Introduction
Pancreatic cancer has the poorest prognosis of any
major malignancy (5-year survival rate, 6%) (1). At present, surgical resection represents
the only potentially curative treatment strategy in these patients,
however, the 5-year survival rate following surgical resection
remains low, at 5.5–21% (2,3). Gemcitabine (GEM)-based chemotherapy
forms the core of multimodal therapy and has improved the prognosis
of patients with pancreatic cancer (3). Multimodal therapies including
preoperative treatments have been investigated, and studies
indicate that preoperative chemoradiotherapy followed by surgery
may improve the clinical outcome by reducing the frequency of local
recurrence and increasing the 5-year survival rate in pancreatic
cancer patients (4–8). However, in cases where preoperative
therapy is not sufficiently effective and extensive tumor growth
occurs, chemotherapy may unnecessarily increase the time between
diagnosis and surgery, and may result in the patient missing the
opportunity for surgical resection. Therefore, it is essential to
identify the specific pre-treatment prognostic factors that can
determine which patients will benefit from preoperative
therapy.
To date, the identified prognostic factors
predominantly consist of various pathological characteristics of
the resected tumor specimen, including tumor size (9), histological grade, vascular invasion
(10), lymph node metastases
(11) and intrapancreatic perineural
invasion (12). However, each of
these factors can only be determined following surgical
resection.
There is increasing evidence demonstrating that
inflammatory cells in the tumor microenvironment are important in
the development of tumors; blood cell counts in peripheral blood,
which in part reflect immune function in cancer patients, are
considered part of the internal environment (13–20). A
number of prognostic factors based on cancer-associated systemic
inflammation have been investigated, including the following: Serum
C-reactive protein (CRP) combined with albumin levels (modified
Glasgow prognostic score; mGPS) (21); Albumin level in combination with
lymphocyte count (Onodera's prognostic nutritional index; PNI)
(22); the neutrophil to lymphocyte
ratio (NLR) (23), combining
neutrophil and lymphocyte counts; and the platelet to lymphocyte
ratio (PLR) (24), combining platelet
and lymphocyte counts. However it is unknown whether such
prognostic markers correlate with the outcome of preoperative
therapy in pancreatic cancer patients.
The present study aimed to determine whether the
presence of systemic inflammation predicts the outcome of
preoperative treatments in patients with pancreatic cancer.
Materials and methods
Patient population
The present retrospective analysis included data
from 56 consecutive patients with histologically confirmed
pancreatic ductal adenocarcinoma, whose tumors were completely
resected by surgery (R0) at Osaka University Hospital (Suita,
Osaka, Japan) between March 2007 and October 2012. None of the
patients had received any prior treatments, and all were newly
diagnosed. During this period, patients with any T stage (cT1–4)
and degree of lymph node involvement, including regional and
distant lymph nodes (N1 and M1 lym), but without distant organ
metastasis, received chemoradiotherapy prior to surgery. All
patients had sufficient renal, hepatic, cardiac and bone marrow
reserve and were able to tolerate the planned chemotherapy and
subsequent surgical procedures.
The disease stages of all patients were determined
prior to preoperative therapy and following surgery, according to
the International Union Against Cancer criteria (25). Pre-treatment clinical staging was
based on computed tomography (CT) scans of the chest and abdomen,
magnetic resonance imaging, and positron emission tomography (PET)
scanning. Lymph nodes measuring ≥1.0 cm in maximum transverse
diameter on CT scans were diagnosed as metastasis-positive; if
lymph nodes were visible but measured <1.0 cm, they were
regarded as metastasis-positive only when the PET scan revealed
focal prominent 18-fluorodeoxyglucose uptake. The study protocol
was approved by the Human Ethics Review Committee of Osaka
University School of Medicine. Written informed consent was
obtained from all patients.
Hematological examination
Routine laboratory tests for leukocyte, neutrophil,
lymphocyte, platelet, C-reactive protein (CRP), albumin,
carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA)
and DUPAN-2 levels were conducted prior to surgery and the
commencement of preoperative therapy. The latex immunonephelometry
method was applied to measure the serum concentration of CRP
(normal range, 0–0.3 mg/dl) using a JCA-BM6070 automated
biochemical analyzer (JEOL Ltd., Tokyo, Japan) and CRP-EX (LSI
Medience Corporation, Tokyo, Japan). The chemiluminescence enzyme
immunoassay method was applied to measure serum levels of CA19-9,
CEA and DUPAN-2 using Lumipulse G1200 (Fujirebio, Inc., Tokyo,
Japan), Access CEA reagent and the UniCel DXI 800 immunoassay
system (Beckman Coulter, Inc., Brea, CA, USA). Serum levels <37
U/ml for CA19-9, <5 ng/ml for CEA and <150 U/ml for DUPAN-2
were considered as normal levels in the present study. Based on the
mGPS (21), which combines CRP and
albumin concentrations, patients who had both elevated CRP levels
(>1 mg/dl) and albumin levels <3.5 g/dl were assigned a score
of 2. Patients with only elevated CRP (>1 mg/dl) were assigned a
score of 1. Patients with neither of these abnormalities were
assigned a score of 0. The PNI was calculated using the following
formula: PNI = [albumin (g/dl) × 10] + [0.005 × total lymphocyte
count (/µl)] (22,26).
