies (cat. no. 1303-1;
dilution, 1:300; Epitomics) and the rabbit anti-human VEGFR-2
polyclonal antibody (cat. no. ab39256; dilution, 1:150; Abcam,
Cambridge, MA, USA), at 4°C overnight. Goat anti-rabbit
Cy3-conjugated Affinipure immunoglobulin (Ig)G (heavy and light
chains) antibody (cat. no. 10285-1-AP; dilution, 1:1,000; Wuhan
Sanying Biotechnology, Wuhan, Hubei, China) was used as a secondary
antibody, and was incubated with the primary antibodies for an
additional 1 h. The cell nuclei were then labeled with
4′,6-diamidino-2-phenylindole. The coverslips were mounted on a
glass slide and visualized under a Zeiss laser confocal scanning
microscope.
Western blot analysis
HUVECs were seeded on 6-well plates
(2×105 cells/well) and cultured for 24 h with various
concentrations of IL-8. Subsequently, the cells were lysed using
150 µl cell lysate buffer (Beyotime Institute of Biotechnology,
Shanghai, China). Proteins in the total cell lysate were separated
by SDS-PAGE (10% separation gel; 5% spacer gel; Beyotime Institute
of Biotechnology) and electrotransferred to polyvinylidene
difluoride film (Bio-Rad, Laboratories, Inc., Hercules, CA, USA).
Blotted films were placed in Tris-buffered saline with Tween (TBST;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Rabbit
anti-human VEGF-A (dilution, 1:250) and VEGFR-1 monoclonal
antibodies (dilution, 1:250), rabbit anti-human VEGFR-2 polyclonal
antibody (dilution, 1:250) and mouse anti-human glyceraldehyde
3-phosphate dehydrogenase (GAPDH) monoclonal antibody (cat. no.
KM9002; dilution, 1:3,000; Tianjin Sungene Biotech Co., Ltd.,
Tianjin, China) were used to probe the blotted films overnight at
4°C. Following a thorough wash with TBST, the blots were incubated
with goat anti-rabbit IgG-hydrogen peroxide (HRP) secondary
antibody (dilution, 1:1,000; Santa Cruz Biotechnology, Inc.) or
goat anti-mouse IgG-HRP secondary antibody (dilution, 1:3,000;
Tianjin Sungene Biotech Co., Ltd.) for 1 h at room temperature. The
blots were visualized using an enhanced chemiluminescence method,
and were exposed to plain X-ray film in a darkroom. Grayscale
reconstruction was performed by Image J software version 1.48
(National Institutes of Health, Bethesda, MD, USA; available from
http://rsb.info.nih.gov./ij/), and the
expression rate of the 3 proteins (VEGF-A, VEGFR-1 and VEGFR-2)
relative to GAPDH protein expression (internal control) was
calculated. All experiments were repeated 3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
HUVECs were seeded on a 12-well plate
(1×105 cells/well) and incubated with various
concentrations of IL-8 for 24 h. Total RNA of the HUVECs was
extracted using TRIzol reagent (Takara Bio, Inc., Otsu, Shiga,
Japan), according to the manufacturer's protocol. RT-qPCR was
performed with SYBR® Green PCR mix in a Bio-Rad iQ5 PCR system
(Bio-Rad Laboratories, Inc.). Each sample was analyzed 3 times. The
PCR cycling conditions were as follows: 1 cycle at 95°C for 2 min;
