Introduction
Worldwide, colorectal cancer (CRC) is ranked the
third most commonly diagnosed cancer types of in both genders after
lung and breast cancers, accounting for almost 10% of the cancer
mortalities each year. Approximately 1.4 million new cases are
diagnosed annually with CRC of which there is an estimated
mortality rate of 50% (1). In the
year 2005, the population of the Kingdom of Saudi Arabia (KSA) was
estimated at 16,945,484, comprising mostly of native Saudis (62%).
In that same year, the Saudi Cancer Registry reported that CRC was
the second most common malignancy among Saudis for all ages (10.3%)
and the number one malignancy in males (11.8%) (2,3).
Additionally, between 1994 and 2003 age-standardized rates for CRC
in KSA almost doubled (2). Between
2001 and 2003, while the annual percent change (APC) of CRC
incidence in Saudi females exhibited an insignificant increase of
6%, a profoundly rising incidence among Saudi males was observed,
with an APC of 20.5% (4). It has been
predicted that by the year 2030, the CRC incidence in KSA may
increase four-fold in the two genders (2). This suggests a foreseeable increase in
cancer burden mainly attributed to population growth, adoption of
unhealthy life styles and its associated risk factors and aging of
the population.
Early diagnosis and treatment of CRC positively
correlates with higher patient survival rates (5). In the U.S., for example, the 5-year
survival rate is as high as 93.2% for stage I as compared to only
8.1% for stage IV (5). Consequently,
early diagnosis is a vital goal for any healthcare system to
achieve a reliable treatment and successful clinical outcome of CRC
patients. Several clinical tests are currently employed for CRC
screening, including fecal occult blood tests (FOBT), radiologic
tests, colonoscopy and stool DNA test. However, none of these
approaches have been regarded as the method of choice due to their
invasiveness, low sensitivity or high cost (6,7). Thus, it
is fundamentally important to search for novel, non-invasive,
specific and sensitive biomarkers for the early diagnosis of
CRC.
Recently, microRNAs (miRNAs or miRs), which
represent a newly-discovered class of short (19–25 nucleotides)
non-coding RNAs, have acquired considerable interest due the roles
they play in a variety of cell processes including development,
cell cycle progression, cell differentiation, proliferation and
apoptosis (8). Bioinformatics and
cloning studies have indicated that miRNAs may
post-transcriptionally regulate almost 60% of all human genes and
control hundreds of cognate gene targets through their oncogenic or
tumor-suppressive activity (9).
Aberrant miRNA expressions have been associated with several types
of hematological and solid malignant tumors (10,11)
including CRC (12–17), highlighting their potential for
diagnostic and prognostic applications, and classification of human
malignancies (18,19). While the discovery of
malignancy-linked RNAs in plasma/serum were first described almost
10 years ago (20), scientific
reports on the existence of miRNAs in body fluids such as blood
plasma/serum, saliva, urine and semen of cancer patients have only
been presented more recently (21–24).
Notably, various studies have recently confirmed the potential
utility of circulating miRNAs as stable biomarkers for the
multi-stage process of carcinogenesis in solid tumors and several
other malignancies (21,23).
The investigation of cancer-specific circulating
miRNA biomarkers is an emerging and promising field of research.
Thus, we aimed in the current study to conduct targeted
transcriptional profiling of a panel of four mature miRNA
signatures (miR-145, miR-195, miR-29 and
miR-92) in the plasma and colorectal tissues of Saudi CRC
patients in relation to healthy controls to assess their potential
diagnostic value using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). These miRNA genes were
particularly selected based on a comprehensive review of relevant
literatures and previously published data on CRC (15,22,25–28).
In addition, the association between the aberrant expression of
these circulating miRNA gene profiles and CRC clinical stages was
also evaluated.
Materials and methods
Patients
The subjects recruited in this study (CRC patients,
n=20; healthy neoplasm-free controls, n=20) were subjected to
rigorous eligibility criteria for the selection (Table I). Patients were excluded if they had
been undergoing chemotherapy or radiotherapy prior to blood
sampling, or clinically diagnosed with familial adenomatous
polyposis or hereditary non-polyposis CRC. The
tumor-node-metastasis classification system accepted by the Union
for International Cancer Control (29) has been employed for staging of
malignant tumors. Signed written and informed consent forms were
collected from all the subjects participating in this study for the
use of their blood and tissue samples. Research protocols conducted
in this project were approved by the Institutional Medical Ethics
Review board at the College of Applied Medical Sciences Affiliated
to King Saud University (Riyadh, Kingdom of Saudi Arabia). All
samples were obtained from the Saudi CRC Biobank at King Khalid
University Hospital.
 | Table I.Eligibility criteria for the
selection and exclusion of study subjects. |
Table I.
Eligibility criteria for the
selection and exclusion of study subjects.
| Clinicopathological
characteristics |
|---|
| General inclusion
criteria |
| 1. Age
≥18 years and ≤80 years |
| 2. Not
currently residing in an institution, such as a nursing home or
shelter |
| 3. Not
severely ill in the intensive care unit |
| 4. With
the capability to give informed consent |
| 5.
Encountered between September 2013 and March 2015 |
| Healthy individuals
(plasma control group) |
| 1.
Underwent the medical check-up in King Khalid University
Hospital |
| 2.