Preoperative therapy and postoperative
follow-up
The preoperative treatment consisted of GEM-based
chemotherapy [GEM alone (600–1,000 mg/m2) or GEM plus
S-1 (60–80 mg/m2), a fourth-generation oral
fluoropyrimidine] combined with 40 or 50.4 Gy irradiation, as
reported previously (4,27). Based on the CONKO-001 study (3), gemcitabine-based adjuvant chemotherapy
has been routinely administered since 2007. Postoperative follow-up
consisted of a routine physical examination and laboratory tests,
including assessment of serum levels of CEA, CA19-9 and DUPAN-2.
Chest X-ray and CT/ultrasonography of the abdomen were performed
every 3 months, and the presence or absence of cancer recurrence
was carefully monitored. Recurrence was defined as the detection of
a new abnormal finding or the gradual enlargement of an abnormal
finding during any imaging study. The median follow-up period of
the 56 patients was 27.1 months (range, 6.1–80.2 months).
Evaluation of response to preoperative
therapies
The preoperative treatment effect was determined
based on the examination of hematoxylin-eosin (Sigma-Aldrich, St.
Louis, MO, USA) stained permanent sections by a gastrointestinal
pathologist; samples were scored using a previously published
grading system, Evans classification (28). A minimal pathological response was
defined as a treatment effect score of grade I or grade IIa (≥90%
or 50–89% viable tumor cells, respectively, remaining following
therapy). Grades IIb (10–49% viable tumor cells remaining) or III
(<10% viable tumor cells remaining) were considered a partial
pathological response. The absence of any remaining viable tumor
cells, corresponding to grade IV, was considered a complete
pathological response.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Clinicopathological parameters were compared using the
Fisher's exact test and χ2 test, and continuous
variables were compared using a Mann-Whitney U test. A receiver
operating characteristic (ROC) curve was constructed to estimate
the optimal cut-off value of the pre-treatment NLR. A logistic
regression analysis was used to analyze the simultaneous influence
of predictive factors. Odds ratios (ORs) estimated from the
logistic analysis were reported as relative risks with
corresponding 95% confidence intervals (CIs). In all analyses, a
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using JMP software
version 10.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The 56 patients in the current study comprised 34
(60.7%) males and 22 (39.3%) females, and the mean age was
65.6±10.8 years (range, 38–84 years). All patients who received
preoperative chemoradiotherapy followed by surgery were enrolled in
the study. With regard to the hematological examination, the mean
NLR value among the 56 patients was 2.6±1.6, the mean PLR value was
165.8±70.2, and the mean PNI value was 44.9±4.7. In 47 patients
(83.9%), the tumor was localized to the pancreatic head. Other
clinical and histopathological information is listed in Table I.
 | Table I.Clinicopathological characteristics
of the included patients (n=56). |
Table I.
Clinicopathological characteristics
of the included patients (n=56).
| Parameter | Value |
|---|
| Age (years) | 65.6±10.8 |
| Gender, n |
|
|
Male | 34 |
|
Female | 22 |
| White blood cells
(/µl)a | 5425.7±1450.4 |
| Neutrophil
(%)a | 60.6±9.4 |
| Lymphocyte
(%)a | 27.6±7.9 |
| Platelets
(104/µl)a | 22.1±6.8 |
| C-reactive protein
(mg/dl)a | 0.36±0.67 |
| Albumin
(g/dl)a | 3.8±0.3 |
| Carbohydrate
antigen 19-9 (U/ml)a | 328.0±391.9 |
| Carcinoembryonic
antigen (ng/ml)a | 5.6±14.8 |
| DUPAN-2
(U/ml)a | 1951.7±7451.4 |
| Neutrophil to
lymphocyte ratioa | 2.6±1.6 |
| Platelet to
lymphocyte ratioa | 165.8±70.2 |
| Modified Glasgow
prognostic score, n |
|
|
1+2 | 6 |
| 0 | 50 |
| Prognostic
nutrition indexa | 44.9±4.7 |
| Location, n |
|
|
Pancreatic head | 47 |
|
Pancreatic body | 3 |
|
Pancreatic tail | 6 |
| cT stage, n |
|
| T1 | 3 |
| T2 | 1 |
| T3 | 51 |
| T4 | 1 |
| cN status, n |
|
|
Positive | 4 |
|
Negative | 52 |
| cStage, n |
|
| I | 4 |
|
IIA | 46 |
|
IIB | 4 |
|
III | 1 |
| IV | 1 |
| Maximal diameter
(mm)a | 21.3±13.6 |
| Histology, n |
|
|
Well-differentiated | 1 |
|
Moderately differentiated | 54 |
| Poorly
differentiated | 1 |
| pT stage, n |
|
| T1 | 14 |
| T2 | 5 |
| T3 | 37 |
| T4 | 0 |
| pN status, n |
|
|
Positive | 17 |
|
Negative | 39 |
| pStage, n |
|
| I | 14 |
|
IIA | 26 |
|
IIB | 15 |
|
III | 0 |
| IV | 1 |
| Evans grade, n |
|
| I | 10 |
|
IIa | 30 |
|
IIb | 14 |
|
III | 2 |
| Adjuvant therapy,
n |
|
|
Yes | 42 |
| No | 14 |
| Recurrence, n |
|
|
Yes | 34 |
| No | 22 |
Comparison of mean NLR values of the
poor and good response groups
In order to assess the association between
hematological factors and the pathological response to preoperative
therapies, the patients who underwent preoperative treatments were
divided into a poor response group (Evans grade I or IIa) and a
good response group (Evans grade IIb or III). The background
clinical and histopathological factors were compared between the
two groups (Table II). The mean NLR
value was significantly higher in the poor response group than in
the good response group, whereas the other examined factors
demonstrated no significant differences between the two groups.