1 cycle at 95°C for 15 sec; 1 cycle at 60°C for 20 sec; 1 cycle at
72°C for 20 sec; and 40 cycles at 72°C for 30 sec. The PCR primers
used for amplification are revealed in Table I. Based on the 2−∆∆Cq value
(25), GAPDH mRNA was co-amplified to
serve as an internal control, and the relative levels of VEGF-A,
VEGFR-1 and VEGFR-2 mRNA expression were calculated.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction.
| mRNA | Primer sequence
(5′-3′) | Product size,
bp |
|---|
| GAPDH |
| 145 |
|
Forward |
GGGTGTGAACCATGAGAAGTATG |
|
|
Reverse |
GATGGCATGGACTGTGGTCAT |
|
| VEGF-A |
| 239 |
|
Forward |
CTGCCATCCAATCGAGACCC |
|
|
Reverse |
TGCATTCACATTTGTTGTGCTG |
|
| VEGFR-1 |
| 91 |
|
Forward |
AAGGCACCCAGCACATCAT |
|
|
Reverse |
ACCATTTCAGGCAAAGACCAT |
|
| VEGFR-2 |
| 233 |
|
Forward |
CGATTATGGAAGTGAGTGAAAGAG |
|
|
Reverse |
CTGCCAATACCAGTGGATGTG |
|
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). All data were
presented as the mean ± standard deviation. Analysis of variance
(ANOVA) of repeated measurement data was used to assess tube
formation. One-way ANOVA was used to assess migration, and protein
and mRNA expression levels. The Fisher's least significant
difference method was used to analyze multiple post-hoc
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
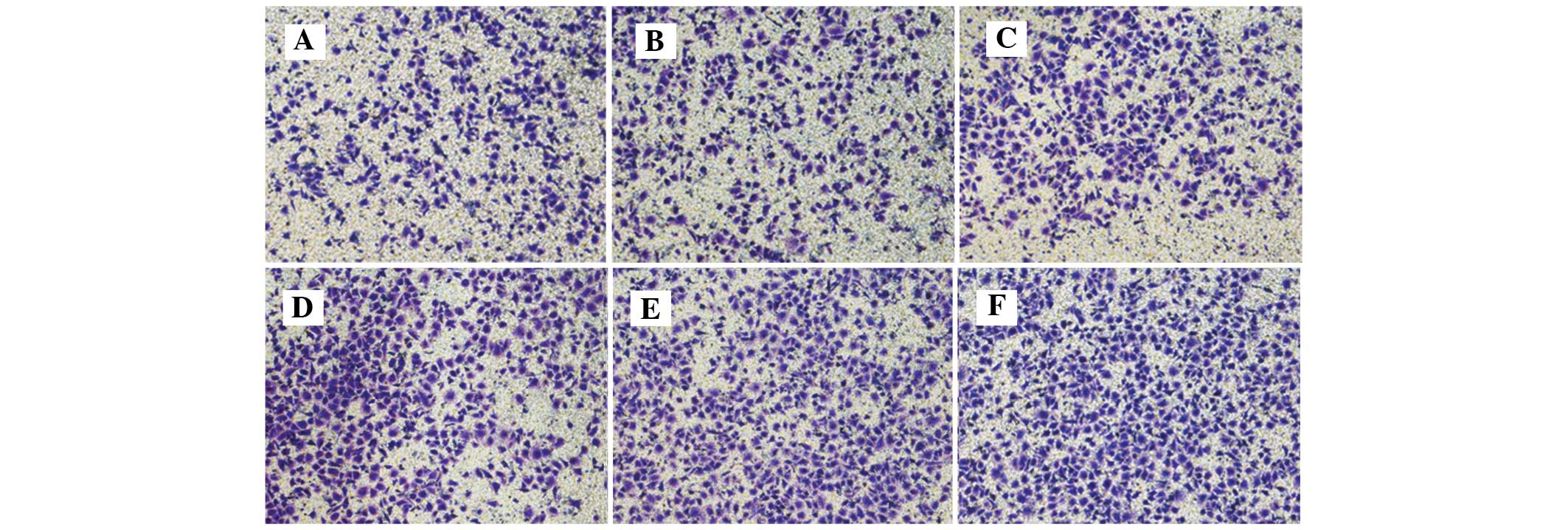
IL-8 promoted HUVEC migration
As demonstrated by Table
II and Fig. 1, the addition of
IL-8 significantly affected HUVEC migration compared with the
control cells under experimental conditions (P<0.001). The pure
SGC-7901 cell culture medium (0.0 ng/ml group) exerted no
significant effect on HUVEC migration (P=0.249). IL-8 at
concentrations of 0.5, 0.8 and 1.0 ng/ml significantly promoted
HUVEC migration (P=0.005, P=0.001 and P<0.001, respectively).
However, no significant differences in HUVEC migration was observed
between the various concentrations administered (P>0.05).
 | Table II.Effect of various concentrations of
IL-8 on human umbilical vein endothelial cells migration and tube
formation. |
Table II.
Effect of various concentrations of
IL-8 on human umbilical vein endothelial cells migration and tube
formation.
|
|
| Tube length,
µm |
|---|
|
|
|
|
|---|
| Group, ng/ml
IL-8 | Migrated cells,
n | 8 h | 12 h | 16 h |
|---|
| Control | 420±38 | 115.01±29.52 |
148.13±23.52 | 187.98±8.13 |
| 0.0 | 490±35 | 125.51±28.36 |
142.78±27.35 |
206.19±15.74 |
| 0.2 | 603±71a | 123.96±20.01 |
157.18±23.13 |
240.51±28.25 |
| 0.5 | 696±90ab | 130.31±31.74 |
173.65±23.54 |
249.03±17.33 |
| 0.8 |
756±125abc | 132.17±27.30 | 174.15±8.08 |
287.25±37.69 |
| 1.0 | 792±71abd | 134.82±28.48 |
183.15±23.22 |
335.86±34.46 |
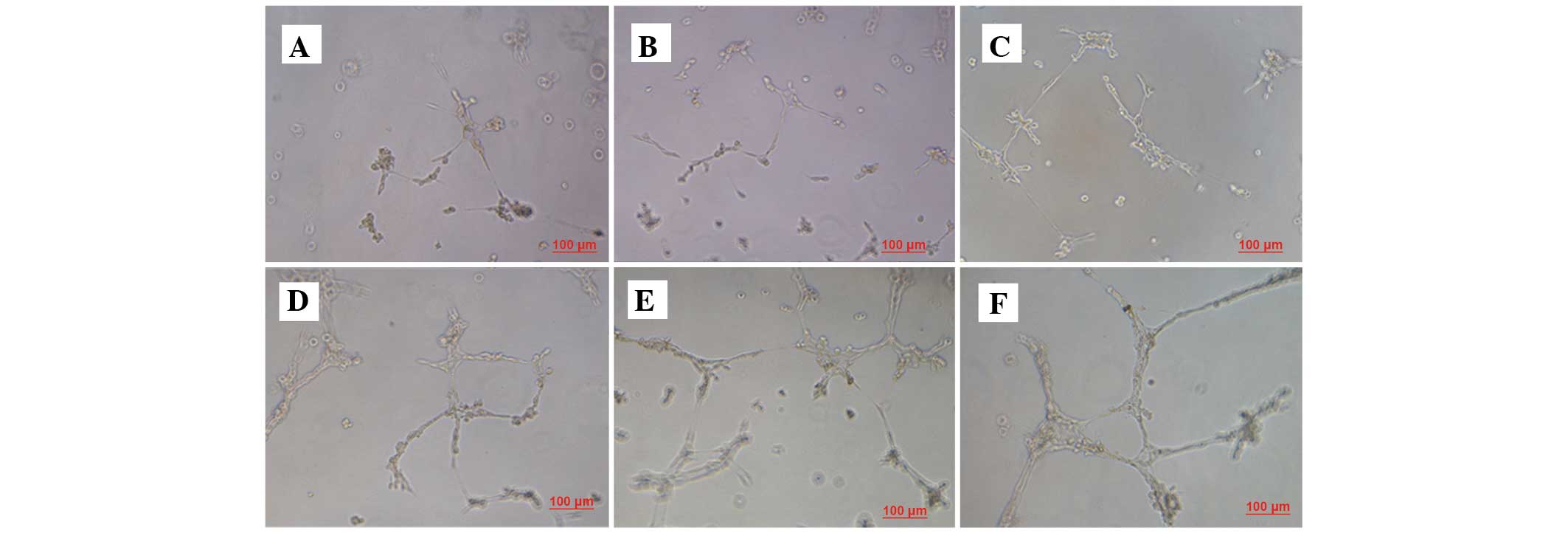
IL-8 promoted HUVEC tube
formation
Tube formation increased gradually subsequent to 8 h
of treatment. The tube lengths between the various time points were
significantly different (P<0.001). As demonstrated by Table II and Fig.