Asymptomatic and apparently healthy without a previous history of
cancer |
| 3.
Confirmed healthy condition without malignancy in the physical
examinations |
| 4. No
system infection (lung, gastrointestinal or urinary tract) |
| Colorectal cancer
patients (CRC group) |
| 1.
Underwent colonoscopy biopsy and colorectal surgical
resections |
| 2.
Diagnosed by 2 experienced pathologists |
| 4. No
pre-operative chemotherapy and radiotherapy |
Tissue isolation and RNA
extraction
Paired fresh CRC tissue specimens and their adjacent
non-cancerous normal mucosa were collected from 20 patients who
underwent surgical resection of tumors by surgeons and subsequently
examined by pathologists. The CRC tissues were histologically
confirmed to be an adenocarcinoma of the colon. Tissue specimens
were collected in RNAlater® RNA Stabilization Reagent (Qiagen,
Hilden, Germany) tubes, snap-frozen in liquid nitrogen, and stored
at −80°C until further analysis. Total RNA including miRNAs was
extracted from 50–100 mg of cryo-preserved tissues by use of
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
as described in the manufacturer's protocol. In order to maximize
RNA yield, a homogenization step was carried out by use of a
TissueLyser LT with 5-mm stainless-steel beads (Qiagen).
Plasma preparation and circulating RNA
extraction
Blood was drawn from the 20 CRC patients recruited
in this study as well as 20 age-matched neoplasm-free healthy
subjects. The cancer-free status of blood-donating healthy control
subjects was confirmed a priori based on their negative health
examination results including: blood test, chest X-ray, abdominal
ultrasound examination, FOBT, rectal examination, computed
tomography scan and colonoscopy. None of the controls had been
previously diagnosed with any types of malignancy. Peripheral whole
blood (8–10 ml) was collected from each individual into BD
Vacutainer® blood collection tubes (BD Biosciences, Franklin Lakes,
NJ, USA) (EDTA spray-coated). Plasma was fractionated from whole
blood samples according to the procedure described by Duttagupta
et al (30). Freshly drawn
whole blood was processed for plasma fractionation and the obtained
plasma was frozen at −80°C within 4 h from blood draw. The plasma
samples were spectrophotometrically analyzed to be free from
haemoglobin (31). Hemolyzed plasma
samples were excluded from further analysis. Whole blood was
centrifuged at 1,700 × g for 10 min. The obtained cloudy
supernatant was transferred to a fresh tub and centrifuged at 2,000
× g for 10 min. Subsequently, the obtained supernatant was
centrifuged at 12,000 × g for 10 min to pellet any remaining
cellular debris. Circulating RNA extraction was essentially
conducted on 1 ml plasma volume using the Plasma/Serum Circulating
and Exosomal RNA Purification kit (Slurry Format; Norgen Biotek
Corp., Thorold, ON, Canada) following the manufacturer's
instructions. The resulting eluate was subjected to an additional
concentration step by the use of the RNA Clean-Up and Concentration
kit (Norgen Biotek Corp., Thorold, ON, Canada) using 20 µl elution
buffer to collect the RNA.
RNA quality
The concentration of the isolated RNA from tissue
specimens was assessed by measuring the optical density at 260 nm
(OD260) and 280 nm (OD280) using a Qubit® 2.0 Fluorometer (Thermo
Fisher Scientific, Waltham, MA, USA) and a NanoDrop 1000
spectrophotometer (Peqlab Biotechnologie GmbH, Erlangen, Germany),
whereas the quality of the RNA purified from plasma was assessed by
PCR amplification curves and efficiencies, due to the absence of
ribosomal RNA. RNA purity was evaluated by the ratio of the
absorbance at OD260/OD280. Analysis of RNA integrity (RIN) was
conducted using the Agilent 2100 Bioanalyzer (Agilent Technologies
GmbH, Waldbronn, Germany) with the RNA 6000 series II Nano LabChip
analysis kit. The 2100 Bioanalyzer generates numerical RIN values.
RIN is an incremental scale spanning 0–10, where increasing RIN
correlates with increasing RIN value. Total RNA samples extracted
from CRC and adjacent neoplasm-free mucosal tissues were of high
integrity as judged by the obtained RIN values of ≥8.0.
miRNA quantification by real-time
(RT)-qPCR
Total RNA including miRNAs, from a fixed plasma
volume of 1 ml or 1 µg RNA from colorectal tissues, was
polyadenylated and reverse transcribed to cDNA in a final volume of
20 µl using the miScript Reverse Transcription kit (Qiagen) and the
5X miScript HiSpec buffer. Prior to qPCR, cDNA was diluted by
adding 200 µl RNase-free water to the 20 µl RT reaction. qPCR
assays were performed in triplicate measurements using the miScript
SYBR-Green PCR kit (Qiagen, Germany) on the Rotor-Gene Q 5-Plex HRM
(Qiagen) according to the manufacturer's instructions. The miRNAs
utilized in this study were purchased from Qiagen.
The amplification profile was denatured at 95°C for
10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min, in which fluorescence was obtained. After the above PCR
cycling program, melting curve analyses and agarose gel
electrophoresis (3.0%) on the amplicons were conducted to validate
the specificity of the expected PCR amplicon. Raw cycle threshold
(Ct) values, defined as the number of cycles required for the
fluorescent signal to cross the threshold in an RT-qPCR experiment,
were calculated using the Rotor-Gene® Q software 2.1 (Qiagen), and
implementing automatic baseline settings and a threshold of 0.1.