 | Table II.Comparison of clinical and
histopathological factors between poor response group (Evans I+IIa)
and good response group (Evans IIb+III). |
Table II.
Comparison of clinical and
histopathological factors between poor response group (Evans I+IIa)
and good response group (Evans IIb+III).
|
| Evans grade |
|
|---|
|
|
|
|
|---|
| Parameter | I/IIa (n=40) | IIb/III (n=16) | P-value |
|---|
| Age
(years)a | 65.9±10.0 | 64.7±12.9 | NS |
| Gender, n |
|
| NS |
|
Male | 25 | 9 |
|
|
Female | 15 | 7 |
|
| CA19-9
(U/ml)a | 313.6±377.3 | 365.3±439.3 | NS |
| CEA
(ng/ml)a | 6.4±17.4 | 3.4±1.8 | NS |
| DUPAN-II
(U/ml)a | 2231.2±8717.9 | 1233.0±1971.5 | NS |
| NLRa | 2.9±1.8 | 1.9±0.6 | 0.0481 |
| PLRa | 172.9±73.4 | 147.3±59.2 | NS |
| mGPS, n |
|
| NS |
|
1+2 | 5 | 0 |
|
| 0 | 35 | 16 |
|
| PNIa | 44.2±4.4 | 46.8±5.1 | NS |
| Location, n |
|
| NS |
|
Pancreatic head | 28 | 8 |
|
|
Pancreatic body | 8 | 6 |
|
|
Pancreatic tail | 4 | 2 |
|
| cT stage, n |
|
| NS |
| T1 | 3 | 0 |
|
| T2 | 1 | 0 |
|
| T3 | 35 | 16 |
|
| T4 | 1 | 0 |
|
| cN status, n |
|
| NS |
|
Positive | 4 | 0 |
|
|
Negative | 36 | 16 |
|
| cStage, n |
|
| NS |
| I | 4 | 0 |
|
|
IIA | 31 | 15 |
|
|
IIB | 4 | 0 |
|
|
III | 1 | 0 |
|
| IV | 0 | 1 |
|
| Maximal diameter
(mm)a | 22.3±14.9 | 18.8±9.4 | NS |
| Histology, n |
|
| NS |
|
Well | 1 | 0 |
|
|
Moderate | 38 | 16 |
|
|
Poor | 1 | 0 |
|
| pT stage, n |
|
| 0.0611 |
| T1 | 7 | 7 |
|
| T2 | 3 | 2 |
|
| T3 | 30 | 7 |
|
| T4 | 0 | 0 |
|
| pN status, n |
|
| NS |
|