2, the administration of IL-8 significantly affected HUVEC tube
formation (P<0.001). The pure SGC-7901 cell culture medium
exerted no significant effect on HUVEC migration compared with the
control cells (P=0.517). IL-8 at concentrations of 0.5, 0.8 and 1.0
ng/ml significantly promoted HUVEC tube formation, compared with
the 0.0 ng/ml group (P=0.039, P=0.003 and P<0.001,
respectively). Furthermore, the tube length of the 1.0 ng/ml group
was increased compared with the tube length of the 0.2 ng/ml
(P=0.001) and 0.5 ng/ml (P=0.011) groups, but not the 0.8 ng/ml
group (P=0.105).
IL-8 promoted VEGF-A, VEGFR-1 and
VEGFR-2 protein expression levels in HUVECs
As demonstrated by Table
III, and Figs. 3 and 4, the addition of IL-8 significantly altered
the VEGF-A, VEGFR-1 and VEGFR-2 protein expression levels compared
with the control cells (P<0.001, P=0.009 and P<0.001,
respectively). The pure SGC-7901 cell culture medium significantly
upregulated the expression of VEGF-A protein (P=0.019). IL-8 at
concentrations >0.2 ng/ml markedly increased VEGF-A protein
levels compared with the 0.0 ng/ml group (P<0.001). The pure
SGC-7901 cell culture medium exerted no significant effect on
VEGFR-1 protein expression (P=0.9999). IL-8 at concentrations of
0.8 and 1.0 ng/ml enhanced VEGFR-1 protein expression (P=0.037 and
P=0.002, respectively). Notably, the pure SGC-7901 cell culture
medium downregulated the expression of the VEGFR-2 protein
(P<0.001). However, IL-8 at concentrations >0.2 ng/ml
markedly increased VEGFR-2 protein levels compared with the 0.0
ng/ml group (0.2 ng/ml vs. 0.0 ng/ml, P=0.034; 0.5 ng/ml vs. 0.0
ng/ml, P<0.001; 0.8 ng/ml vs. 0.0 ng/ml, P<0.001; 1.0 ng/ml
vs. 0.0 ng/ml, P<0.001). The VEGFR-2 protein level following the
addition of 1.0 ng/ml of IL-8 revealed no significant difference
compared with the control group (P=0.876).
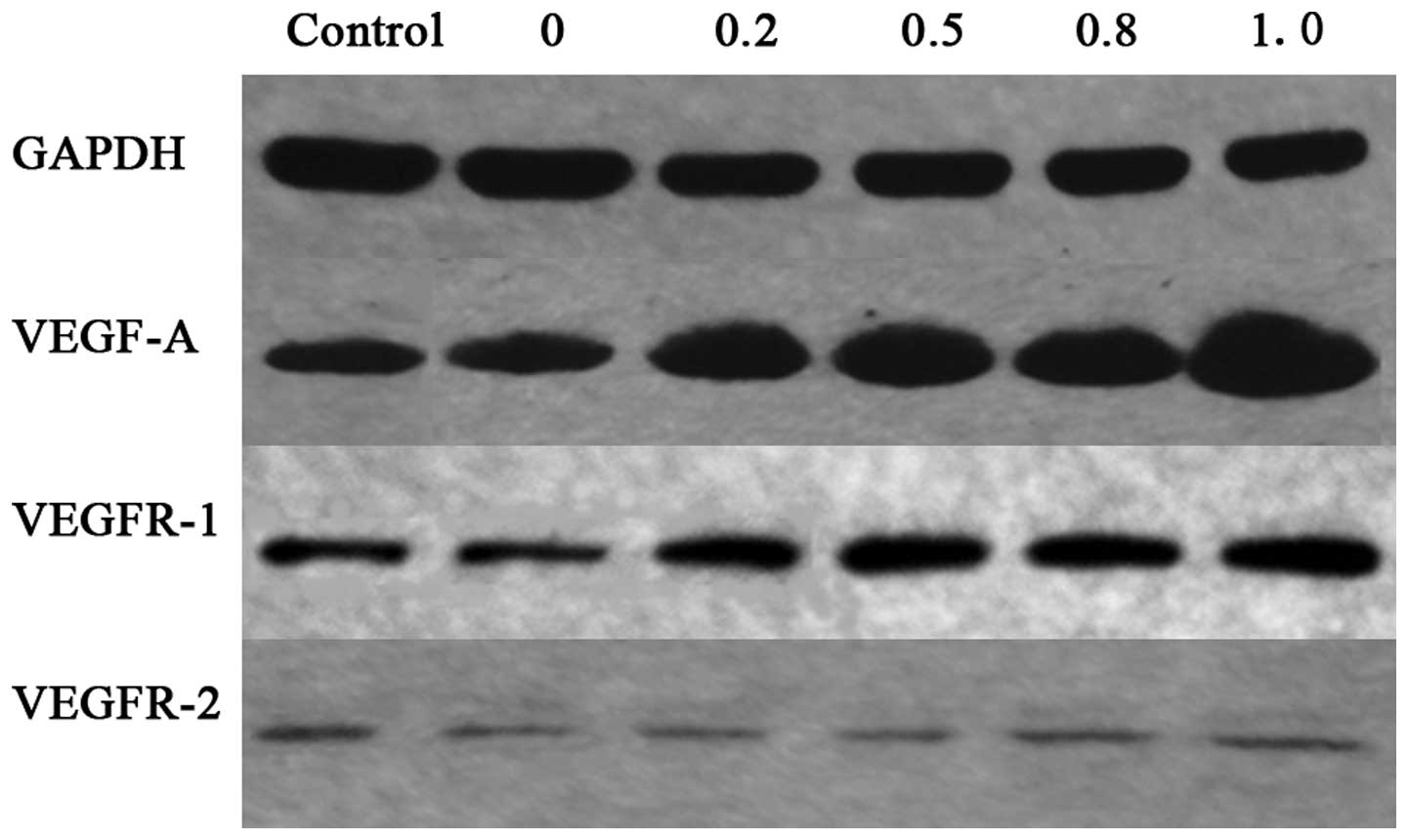 | Figure 4.Effect of various concentrations of
IL-8 on the protein expression levels of VEGF-A, VEGFR-1 and
VEGFR-2 in HUVECs, assessed using western blot analysis. The pure
SGC-7901 cell culture medium significantly upregulated the