The ΔCt was then calculated by subtracting the Ct values of the
control [housekeeping (HK)] gene from the Ct values of the gene of
interest. ΔΔCt was then calculated by subtracting ΔCt of the
appropriate controls (plasma, miR-191; tissues, RNUB-6) from the
ΔCt of the CRC plasma or tissue sample. The expression level of
each miRNA gene was represented by fold change, which was
calculated using the equation 2−ΔΔCt. The efficiency of
each miRNA assay was determined by constructing a standard curve
using a series of total RNA dilutions. Assays exhibited good
linearity (R2>0.96) across the obtained Ct values and
the log of the initial total RNA quantity of each dilution (data
not shown).
Statistical analysis
Data were presented as mean ± standard deviation.
The Mann-Whitney U test was employed to evaluate the differential
expression level of miRNAs between CRC patients and healthy
controls using SPSS Data Analysis Software version 19 (SPSS, Inc.,
Chicago, IL, USA).
Results and Discussion
Clinicopathological characteristics of
CRC patients
Inclusion criteria for the 20 CRC patients and 20
healthy individuals (plasma control group) are detailed in Table I. The age of the participants was
within the range of ≥18 and ≤80 years. Clinicopathological
parameters of the 20 participants (9 men and 11 women) recruited in
the study are shown in Table II. The
mean age of the subjects was 61 years (±10.6 standard deviation).
None of the participants exhibited any evidence of other disease
complications. In addition, 5 participants (25%) had stage I CRC, 3
(15%) had stage II, 7 (25%) had stage III, while the remaining 5
(35%) were diagnosed with stage IV of the disease (Table II). In terms of tumor location, 7
patients had tumors which were localized to the rectum, 5 to the
distal colon and 8 to the proximal colon. Histologic examination
revealed that the diagnosed CRC tumors were of the adenocarcinoma
type: 16 adenocarcinoma, 2 mucous adenocarcinoma and 2 signet ring
cell (Table II).
 | Table II.Clinicopathological characteristics
of CRC patients. |
Table II.
Clinicopathological characteristics
of CRC patients.
| Variable | Frequency,
n=20 |
|---|
| Gender |
|
|
Male | 9 |
|
Female | 11 |
| Age at
diagnosis |
| Mean ±
standard deviation | 61±10.6 |
| Median
range | 61 (26–84) |
| TNM stage |
|
| I | 5 |
| II | 3 |
|
III | 7 |
| IV | 5 |
| Nodal stage |
|
|
Positive | 12 |
|
Negative | 8 |
| T stage |
|
| T1 | 3 |
| T2 | 4 |
| T3 | 9 |
| T4 | 4 |
| Tumor location |
|
|
Rectum | 7 |
| Distal
colon | 5 |
|
Proximal colon | 8 |
| Histology |
|
|
Adenocarcinoma | 16 |
| Mucous
adenocarcinoma | 2 |
| Signet
ring cell | 2 |
qPCR analyses of mature miRNAs
Mounting evidence suggests miRNAs function in
carcinogenesis as oncogenes or tumor suppressors (8,19,27). Thus, it was fundamentally important to
identify CRC-related miRNA signatures by comprehensive quantitative
techniques such as qPCR to broaden our understanding of their
functional roles in CRC biology and pathogenesis. In this context,
we selected four mature miRNAs genes, i.e., miR-145, miR-195,
miR-29 and miR-92, to profile their expression levels in the plasma
and tissue samples of CRC patients in relation to healthy normal
specimens. Previously, the most frequently used method for the
quantification of miRNAs was Northern blotting. Alsmost a decade
ago, several assays including cDNA arrays, modified invader and
flow cytometry were introduced for the quantitative determination
of miRNAs (18,32–34).
However, these assays often suffer from a serious limitation of low
sensitivity due to the fact that they lack a miRNA amplification
step (13). Thus, being more
sensitive and RT-qPCR technology was employed to overcome the
relatively low sensitivity issues of these assays (35). Traditionally, miRNA discovery
workflows using global profiling are firstly conducted with either
cDNA microarrays or deep sequencing (next generation sequencing).
Subsequently, the RT-qPCR assay is employed as the method of choice
to evaluate the diagnostic potential on a panel of selected and
defined miRNAs of interest (36).