Positive | 13 | 3 |
|
|
Negative | 27 | 13 |
|
| pStage, n |
|
| NS |
| I | 7 | 7 |
|
|
IIA | 20 | 6 |
|
| pStage, n |
|
| NS |
|
IIB | 12 | 3 |
|
|
III | 0 | 0 |
|
| IV | 1 | 0 |
|
| Adjuvant therapy,
n |
|
| NS |
|
Yes | 28 | 14 |
|
| No | 12 | 2 | NS |
| Recurrence, n |
|
| 0.0695 |
|
Yes | 21 | 13 |
|
| No | 19 | 3 |
|
Optimal cut-off level of the
pre-therapeutic NLR
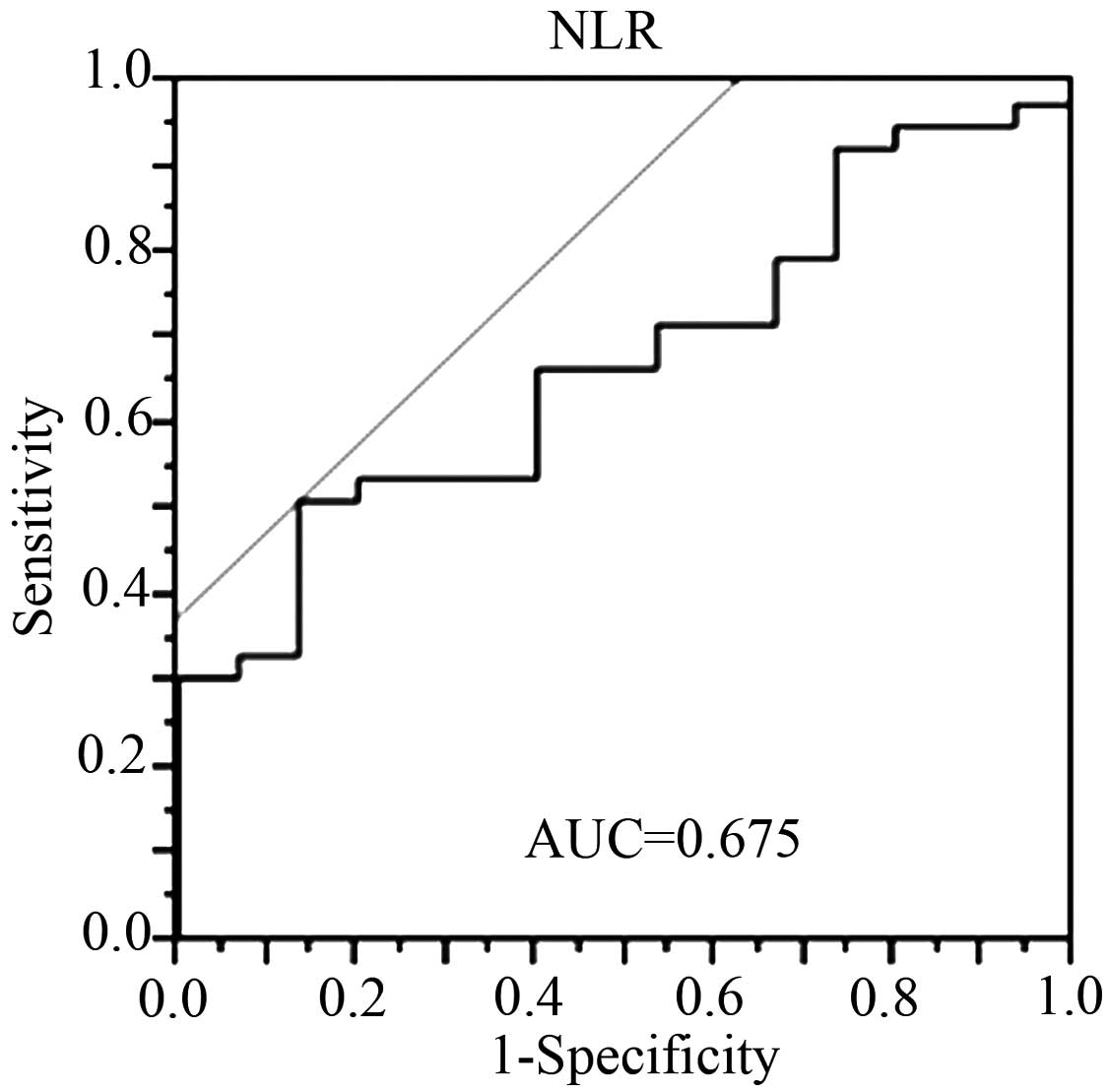
An ROC curve was prepared by plotting sensitivity
values against specificity values at the indicated NLR (Fig. 1). From the ROC curve, the optimal
cut-off level of the pre-therapeutic NLR for predicting
pathological non-responders (Evans I/IIa) was determined to be
2.2.
NLR is an independent predictive
factor for pathological response
The evaluation of predictive factors for the
pathological response among clinical information were assessed,
including a number of prognostic markers that have been previously
reported: NLR (23), PLR (24), mGPS (21) and PNI (22). Upon univariate analysis, NLR and mGPS
were determined to be significantly associated with the
pathological response, whilst the other prognostic markers were not
(Table IIIA). Furthermore,
multivariate analysis identified NLR as a significant and
independent predictive factor (Table
IIIB). NLR and mGPS are both closely related to inflammation;
therefore, mGPS was not included in the multivariate analysis.
Subsequently, the association between the blood NLR value and
features of the corresponding clinical specimen were examined.