expression of VEGF-A protein, but downregulated the expression of
VEGFR-2 protein, and exerted no significant effect on the
expression of VEGFR-1 protein. IL-8 at concentrations of 0.2, 0.5,
0.8 and 1.0 ng/ml increased VEGF-A and VEGFR-2 protein levels
compared with the 0 ng/ml group. IL-8 at concentrations of 0.8 and
1.0 ng/ml enhanced VEGFR-1 protein expression. IL-8, interleukin-8;
VEGF, vascular endothelial growth factor; VEGFR, vascular
endothelial growth factor receptor; HUVEC, human umbilical vein
endothelial cells; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
 | Table III.Effect of various concentrations of
IL-8 on VEGF-A, VEGFR-1 and VEGFR-2 protein expression levels in
human umbilical vein endothelial cells. |
Table III.
Effect of various concentrations of
IL-8 on VEGF-A, VEGFR-1 and VEGFR-2 protein expression levels in
human umbilical vein endothelial cells.
| Group, ng/ml
IL-8 | VEGF-A | VEGFR-1 | VEGFR-2 |
|---|
| Control | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| 0.0 |
1.17±0.05a | 1.00±0.01 |
0.69±0.02b |
| 0.2 |
1.70±0.00bd | 1.00±0.04 |
0.74±0.04bc |
| 0.5 |
1.75±0.03bd | 1.03±0.03 |
0.85±0.02bdf |
| 0.8 |
2.10±0.13bdfg |
1.10±0.03ace |
0.86±0.03bdfg |
| 1.0 |
2.78±0.12bdfgh |
1.17±0.12bdfg |
1.00±0.03dfh |
IL-8 promoted VEGF-A, VEGFR-1 and
VEGFR-2 mRNA expression levels in HUVECs
As demonstrated by Table
IV and Fig. 5, the administration
of IL-8 significantly affected the VEGF-A, VEGFR-1 and VEGFR-2 mRNA
expression levels compared with the control cells (P<0.001). The
pure SGC-7901 cell culture medium exerted no significant effect on
VEGF-A and VEGFR-2 mRNA expression (P=0.376 and P=0.487,
respectively), but downregulated the expression of VEGFR-1 mRNA
(P<0.001). IL-8 at concentrations of 0.5, 0.8 and 1.0 ng/ml
markedly increased the mRNA expression of VEGF-A (P=0.046, P=0.001
and P<0.001, respectively) and VEGFR-1 (P=0.042, P<0.001 and
P<0.001, respectively) compared with the 0.0 ng/ml group. IL-8
at concentrations >0.2 ng/ml markedly increased VEGFR-2 mRNA
levels compared with the 0.0 ng/ml group (0.2 ng/ml, P=0.003; 0.5
ng/ml, P=0.005; 0.8 ng/ml, P<0.001; and 1.0 ng/ml,
P<0.001).