Determination of the most
invariantly-expressed housekeeping miRNA gene in plasma and
tissues
A critical prerequisite for any RT-qPCR assay to
robustly profile circulating miRNA species in body fluids including
plasma is the ability to isolate and accurately measure those
miRNAs under study (30). In order to
be able to accurately profile the expression of miRNA biomarkers in
CRC plasma samples in relation to normal controls we first
identified the most invariantly-expressed miRNA gene to utilize as
a reference (HK) gene for RT-qPCR. Thus, we examined the expression
of eight different miRNA genes and their differential expression
between CRC and cancer-free plasma samples which was calculated by
subtracting their obtained Ct values
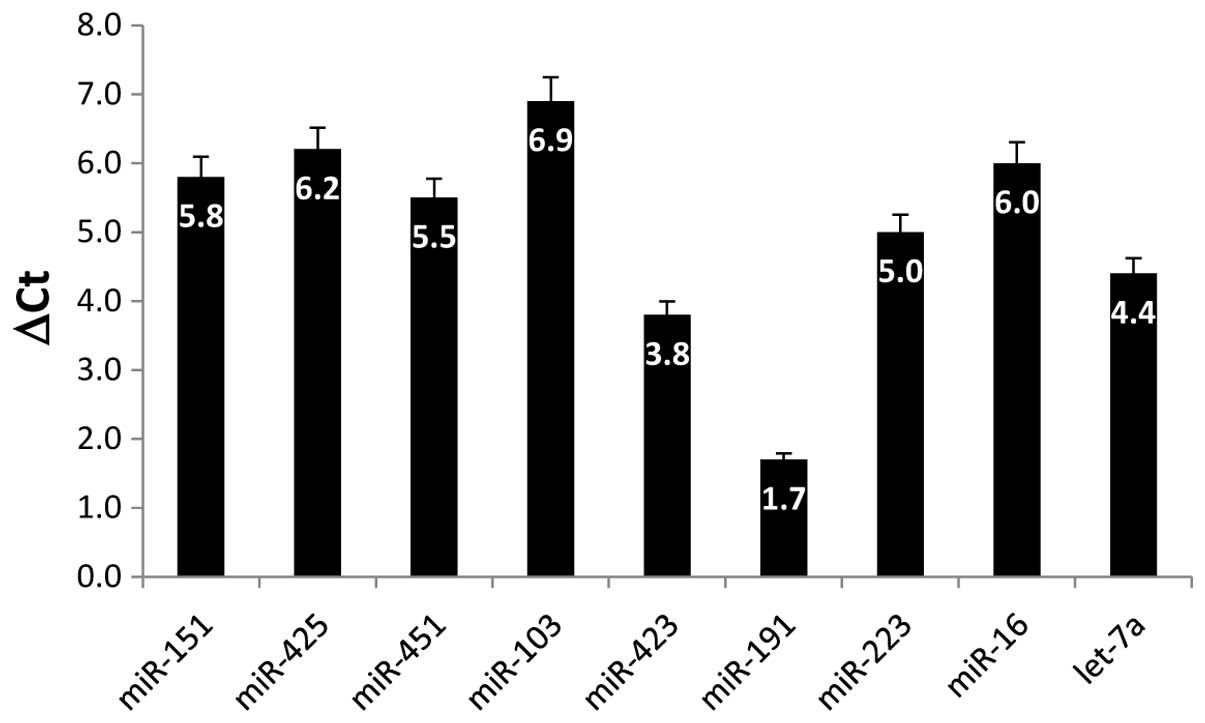
(ΔCt=Cttumor-Ctcontrol). The results shown in
Fig. 1 indicated that the least
variable gene among the studied miRNA genes is miR-191 with a ΔCt
value of only 1.7. Therefore, it was employed as a reliable HK gene
for fold change calculations. Previous reports have recommended
using miR-16, miR-223 or let-7a as normalizers for RT-qPCR data as
they maintain a high and constantly expressed level of expression
in plasma/serum samples (37).
However, based on our obtained results the abovementioned candidate
normalizer miRNAs exhibited the most variable ΔCt values among the
9 genes examined (Fig. 1) and were
therefore disregarded as HK genes in miRNA gene expression
profiling in plasma. Nevertheless, more empirical validations
remain to be systematically conducted in order for a consensus on
robust and accurate normalization controls to be identified. As for