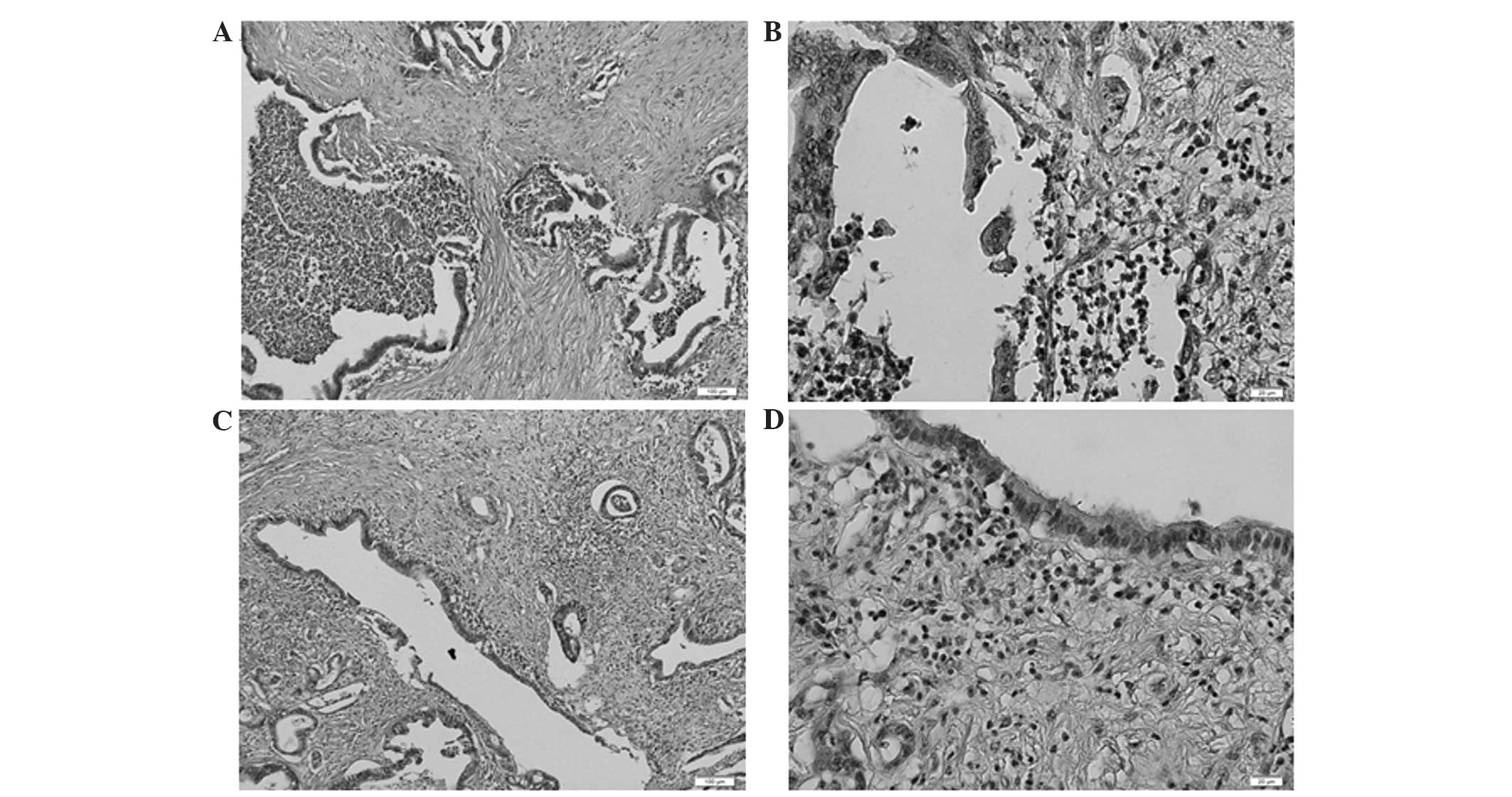
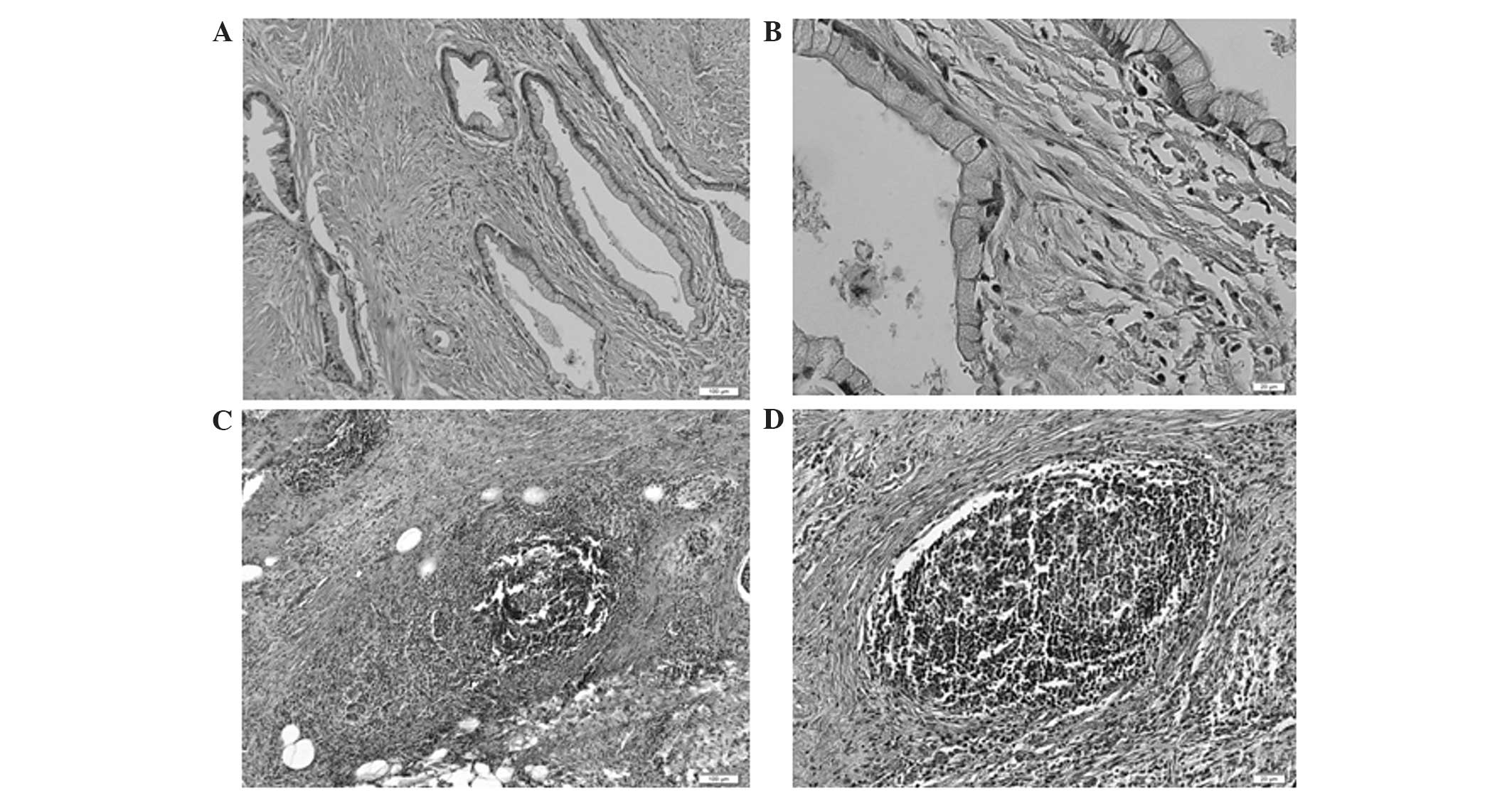
Notably, numerous masses of neutrophils were detected in pancreatic
ductal adenocarcinoma in cases with particularly high NLRs
(Fig. 2), and the formation of
lymphoid follicles in the stromal tissue adjacent to the tumor was
observed in cases with particularly low NLRs (Fig. 3). This finding indicated that the NLR
determined by blood examination at least partially reflected the
state of the inflammation in the corresponding clinical
specimens.
 | Table III.Predictive factors for the
pathological response in clinical information. |
Table III.
Predictive factors for the
pathological response in clinical information.
| A, Univariate
analysis |
|---|
|
|---|
| Variable | OR (95% CI) | P-value |
|---|
| NLR
(≥2.2/<2.2) | 6.84
(1.61–47.58) |
0.00740 |
| mGPS (1+2/0) | NA | 0.0407 |
| cT
(T1,T2/T3,T4) | NA | 0.0935 |
| cN (+/-) | NA | 0.0935 |
|
| B, Multivariate
analysis |
|
|
|
|
|
| Variable | OR (95% CI) | P-value |
|
| NLR
(≥2.2/<2.2) | 5.35
(1.21–38.03) |
0.0257 |
| cT
(T1,T2/T3,T4) | NA | 0.175 |
| cN (+/-) | NA | 0.175 |
Finally, the predictive ability of the NLR with
regard to the pathological response to preoperative therapies was
evaluated. Table IV shows the
prediction of pathological responses using the pre-treatment NLR
values (≥2.2/<2.2). The NLR was revealed to be a significant
predictive marker of pathological response (P=0.00699): The good
response rates were 9.1% in patients with an NLR ≥2.2, and 44.1% in
patients with an NLR <2.2.
 | Table IV.Association between pathological
response and pretreatment NLR. |
Table IV.
Association between pathological
response and pretreatment NLR.