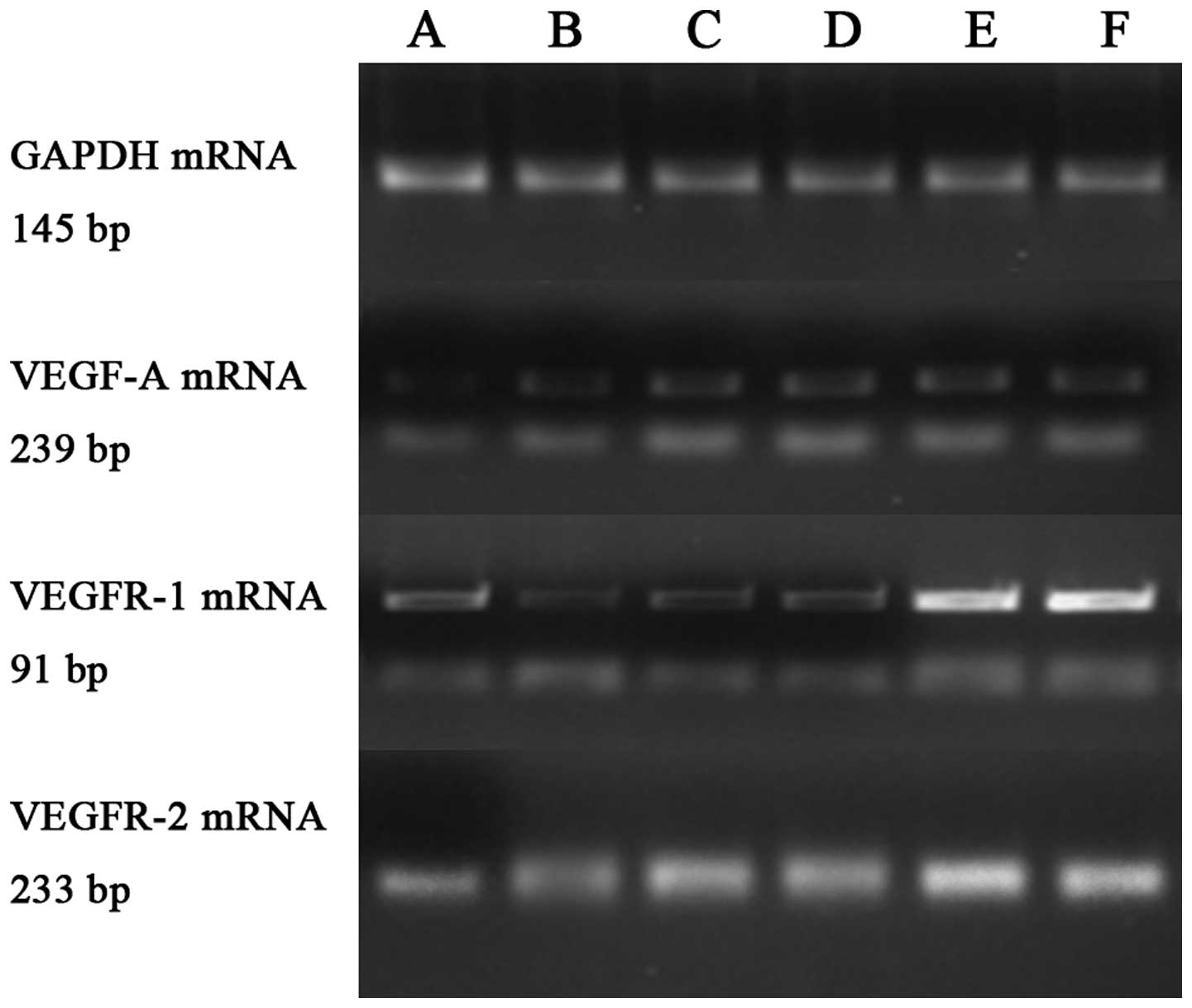 | Figure 5.Effect of various concentrations of
IL-8 on VEGF-A, VEGFR-1 and VEGFR-2 mRNA expression levels in human
umbilical vein endothelial cells using reverse
transcription-quantitative polymerase chain reaction. Lanes A-F:
Control and 0.0, 0.2, 0.5, 0.8 and 1.0 ng/ml IL-8 groups,
respectively. The pure SGC-7901 cell culture medium did not affect
VEGF-A and VEGFR-2 mRNA expression, but downregulated VEGFR-1 mRNA
expression. IL-8 at concentrations >0.5 ng/ml upregulated VEGF-A
and VEGFR-1 mRNA levels. IL-8 at concentrations >0.2 ng/ml
upregulated VEGFR-2 mRNA levels. IL-8, interleukin-8; VEGF,
vascular endothelial growth factor; VEGFR, vascular endothelial
growth factor receptor; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
 | Table IV.Effect of various concentrations of
IL-8 on VEGF-A, VEGFR-1 and VEGFR-2 mRNA expression levels in human
umbilical vein endothelial cells. |
Table IV.
Effect of various concentrations of
IL-8 on VEGF-A, VEGFR-1 and VEGFR-2 mRNA expression levels in human
umbilical vein endothelial cells.
|
| mRNA |
|---|
|
|
|
|---|
| Group, ng/ml
IL-8 | VEGF-A | VEGFR-1 | VEGFR-2 |
|---|
| Control | 1.01±0.15 | 1.00±0.09 | 1.00±0.04 |
| 0.0 | 1.12±0.06 |
0.50±0.09b | 0.83±0.01 |
| 0.2 |
1.33±0.02a |
0.64±0.00b |
1.73±0.09bd |
| 0.5 |
1.38±0.04bc |
0.73±0.10ac |
1.63±0.28bd |
| 0.8 |
1.61±0.25bde |
1.61±0.11bdfg |
3.06±0.49bdfg |
| 1.0 |
1.77±0.20bdfg |
1.56±0.24bdfg |
2.90±0.42bdfg |
Discussion
Angiogenesis is a complex process involving multiple
pro- and anti-angiogenic factors. Currently, angiogenesis is
acknowledged as a key and validated cancer therapeutic target due
to its pivotal role in the progression and metastasis of
malignancies (26,27). Worldwide, gastric cancer is one of the
leading causes of cancer-associated mortality (2). Tumor angiogenesis is closely associated
with the prognosis and hematogenous metastasis of gastric cancer
(8). IL-8 is a chemokine and a
pro-inflammatory mediator (14). The
present study evaluated the role of IL-8 in the angiogenesis of
gastric cancer. The present data demonstrated that IL-8 may be a
potent promoter of angiogenesis in gastric cancer. This result
directly supports the hypothesis that IL-8 may be a promising
therapeutic target for gastric cancer.