colorectal tissues, we established RNU6B (a small nucleolar RNA
gene) as an ideal internal control HK gene, as it exhibited a
consistent, stable and high expression across all the tested tissue
samples
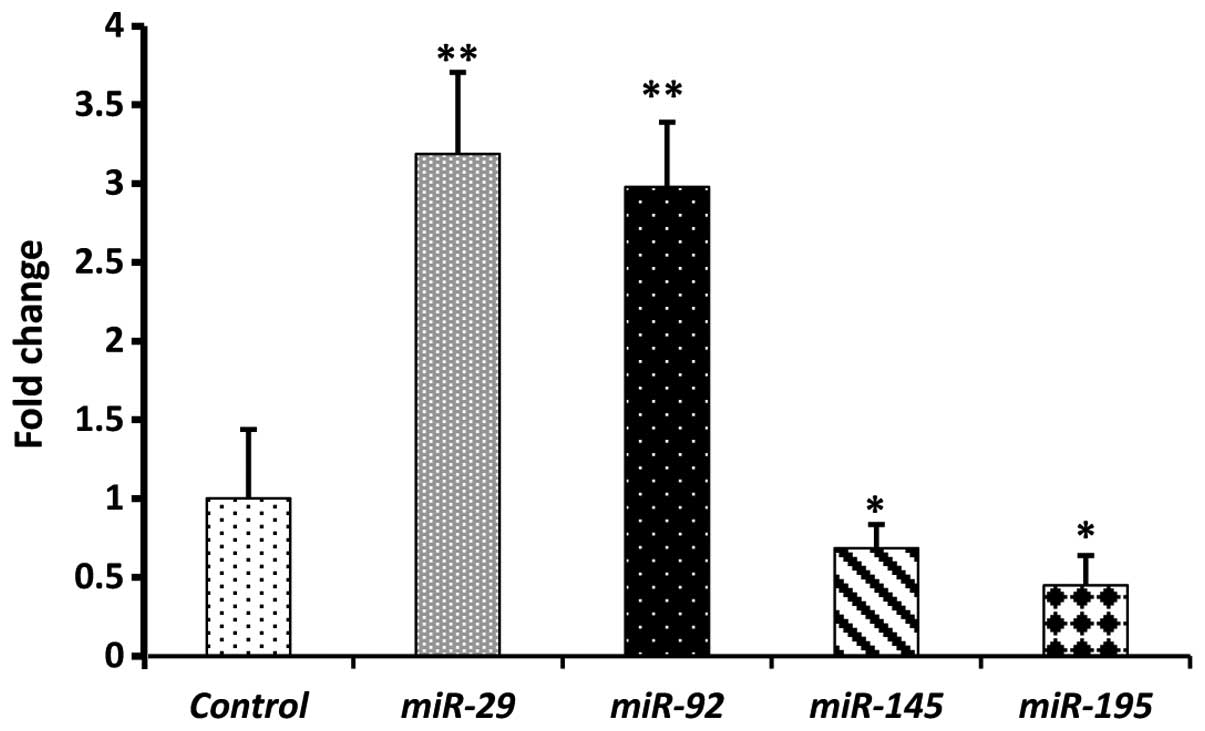
Results of qPCR profiling of 20 clinical CRC plasma
samples showed that the expression levels of circulating
miR-29 and miR-92 were significantly higher
(P<0.01) than in the age-matched normal plasma, with mean
fold-change values of (3.2) and (2.9), respectively (Fig. 2). We then investigated the expression
levels of miR-145 and miR-195. The mean expression
levels of miR-145 and miR-195 were significantly
lower (P<0.05) in CRC plasma than in the healthy control plasma,
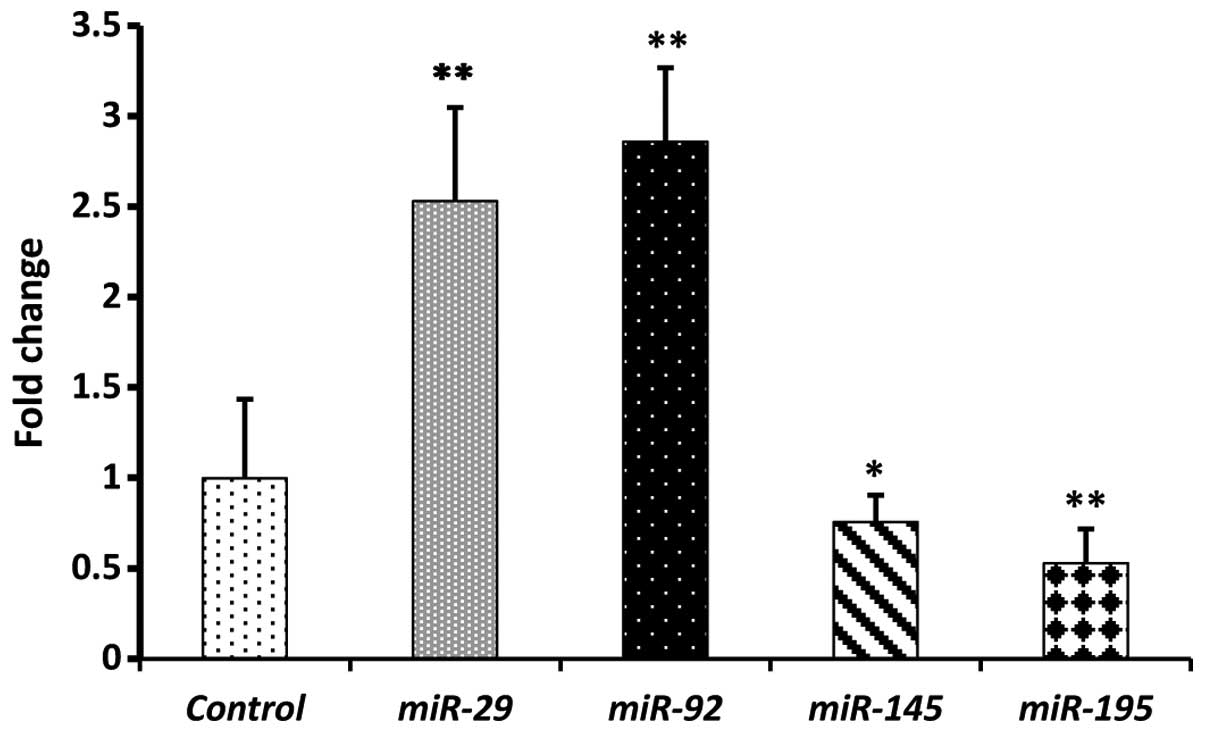
with means of 0.69 and 0.45, respectively (Fig. 2). In a paired-study design employing
RT-qPCR, statistically significant (P<0.01) increases in
miR-29 (2.5) and miR-92 (2.6) were observed in the 20
CRC tissue samples compared with adjacent non-cancerous colorectal
mucosa (Fig. 3). By contrast,
miR-145 (0.75) and miR-195 (0.53) exhibited
statistically significant (P<0.05) decreases in their mean
expression levels in CRC tissue samples in relation to normal
tissues (Fig. 3). Taken together, our
data clearly reflect similar trends in the expression levels of the
four miRNAs in CRC tissues and plasma, as well as the positive
correlation between tissue and circulating miRNAs. Moreover, the
obtained results are in strong agreement with previous reports on
the expression level of the four miRNAs under investigation in CRC
tissues and plasma (15,22,25–28),
thereby confirming their potential as diagnostic biomarkers.