| Pathological
response | High NLR (≥2.2),
n | Low NLR (<2.2),
n | P-value |
|---|
| Evans grade
I/IIa | 20 | 19 | 0.00699 |
| Evans grade
IIb/III | 2 | 15 |
|
Discussion
The NLR, which is an inexpensive and widely
available blood test, has been demonstrated to be an important
prognostic predictor in numerous types of cancer, including
colorectal cancer (29), gastric
cancer (30), ovarian cancer
(31), intrahepatic
cholangiocarcinoma (32),
hepatocellular carcinoma (33), and
pancreatic cancer (34). Furthermore,
it has been reported that the NLR is correlated with the
pathological response to preoperative therapy (35,36).
However, there have been no reports focusing on the association
between high NLR and poor response to neoadjuvant therapies in
pancreatic cancer. In the present study, various pre-treatment
hematological factors related to pathological response were
assessed.
Biologically, the significance of high neutrophil
counts in malignant tumors is based on a combination of T-cell
suppression via the production of certain active substances, such
as reactive oxygen species, nitric oxide and arginase (37,38), and
stimulation of tumor angiogenesis through the production of IL-8,
vascular endothelial growth factor, elastase and matrix
metalloproteinase (39–41). By contrast, previous reports have
suggested that a high number of tumor-infiltrating lymphocytes was
strongly associated with favorable outcomes in patients with
various types of cancer (42,43). Furthermore, lymphocytes, particularly
T cells, are considered to play a central role in antitumor
immunity; thus, the lymphocyte count is thought to reflect the
ability of the body to eliminate tumor cells (44).