IL-8 is a member of the CXC chemokine family, and is
released through the nuclear factor-κB (NF-κB) signaling pathway.
Increased IL-8 expression levels have been detected in numerous
types of human cancer, including human melanoma (28), squamous cell carcinoma (29) and cervical (30), ovarian (31), non-small cell lung (32), colon (33) and gastric cancers (15). Previous evidence has revealed that
IL-8 is markedly associated with the development and metastasis of
gastric cancer via autocrine and paracrine mechanisms (34). In vivo, IL-8 is important in
the depth of invasion and venous and lymphatic invasion of tumors,
and may be an independent prognostic factor in human gastric
carcinoma (35). In vitro, the
IL-8 level is significantly associated with the adhesion,
migration, invasion and chemosensitivity of human gastric cancer
cells (36,37).
It is widely accepted that IL-8 acts as a
pro-angiogenic mediator (14,16,17). Due
to its pro-angiogenic characteristics, IL-8 may be a key mediator
in the angiogenesis and progression of various tumors. IL-8 has
been revealed to be involved in seminal plasma-induced regulation
of vascular function in cervical cancer (20), transition between liver cirrhosis and
highly vascularized hepatocellular carcinoma (18), the malignant phenotype of
hematological malignancies (38),
Epstein-Barr virus latent membrane protein-1-induced angiogenesis
in nasopharyngeal carcinoma through the NF-κB-binding site
(22) and tumor growth and
angiogenesis in melanoma via the secretion of tumor necrosis factor
α and IL-1α (21). A previous study
has identified that gastric cancer cells secret various levels of
IL-8 protein and, more notably, the level of IL-8 mRNA in the
neoplasms is markedly associated with vascularization in
vivo, suggesting that IL-8 may regulate neovascularization and
the growth and spread of gastric cancer (39). However, the role of IL-8 in gastric
cancer angiogenesis remains unclear, and few studies have
investigated its role in gastric cancer angiogenesis in
vitro. The present data reveal that IL-8 significantly promotes
HUVEC migration and tube formation, which were co-cultured with
human gastric cancer SGC-7901 cells, therefore supporting the
hypothesis that IL-8 promotes angiogenesis in gastric cancer.
VEGFs belong to a platelet-derived growth factor
supergene family. It is widely accepted that these factors are
important signaling proteins involved in lymphangiogenesis and
angiogenesis (40). VEGF-A, the
prototype VEGF ligand, which was originally isolated from tumor
cells, is a key factor in angiogenesis and vascular permeability
(7,24). As a tumor angiogenesis factor, VEGF-A
is crucial for the pathological angiogenesis of various cancers,
including chronic lymphocytic leukemia (41), astrocytic tumors (42) and breast (43), non-small cell lung (44), colorectal (45) and gastric cancers (46). Therefore, VEGF-A is regarded as a
marker for angiogenesis, and currently anti-VEGF therapy is widely
used in clinical settings to treat various cancers (40). A previous study reported that IL-8
stimulates VEGF-A expression in endothelial cells (47); however, the interaction between IL-8
and VEGF-A in gastric cancer remains unknown. The present study
revealed that IL-8 enhanced VEGF-A protein and mRNA expression
in vitro, indicating that IL-8 may be a promoter of
angiogenesis in gastric cancer.
The VEGF-VEGFR signaling system is important, not
only in physiological angiogenesis from early embryonic to adult
stages, but also in pathological angiogenesis, including in cancers
(48–52). VEGF-A binds and activates the two
tyrosine kinase (TK) receptors VEGFR-1 and VEGFR-2. VEGFR-2
(200–230 kDa) is a key factor in vascular and hematopoietic
development and activates almost all endothelial cell responses by
binding to VEGF-A (53). VEGFR-2 is
abundant in various types of cancer. Furthermore, the localization
of VEGFR-2 expression is important in cancer pathogenesis (53), and VEGFR-2 exhibits strong TK activity
towards pro-angiogenic signals (40).
Therefore, VEGFR-2 is usually investigated as a key marker of
angiogenesis in cancer (53–55). It has previously been reported that
IL-8 stimulates the autocrine activation of VEGFR-2 in endothelial
cells by the activation of NF-κB through the caspase recruitment
domain and membrane-associated guanylate kinase-like
domain-containing protein/B-cell lymphoma-10/mucosa-associated
lymphoid tissue lymphoma translocation-1 complex (47). IL-8 activates chemokine (C-X-C motif)
receptor 1/2 on endothelial cells, leading to VEGFR-2
transactivation, and this is required for IL-8-induced endothelial
permeability (56,57). However, there is little evidence
concerning the association between IL-8 and VEGFR-2 in gastric
cancer. The present study demonstrated that IL-8 elevated VEGFR-2
protein and mRNA levels, supporting the hypothesis that IL-8 may be
a pro-angiogenesis factor in gastric cancer.