The ideal biomarker should possess a number of
essential criteria including non-invasiveness, specificity,
reliability in indicating the presence of disease prior to the
manifestation of any clinical symptoms, and more importantly
sensitivity to changes in the pathology (disease progression or
therapeutic response) (38).
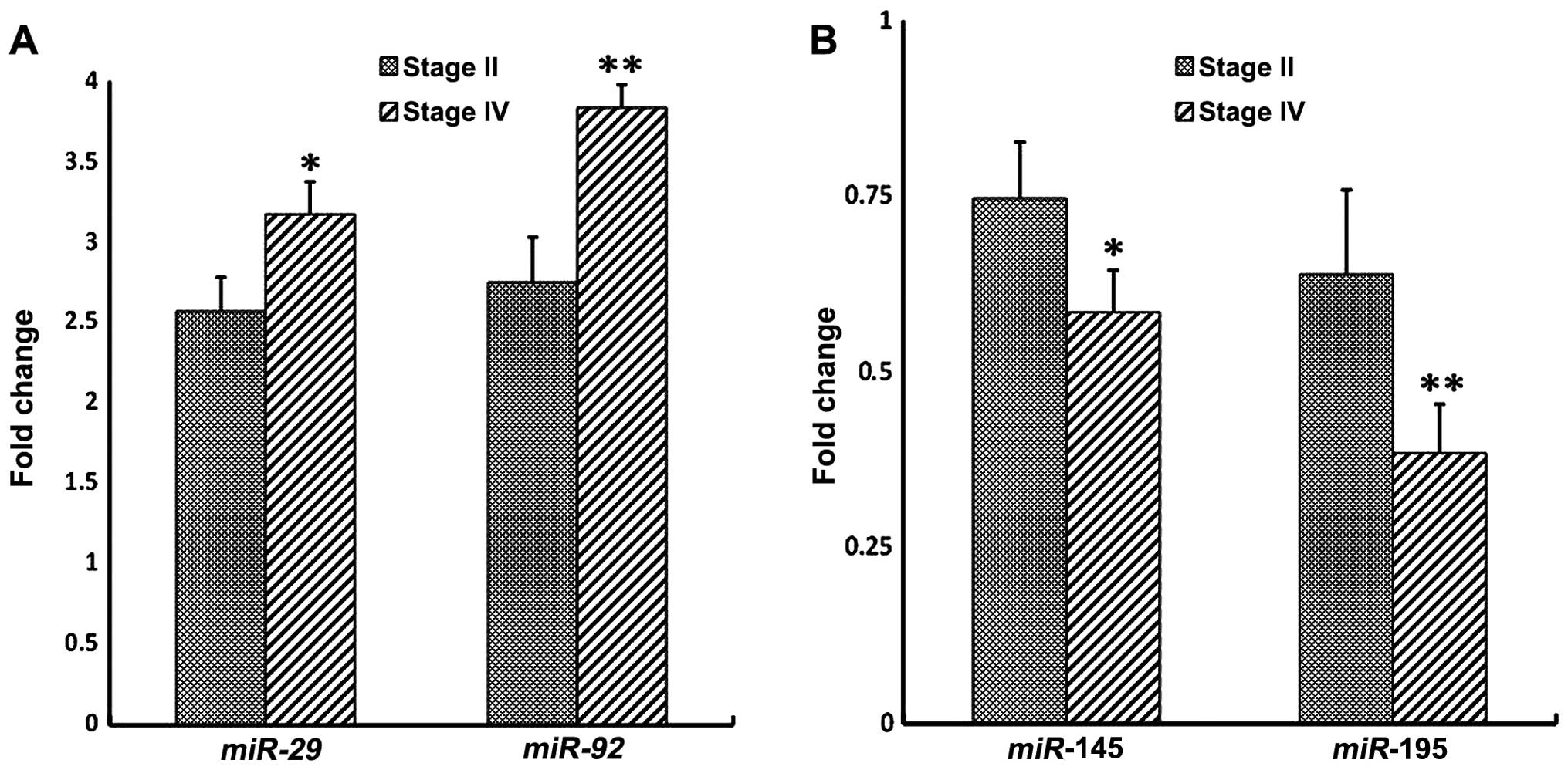
Therefore, we examined whether there is an association between the
aberrant expression of these circulating miRNAs genes in the plasma
and CRC clinical stages (Fig. 4).
Distinct stage-dependent changes in the expression level of the
four miRNA gene profiles were observed between stages II and IV
plasma of CRC patients relative to control plasma (Fig. 4). This observation clearly indicates
that the four miRNAs under study exhibit some dynamic changes in
their levels of expression as a function of the clinical cancer
stage.