Recently, a number of combination therapies,
consisting of preoperative chemoradiotherapy, surgery and
postoperative chemotherapy, have been used in clinical trials,
which were found to improve the poor prognosis of pancreatic cancer
(27,45). In cases where combined therapies are
used, it is essential to identify predictors of response to
preoperative therapy in order to inform the assessment of risk and
patient counselling. Similar multimodal therapies have been used
for the treatment of esophageal and rectal cancers, as well as
pancreatic cancer; NLR has been reported to be a useful and
available predictive marker associated with pathological response
to neoadjuvant chemotherapy or preoperative chemoradiotherapy in
esophageal and rectal cancers, respectively (35,36).
However, to the best of our knowledge, the present study is the
first to demonstrate that pre-treatment NLR is significantly higher
in pancreatic cancer patients who respond poorly to treatment
compared with that of patients who exhibit a favorable response.
NLR was identified as a significant independent risk factor among
pre-treatment clinical factors, and the ratio of pathologically
favorable responses was significantly lower in patients with an NLR
≥2.2 compared with that of the patients with an NLR <2.2. This
finding suggests that pre-treatment NLR may be used to predict
which patients will benefit from preoperative therapy.
In conclusion, pre-treatment NLR is an independent
predictive marker of the pathological response to preoperative
therapy in pancreatic cancer patients. However, long term analysis
to investigate the association between pre-treatment NLR and
disease free or overall survival has not yet been performed. Thus,
further large scale, long-term studies are required to establish a
cut-off value for the NLR which may be used to guide preoperative
treatment choices.
Acknowledgements
The authors would like to thank Professor Eiichi
Morii and Dr Satoshi Nojima (Department of Pathology, Graduate
School of Medicine, Osaka University) for evaluating the
pathological findings.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neoptolemos JP, Stocken DD, Friess H,
Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L,
Dervenis C, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eguchi H, Nagano H, Tanemura M, Takeda Y,
Marubashi S, Kobayashi S, Kawamoto K, Wada H, Hama N, Akita H, et
al: Preoperative chemoradiotherapy, surgery and adjuvant therapy
for resectable pancreatic cancer. Hepatogastroenterology.
60:904–911. 2013.PubMed/NCBI
|
|
5
|
Ohigashi H, Ishikawa O, Eguchi H,
Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H,
Tomita Y and Nishiyama K: Feasibility and efficacy of combination
therapy with preoperative full-dose gemcitabine, concurrent
three-dimensional conformal radiation, surgery and postoperative
liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg.
250:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans DB, Varadhachary GR, Crane CH, Sun
CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA,
et al: Preoperative gemcitabine-based chemoradiation for patients
with resectable adenocarcinoma of the pancreatic head. J Clin
Oncol. 26:3496–3502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi H, Ohigashi H, Gotoh K,
Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M and
Ishikawa O: Preoperative gemcitabine-based chemoradiation therapy
for resectable and borderline resectable pancreatic cancer. Ann
Surg. 258:1040–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuda T, Taniguchi F, Minato H, Nomura
H, Tsuda T and Aikawa I: Successful resection of advanced
pancreatic tail cancer after neoadjuvant gemcitabine chemotherapy:
Report of a case. Surg Today. 36:754–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fortner JG, Klimstra DS, Senie RT and
Maclean BJ: Tumor size is the primary prognosticator for pancreatic
cancer after regional pancreatectomy. Ann Surg. 223:147–153. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Griffanti-Bartoli F, Arnone GB, Ceppa P,
Ravera G, Carrabetta S and Civalleri D: Malignant tumors in the
head of the pancreas and the periampullary region. Diagnostic and
prognostic aspects. Anticancer Res. 14:657–666. 1994.PubMed/NCBI
|
|
11
|
Raut CP, Tseng JF, Sun CC, Wang H, Wolff
RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE, et al:
Impact of resection status on pattern of failure and survival after
pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg.
246:52–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozaki H, Hiraoka T, Mizumoto R, Matsuno S,
Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y,
et al: The prognostic significance of lymph node metastasis and
intrapancreatic perineural invasion in pancreatic cancer after
curative resection. Surg Today. 29:16–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bambury RM, Teo MY, Power DG, Yusuf A,
Murray S, Battley JE, Drake C, O'Dea P, Bermingham N, Keohane C, et
al: The association of pre-treatment neutrophil to lymphocyte ratio
with overall survival in patients with glioblastoma multiforme. J
Neurooncol. 114:149–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fox P, Hudson M, Brown C, Lord S, Gebski
V, De Souza P and Lee CK: Markers of systemic inflammation predict
survival in patients with advanced renal cell cancer. Br J Cancer.