VEGFR-1 (180 kDa) has an extremely high affinity for
its ligand, VEGF; however, the kinase activity of VEGFR-1 is
one-tenth lower compared to that of VEGFR-2 (40). The role of VEGFR-1 in the angiogenesis
of cancer remains ambiguous and ambivalent. A previous study
revealed that the mechanism of VEGF regulation of angiogenesis may
be due to the enhanced proliferation of VEGFRs, particularly
VEGFR-1 (54). However, an additional
study identified that VEGFR-1 may negatively regulate angiogenesis
under certain conditions, and VEGFR-1 is a suppressor of VEGFR-2
signaling (40). The current study
demonstrated that IL-8 promoted VEGFR-1 protein and mRNA
expression. Placental growth factor (PlGF) is an additional VEGF
family member that also binds VEGFR-1 (58). PlGF has been revealed to stimulate
angiogenesis and collateral growth in ischemic heart and limbs,
with a comparable efficiency to VEGF (58). PlGF and VEGFR-1 have been hypothesized
to be novel therapeutic targets for angiogenesis (58). The present study hypothesizes that the
effect of IL-8 in enhancing the VEGFR-1 level, involved in the
promotion of angiogenesis, may be due to IL-8-induced PlGF
overexpression. The potential regulatory mechanisms of the
interaction between IL-8 and VEGFR-1 require additional
investigation.
In conclusion, the present data revealed that IL-8
significantly promotes HUVEC migration and tube formation, and
increases the expression levels of the VEGF-A, VEGFR-1 and VEGFR-2
proteins and mRNA, suggesting that IL-8 may be a potent promoter of
angiogenesis in gastric cancer.
Acknowledgements
The present study was supported by grants from the
3-Year Action Plan Fund of Traditional Chinese Medicine, Shanghai
City Health Administration (grant no's. ZYSNXD-CC-ZDYJ024 and
ZY3-RCPY-3-1016) and the National Natural Science Foundation of
China (grant no. 81573762).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Ervik M, et al:
GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide.
(11)http://globocan.iarc.fr/Accessed. August
08–2014
|
|
3
|
Suh YS and Yang HK: Screening and early
detection of gastric cancer: East versus west. Surg Clin North Am.
95:1053–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group. Paoletti X, Oba
K, et al: Benefit of adjuvant chemotherapy for resectable gastric
cancer: A meta-analysis. JAMA. 303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Angiogenesis in cancer
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito H and Tsujitani S: Angiogenesis,
angiogenic factor expression and prognosis of gastric carcinoma.
Anticancer Res 21 (6B). 4365–4372. 2001.
|
|
9
|
Folkman J: Angiogenesis: A n organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel-Hett S and D'Amore PA: Signal
transduction in vasculogenesis and developmental angiogenesis. Int
J Dev Biol. 55:353–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Spiegelaere W, Casteleyn C, Van den
Broeck W, et al: Intussusceptive angiogenesis: A biologically
relevant form of angiogenesis. J Vasc Res. 49:390–404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koch S: Neuropilin signalling in
angiogenesis. Biochem Soc Trans. 40:20–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tung JJ, Tattersall IW and Kitajewski J:
Tips, stalks, tubes, Notch-mediated cell fate determination and
mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb
Perspect Med. 2:a0066012012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takagi A, Kamiya S, Koga Y, et al:
Analysis of interleukin-8 secretion induced by Helicobacter pylori
from the gastric epithelial cell line MKN45: A mechanism
independent of the intensity of cytotoxicity. J Gastroenterol
Hepatol. 12:368–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koch AE, Polverini PJ, Kunkel SL, et al:
Interleukin-8 as a macrophage-derived mediator of angiogenesis.
Science. 258:1798–1801. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strieter RM, Kunkel SL, Elner VM, et al:
Interleukin-8. A corneal factor that induces neovascularization. Am
J Pathol. 141:1279–1284. 1992.PubMed/NCBI
|
|
18
|
Nomura T, Morishita A, Jian G, et al:
Expression of angiogenic factors in hepatocarcinogenesis:
Identification by antibody arrays. Oncol Rep. 30:2476–2480.
2013.PubMed/NCBI
|
|
19
|
Akiba J, Yano H, Ogasawara S, Higaki K and
Kojiro M: Expression and function of interleukin-8 in human
hepatocellular carcinoma. Int J Oncol. 18:257–264. 2001.PubMed/NCBI
|
|
20
|
Sales KJ, Sutherland JR, Jabbour HN and
Katz AA: Seminal plasma induces angiogenic chemokine expression in
cervical cancer cells and regulates vascular function. Biochim
Biophys Acta. 1823:1789–1795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torisu H, Ono M, Kiryu H, et al:
Macrophage infiltration correlates with tumor stage and
angiogenesis in human malignant melanoma: Possible involvement of
TNFalpha and IL-1alpha. Int J Cancer. 85:182–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshizaki T, Horikawa T, Qing-Chun R, et
al: Induction of interleukin-8 by Epstein-Barr virus latent
membrane protein-1 and its correlation to angiogenesis in
nasopharyngeal carcinoma. Clin Cancer Res. 7:1946–1951.
2001.PubMed/NCBI
|
|
23
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrara N: Vascular endothelial growth
factor. Arterioscler Thromb Vasc Biol. 29:789–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−∆∆CT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meadows KL and Hurwitz HI: Anti-VEGF
therapies in the clinic. Cold Spring Harb Perspect Med.