The identification of circulating miRNAs in
plasma/serum has led to scientific interest. Therefore, we
investigated the circulating levels of two overexpressed and two
downregulated miRNAs previously identified in plasma samples of CRC
patients compared to healthy individuals. Colorectal adenomas are a
precursor stage of adenocarinoma. Notably, it has been previously
reported that the aberrantly expressed plasma miR-29a and
miR-92a were equally efficient in discriminating advanced
adenoma patients from neoplasm-free individuals, albeit with a
lesser discriminatory power than for CRC patients (22,39).
Similar findings have been recently described in matched CRC and
normal tissue samples for miR-145 at different clinical
stages, emphasizing its potential as an early diagnostic biomarker
for CRC (15). Furthermore, it has
been reported that miR-145 acts as a powerful tumor
suppressor that regulates multiple cell pathways, making it an
ideal biomarker for the diagnosis of various carcinomas (27,28).
However, further studies with larger numbers of patients and
simultaneous measurements of miRNA expression in plasma and tissue
are required to clarify this issue.
There are certain limitations that need to be
considered when interpreting the findings of the current study.
Firstly, the sample size of this study remains critically small,
particularly in the context of miRNA-based biomarker identification
and validation. Secondly, numerous miRNA signatures in plasma
samples are generally too low to be accurately detected and
quantified by RT-qPCR, a well-known limitation of this technique.
Therefore, some potentially relevant markers may not be efficiently
identified. In addition, the fact that no consensus endogenous
control has been established yet in circulating miRNA studies
complicates its reliable investigation. Some reports have advocated
the use of miR-16 as an endogenous and reliable control
because of its relative stability and abundance in plasma/serum
(22,40). However, other reports have challenged
this suggestion by showing that miR-16 exhibits an
aberrantly expression level in plasma/serum which was associated
with the risk of prostate cancer and lymphoma (41,42). We
haveinitiated a high throughput miRNome profiling strategy based on
next generation sequencing technology to aid with the
identification of certain novel miRNA biomarkers that are
differentially expressed in CRC patients (unpublished data).
Thirdly, it is not clear whether the observed mis-expression levels
of the four miRNAs signatures, which were examined in the current
study, are CRC-specific in a Saudi population context. Additional
functional dissection is required to confirm the role of the
selected miRNA signatures in CRC and to validate their potential
diagnostic and prognostic value.
In conclusion, the qPCR results identified
alterations of miRNA expression in CRC plasma and tissues with two
downregulated miRNAs (miR-145 and miR-195), and two
upregulated miRNAs (miR-29 and miR-92), which are
potential candidate biomarkers for CRC. Plasma miRNAs appear to be
potentially useful biomarkers for the early detection and diagnosis
of CRC. While research on plasma-based miRNA profiling remains at
its infancy compared to investigations on tissue-based miRNA
profiling, it holds great potential for the development of novel
non-invasive blood-based CRC screening approaches. Nevertheless,
the diagnostic value of the identified panel of miRNA biomarker
signatures may not yet be capable of competing with the performance
of other traditional non-invasive tests, such as immunochemical
FOBTs (39). Additional
investigations on a multi-marker blood-based test, potentially
including the panel of miRNAs profiled in this study among those
proposed by other study groups may constitute a promising strategy
to enhance the repertoire for non-invasive cancer screening
programs. A panel of plasma miRNA multi-markers assays may possess
a superior sensitivity and specificity as compared with other
single-marker ones for CRC screening. Therefore, patients
exhibiting elevated plasma miRNAs, as in the case with
miR-29 and miR-92 for example, may initiate more
targeted and accurate clinical examinations such as
colonoscopy.
Acknowledgements
This study was financially supported by the National
Plan for Science, Technology and Innovation (MAARIFAH), King
Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia,
award no. (11-MED1770-02).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.1. Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 (Internet). Lyon, France:
International Agency for Research on Cancer. 2014.http://globocan.iarc.frAccessed. Jan 16–2015
|
|
2
|
Saudi Cancer Registry: Cancer Incidence
Report Saudi Arabia 2005. Kingdom of Saudi Arabia, Ministry of
Health, Saudi Cancer Registry. http://www.scr.org.sa/reports/SCR2005.pdfAccessed.