109:147–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimazaki J, Goto Y, Nishida K and Tabuchi
T, Motohashi G, Ubukata H and Tabuchi T: In patients with
colorectal cancer, preoperative serum interleukin-6 level and
granulocyte/lymphocyte ratio are clinically relevant biomarkers of
long-term cancer progression. Oncology. 84:356–361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A Glasgow Inflammation Outcome Study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
23
|
Garcea G, Ladwa N, Neal CP, Metcalfe MS,
Dennison AR and Berry DP: Preoperative neutrophil-to-lymphocyte
ratio (NLR) is associated with reduced disease-free survival
following curative resection of pancreatic adenocarcinoma. World J
Surg. 35:868–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors (7th). Oxford:
Wiley-Blackwell. 2010.
|
|
26
|
Buzby GP, Mullen JL, Matthews DC, Hobbs CL
and Rosato EF: Prognostic nutritional index in gastrointestinal
surgery. Am J Surg. 139:160–167. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eguchi H, Nagano H, Kobayashi S, Kawamoto
K, Wada H, Hama N, Tomimaru Y, Akita H, Sakai D, Satoh T, et al: A
phase I trial of combination therapy using gemcitabine and S-1
concurrent with full-dose radiation for resectable pancreatic
cancer. Cancer Chemother Pharmacol. 73:309–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishi Y, Kopetz S, Chun YS, Palavecino M,
Abdalla EK and Vauthey JN: Blood neutrophil-to-lymphocyte ratio
predicts survival in patients with colorectal liver metastases
treated with systemic chemotherapy. Ann Surg Oncol. 16:614–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang N, Deng JY, Liu Y, Ke B, Liu HG and
Liang H: The role of preoperative neutrophil-lymphocyte and
platelet-lymphocyte ratio in patients after radical resection for
gastric cancer. Biomarkers. 19:444–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pre-treatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Immunother. 58:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gomez D, Morris-Stiff G, Toogood GJ, Lodge
JP and Prasad KR: Impact of systemic inflammation on outcome
following resection for intrahepatic cholangiocarcinoma. J Surg
Oncol. 97:513–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gomez D, Farid S, Malik HZ, Young AL,
Toogood GJ, Lodge JP and Prasad KR: Preoperative
neutrophil-to-lymphocyte ratio as a prognostic predictor after
curative resection for hepatocellular carcinoma. World J Surg.
32:1757–1762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stotz M, Gerger A, Eisner F, Szkandera J,
Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner
C, et al: Increased neutrophil-lymphocyte ratio is a poor
prognostic factor in patients with primary operable and inoperable
pancreatic cancer. Br J Cancer. 109:416–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato H, Tsubosa Y and Kawano T:
Correlation between the pretherapeutic neutrophil to lymphocyte
ratio and the pathologic response to neoadjuvant chemotherapy in
patients with advanced esophageal cancer. World J Surg. 36:617–622.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dou X, Wang RB, Yan HJ, Jiang SM, Meng XJ,
Zhu KL, Xu XQ and Mu DB: Circulating lymphocytes as predictors of
sensitivity to preoperative chemoradiotherapy in rectal cancer
cases. Asian Pac J Cancer Prev. 14:3881–3885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Müller I, Munder M, Kropf P and Hänsch GM:
Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows
or brothers in arms? Trends Immunol. 30:522–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rodriguez PC, Ernstoff MS, Hernandez C,
Atkins M, Zabaleta J, Sierra R and Ochoa AC: Arginase I-producing
myeloid-derived suppressor cells in renal cell carcinoma are a
subpopulation of activated granulocytes. Cancer Res. 69:1553–1560.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shamamian P, Schwartz JD, Pocock BJ, Monea
S, Whiting D, Marcus SG and Mignatti P: Activation of progelatinase
A (MMP-2) by neutrophil elastase, cathepsin G and proteinase-3: A
role for inflammatory cells in tumor invasion and angiogenesis. J
Cell Physiol. 189:197–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scapini P, Nesi L, Morini M, Tanghetti E,
Belleri M, Noonan D, Presta M, Albini A and Cassatella MA:
Generation of biologically active angiostatin kringle 1–3 by
activated human neutrophils. J Immunol. 168:5798–5804. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Di Carlo E, Forni G and Musiani P:
Neutrophils in the antitumoral immune response. Chem Immunol
Allergy. 83:182–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density and location of immune cells
within human colorectal tumors predict clinical outcome. Science.
313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morris M, Platell C and Iacopetta B:
Tumor-infiltrating lymphocytes and perforation in colon cancer
predict positive response to 5-fluorouracil chemotherapy. Clin
Cancer Res. 14:1413–1417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Galon J, Pagès F, Marincola FM, Thurin M,
Trinchieri G, Fox BA, Gajewski TF and Ascierto PA: The immune score
as a new possible approach for the classification of cancer. J
Transl Med. 10:12012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Motoi F, Unno M, Takahashi H, et al:
Influence of preoperative anti-cancer therapy on resectability and
perioperative outcomes in patients with pancreatic cancer: Project
study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery.
J Hepatobiliary Pancreat Sci. 21:148–158. 2014. View Article : Google Scholar : PubMed/NCBI
|