2:a0065772012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friis T, Engel AM, Bendiksen CD, Larsen LS
and Houen G: Influence of levamisole and other angiogenesis
inhibitors on angiogenesis and endothelial cell morphology in
vitro. Cancers (Basel). 5:762–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabellini C, Trisciuoglio D, Desideri M,
et al: Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on
human malignant melanoma progression. Eur J Cancer. 45:2618–2627.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christofakis EP, Miyazaki H, Rubink DS and
Yeudall WA: Roles of CXCL8 in squamous cell carcinoma proliferation
and migration. Oral Oncol. 44:920–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu S, Shang H, Cui L, Zhang Z, Zhang Y, Li
Y, Wu J, Li RK and Xie J: Targeted blockade of interleukin-8
abrogates its promotion of cervical cancer growth and metastasis.
Mol Cell Biochem. 375:69–79. 2013.PubMed/NCBI
|
|
31
|
Wang Y, Xu RC, Zhang XL, et al:
Interleukin-8 secretion by ovarian cancer cells increases
anchorage-independent growth, proliferation, angiogenic potential,
adhesion and invasion. Cytokine. 59:145–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ning Y, Manegold PC, Hong YK, et al:
Interleukin-8 is associated with proliferation, migration,
angiogenesis and chemosensitivity in vitro and in
vivo in colon cancer cell line models. Int J Cancer.
128:2038–2049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitadai Y, Haruma K, Mukaida N, et al:
Regulation of disease-progression genes in human gastric carcinoma
cells by interleukin 8. Clin Cancer Res. 6:2735–2740.
2000.PubMed/NCBI
|
|
35
|
Kido S, Kitadai Y, Hattori N, et al:
Interleukin 8 and vascular endothelial growth factor - prognostic
factors in human gastric carcinomas? Eur J Cancer. 37:1482–1487.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ju D, Sun D, Xiu L, et al: Interleukin-8
is associated with adhesion, migration and invasion in human
gastric cancer SCG-7901 cells. Med Oncol. 29:91–99. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuai WX, Wang Q, Yang XZ, et al:
Interleukin-8 associates with adhesion, migration, invasion and
chemosensitivity of human gastric cancer cells. World J
Gastroenterol. 18:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Negaard HF, Iversen N, Bowitz-Lothe IM, et
al: Increased bone marrow microvascular density in haematological
malignancies is associated with differential regulation of
angiogenic factors. Leukemia. 23:162–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitadai Y, Haruma K, Sumii K, et al:
Expression of interleukin-8 correlates with vascularity in human
gastric carcinomas. Am J Pathol. 152:93–100. 1998.PubMed/NCBI
|
|
40
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: P hysiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ayad MW: ElN aggar AA: Angiogenic factor
VEGF and its relationship with biological prognostic markers in
chronic lymphocytic leukemia. Egypt J Immunol. 17:59–71.
2010.PubMed/NCBI
|
|
42
|
Ido K, Nakagawa T, Sakuma T, et al:
Expression of vascular endothelial growth factor-A and mRNA
stability factor HuR in human astrocytic tumors. Neuropathology.
28:604–611. 2008.PubMed/NCBI
|
|
43
|
Xu X, Wang B, Ye C, et al: Overexpression
of macrophage migration inhibitory factor induces angiogenesis in
human breast cancer. Cancer Lett. 261:147–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: Coexpression of MUC1 glycoprotein with multiple
angiogenic factors in non-small cell lung cancer suggests
coactivation of angiogenic and migration pathways. Clin Cancer Res.
6:1917–1921. 2000.PubMed/NCBI
|
|
45
|
Rasheed S, McDonald PJ, Northover JM and
Guenther T: Angiogenesis and hypoxic factors in colorectal cancer.
Pathol Res Pract. 204:501–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He LF, Wang TT, Gao QY, et al:
Stanniocalcin-1 promotes tumor angiogenesis through up-regulation
of VEGF in gastric cancer cells. J Biomed Sci. 18:392011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Martin D, Galisteo R and Gutkind JS:
CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF)
expression and the autocrine activation of VEGFR2 in endothelial
cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1)
complex. J Biol Chem. 284:6038–6042. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shibuya M: Involvement of Flt-1 (VEGF
receptor-1) in cancer and preeclampsia. Proc Jpn Acad Ser B, Phys
Biol Sci. 87:167–178. 2011. View Article : Google Scholar
|
|
50
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alitalo K and Carmeliet P: Molecular
mechanisms of lymphangiogenesis in health and disease. Cancer Cell.
1:219–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jakobsson L, Bentley K and Gerhardt H:
VEGFRs and Notch, A dynamic collaboration in vascular patterning.
Biochem Soc Trans. 37:1233–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kampen KR: The mechanisms that regulate
the localization and overexpression of VEGF receptor-2 are
promising therapeutic targets in cancer biology. Anticancer Drugs.
23:347–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tas F, Duranyildiz D, Oguz H, et al:
Circulating serum levels of angiogenic factors and vascular
endothelial growth factor receptors 1 and 2 in melanoma patients.
Melanoma Res. 16:405–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hanrahan EO, Lin HY, Kim ES, et al:
Distinct patterns of cytokine and angiogenic factor modulation and
markers of benefit for vandetanib and/or chemotherapy in patients
with non-small-cell lung cancer. J Clin Oncol. 28:193–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petreaca ML, Yao M, Liu Y, Defea K and
Martins-Green M: Transactivation of vascular endothelial growth
factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for
IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell.
18:5014–5023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen SU, Chou CH, Lin CW, et al: Signal
mechanisms of vascular endothelial growth factor and interleukin-8
in ovarian hyperstimulation syndrome: Dopamine targets their common
pathways. Hum Reprod. 25:757–767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luttun A, Tjwa M and Carmeliet P:
Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1):
Novel therapeutic targets for angiogenic disorders. Ann NY Acad
Sci. 979:80–93. 2002. View Article : Google Scholar : PubMed/NCBI
|