Oct 12–2015
|
|
3
|
Mosli MH and Al-Ahwal MS: Colorectal
cancer in the Kingdom of Saudi Arabia: need for screening. Asian
Pac J Cancer Prev. 13:3809–3813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibrahim EM, Zeeneldin AA, El-Khodary TR,
Al-Gahmi AM and Sadiq Bin BM: Past, present and future of
colorectal cancer in the Kingdom of Saudi Arabia. Saudi J
Gastroenterol. 14:178–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levin B, Lieberman DA, McFarland B, Smith
RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR,
et al: American Cancer Society Colorectal Cancer Advisory Group; US
Multi-Society Task Force; American College of Radiology Colon
Cancer Committee: Screening and surveillance for the early
detection of colorectal cancer and adenomatous polyps, 2008: a
joint guideline from the American Cancer Society, the US
Multi-Society Task Force on Colorectal Cancer, and the American
College of Radiology. CA Cancer J Clin. 58:130–160. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandel JS: Screening for colorectal
cancer. Gastroenterol Clin North Am. 3797–115. (vii)2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herranz H and Cohen SM: MicroRNAs and gene
regulatory networks: managing the impact of noise in biological
systems. Genes Dev. 24:1339–1344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hannafon BN, Sebastiani P, de las Morenas
A, Lu J and Rosenberg P: Expression of microRNA and their gene
targets are dysregulated in preinvasive breast cancer. Breast
Cancer Res. 13:R242011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miko E, Czimmerer Z, Csánky E, Boros G,
Buslig J, Dezso B and Scholtz B: Differentially expressed microRNAs
in small cell lung cancer. Exp Lung Res. 35:646–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akao Y, Nakagawa Y and Naoe T:
MicroRNA-143 and −145 in colon cancer. DNA Cell Biol. 26:311–320.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M, et
al: Identification by Real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissues. Mol Cancer. 5:29–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michael MZ, O'Connor SM, van Holst
Pellekaan NG, Young GP and James P: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
15
|
Peng J, Xie Z, Cheng L, Zhang Y, Chen J,
Yu H, Li Z and Kang H: Paired design study by real-time PCR:
miR-378* and miR-145 are potent early diagnostic biomarkers of
human colorectal cancer. BMC Cancer. 15:158–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schepeler T, Reinert JT, Ostenfeld MS,
Christensen LL, Silahtaroglu AN, Dyrskjøt L, Wiuf C, Sørensen FJ,
Kruhøffer M, Laurberg S, et al: Diagnostic and prognostic microRNAs
in stage II colon cancer. Cancer Res. 68:6416–6424. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waldman SA and Terzic A: MicroRNA
signatures as diagnostic and therapeutic targets. Clin Chem.
54:943–944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsang JC and Lo YM: Circulating nucleic
acids in plasma/serum. Pathology. 39:197–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: a novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weber JA, Baxter DH, Zhang S, Huang DY,
Huang KH, Lee MJ, Galas DJ and Wang K: The microRNA spectrum in 12
body fluids. Clin Chem. 56:1733–1741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Chen L, Xu Y, Li R and Du X:
microRNA-195 promotes apoptosis and suppresses tumorigenicity of
human colorectal cancer cells. Biochem Biophys Res Commun.
400:236–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Wang Q, Zheng Y, Lv S, Ning S, Sun
J, Huang T, Zheng Q, Ren H, Xu J, et al: Prioritizing human cancer
microRNAs based on genes' functional consistency between microRNA
and cancer. Nucleic Acids Res. 39:e1532011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui SY, Wang R and Chen LB: MicroRNA-145:
A potent tumour suppressor that regulates multiple cellular
pathways. J Cell Mol Med. 18:1913–1926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou Y, Wang X, Chen Y and Mu S:
MicroRNA-145 as an ideal biomaker for the diagnosis of various
carcinomas. Tumour Biol. 36:2641–2649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
UICC: TNM Classification of Malignant
Tumours. Sobin LH and Wittekind Ch: (6th). Hoboken, NJ: John Wiley
& Sons. 2002.
|
|
30
|
Duttagupta R, Jiang R, Gollub J, Getts RC
and Jones KW: Impact of cellular miRNAs on circulating miRNA
biomarker signatures. PLoS One. 6:e207692011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirschner MB, Kao SC, Edelman JJ,
Armstrong NJ, Vallely MP, van Zandwijk N and Reid G: Haemolysis
during sample preparation alters microRNA content of plasma. PLoS
One. 6:e241452011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allawi HT, Dahlberg JE, Olson S, Lund E,
Olson M, Ma WP, Takova T, Neri BP and Lyamichev VI: Quantitation of
microRNAs using a modified Invader assay. RNA. 10:1153–1161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu CG, Calin GA, Meloon B, Gamliel N,
Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M, et
al: An oligonucleotide microchip for genome-wide microRNA profiling
in human and mouse tissues. Proc Natl Acad Sci USA. 101:9740–9744.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andrews WJ, Brown ED, Dellett M, Hogg RE
and Simpson DA: Rapid quantification of microRNAs in plasma using a
fast real-time PCR system. Biotechniques. 58:244–252.
2015.PubMed/NCBI
|
|
37
|
Zubakov D, Boersma AW, Choi Y, van Kuijk
PF, Wiemer EA and Kayser P: MicroRNA markers for forensic body
fluid identification obtained from microarray screening and
quantitative RT-PCR confirmation. Int J Legal Med. 124:217–226.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Etheridge A, Lee I, Hood L, Galas D and
Wang K: Extracellular microRNA: a new source of biomarkers. Mutat
Res. 717:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luo X, Stock C, Burwinkel B and Brenner H:
Identification and evaluation of plasma microRNAs for early
detection of colorectal cancer. PLoS One. 8:e628802013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song J, Bai Z, Han W, Zhang J, Meng H, Bi
J, Ma X, Han S and Zhang Z: Identification of suitable reference
genes for qPCR analysis of serum microRNA in gastric cancer
patients. Dig Dis Sci. 57:897–904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang C, Zhu DX, Dong HJ, Zhou ZJ, Wang YH,
Liu L, Fan L, Miao KR, Liu P, Xu W, et al: Serum microRNAs are
promising novel biomarkers for diffuse large B cell lymphoma. Ann
Hematol. 91:553–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Watahiki A and Wang Y, Morris J, Dennis K,
O'Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PLoS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|