Introduction
Gallbladder carcinoma (GBC) is the most commonly
observed malignancy of the biliary tract, representing 80–95% of
all the cases of biliary tract cancer worldwide, and is the sixth
most frequent malignant neoplasm of the digestive tract (1). In 2012, ~76,844 patients were diagnosed
with GBC, and ~60,334 patients succumbed to disease (2). Currently, the overall mean survival time
for patients exhibiting GBC is 6 months, and the 5-year survival
rate is 5% (3). A positive clinical
outcome may depend on early and timely surgical resection of GBC
(4). However, >90% of patients do
not undergo surgical resection due to the advanced stage of their
tumors at the time of diagnosis. Invasion of adjacent organs or
distant metastases is observed in patients with advanced GBC, and
almost 50% of them exhibit lymph node metastasis (5,6). Thus, it
is essential to identify novel prognostic biomarkers and
therapeutic targets for the treatment of GBC.
The mammalian calpain protease family comprises
intracellular Ca2+-regulated cysteine proteases that
mediate regulatory cleavage of specific substrates (7). Calpains are involved in various
physiological functions, including cell differentiation,
transcriptional regulation, cytokine processing, cell cycle, signal
transduction, migration, apoptosis and protein renewal during
growth and tissue regeneration (7,8). In
addition, the calpain family, including calpain-1, has been
observed to be involved in the progression of cancer (8,9). The
expression of calpain-1 has been reported to be associated with
relapse-free survival in patients with breast cancer treated with
trastuzumab following adjuvant chemotherapy (8), and was also correlated with increased
malignancy in renal cell carcinoma (10).
Glypican-3 is a cell surface protein that attaches
to the cell membrane via a glycosylphosphatidylinositol (GPI)
anchor (11). Glypican-3 is able to
combine with Wnt molecules to form a complex, thereby promoting
cancer growth via stimulation of canonical Wnt signaling (12). It has been reported that glypican-3 is
able to regulate developmental growth by interacting with the
Hedgehog signaling pathway (13).
Previous studies have revealed that mutated glypican-3 lacking its
GPI anchor domain is able to block Wnt signaling and inhibit the
growth of Wnt-dependent tumors (14,15).
Additional reports have demonstrated that glypican-3 expression is
involved in various human malignancies, including hepatocellular
carcinoma (HCC) (16), melanoma
(17), ovarian clear cell carcinoma
(18), yolk sac tumor (YST) (19), neuroblastoma, hepatoblastoma and
Wilms' tumor, among others (20,21).
However, to the best of our knowledge, the
expression of calpain-1 and glypican-3 in GBC has not been
investigated thus far. In the present study, the expression of
calpain-1 and glypican-3 was detected in 100 patients with GBC and
30 patients with cholecystitis by immunohistochemistry, and the
correlations between calpain-1 and glypican-3 expression and
certain clinicopathological characteristics of the patients were
analyzed.
Materials and methods
Clinical samples
The present study was performed according to
REporting recommendations for tumor MARKer prognostic studies
(REMARK) criteria (22). A total of
100 patients with GBC and 30 patients with cholecystitis who
accepted surgical treatment between January 2007 and December 2011
in The First Affiliated Hospital, School of Medicine, Zhejiang
University (Hangzhou, China) were enrolled in the study. Written
informed consent was obtained from the patients prior to
commencement of the study. The present study was approved by the
Ethics Review Committee of The First Affiliated Hospital, School of
Medicine, Zhejiang University (reference number 2014-332). The
inclusion criteria of the patients with GBC were set as follows: i)
The postoperative pathological diagnosis was GBC; ii) no
radiotherapy or chemotherapy had been administered prior to
surgery; iii) no comorbid diseases were present; and iv) complete
pathological and clinical information was available, including age,
gender, degree of tumor differentiation, tumor-node-metastasis
(TNM) classification (23) and
presence of distant metastases. The detailed clinicopathological
variables of the patient cohort are presented in Table I.
 | Table I.Clinicopathological variables of the
patient cohort. |
Table I.
Clinicopathological variables of the
patient cohort.
| Clinicopathological
variables | Gallbladder
carcinoma (n=100) | Cholecystitis
(n=30) |
|---|
| Age, years |
63.57±0.99a |
54.13±1.99b |
| Tumor size,
mm3 |
29.55±7.20c |
|
| Gender, n (%) |
|
|
|
Female | 57 (57) | 10 (33) |
|
Male | 43 (43) | 20 (67) |
| Differentiation
degree, n (%) |
|
|
| Poor
and moderate | 73 (73) |
|
|
Well | 27 (27) |
|
|
Tumor-node-metastasis classification, n
(%) |
|
|
|
I+II | 17 (17) |
|
III+IV | 83 (83) |
| Distant metastases,
n (%) |
|
|
|
Positive | 72 (72) |
|
Negative | 28 (28) |
Immunohistochemical staining
Paraffin-embedded GBC and cholecystitis tissues were
sectioned with a thickness of 4 µm, and deparaffinized using xylene
(Sangon Biotech Co., Ltd., Shanghai, China). The slides were
immersed into various concentrations of alcohol (100%, 95%, 75% and
50%; Sangon Biotech Co., Ltd.) diluted with double distilled
H2O for rehydration, and subsequently treated with 3%
H2O2 (product code, M82228702; Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) to block endogenous
peroxidase activity. For antigen retrieval, the slides were
immersed in boiling (95–100°C) citrate buffer (pH 6.0; Sangon
Biotech Co., Ltd.) for 20 min. Upon washing with phosphate-buffered
saline (PBS; Sangon Biotech Co., Ltd.), the slides were immersed
into blocking solution (3% bovine serum albumin; Hangzhou Sijiqing
Bioengineering Material Co., Ltd., Hangzhou, China) at room
temperature for 30 min. Next, the slides were incubated overnight
at 4°C with primary monoclonal mouse anti-rabbit calpain-1 (cat.
no. ab3589; 1:1,000) and polyclonal rabbit anti-mouse glypican-3
(cat. no. ab66596; 1:1,000; Abcam, Cambridge, MA, USA) antibodies
diluted in blocking serum. Following rinsing with PBS three times
at room temperature, a horseradish peroxidase (HRP) polymer
(SuperPicture™ Polymer Detection Kit, HRP, broad spectrum; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) conjugated to undiluted
anti-rabbit (cat no. PV-6001) or anti-mouse (cat no. PV-6002)
secondary antibodies (Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) were added to the slides for 10 min, and
3,3′-diaminobenzidine chromogen was then added for 5 min. Following
each incubation step, the slides were washed in PBS for 5 min.
Mayer's Hematoxylin Solution (Sigma-Aldrich, St. Louis, MO, USA)
was utilized for counterstaining. Subsequently, the slides were
dehydrated, air-dried and mounted with neutral resins (product
code, ZLI-9555; Zhongshan Golden Bridge Biotechnology Co.,
Ltd.).
Assessment of staining was conducted by scanning the
slides with an inverted microscope (BX41; Olympus Corporation,
Tokyo, Japan) at magnification, ×200. The expression of calpain-1
in tumor and cholecystitis cells was manually assessed by
immunohistochemical H-score, with a slight modification to the
method previously described (8).
Staining intensity was assessed as negative (0), weak (1), medium (2)
or strong (3) over each stained area.
The stained area score was assessed as <50% (1) or ≥50% (2).
H-scores were calculated by multiplying the stained area score by
the staining intensity score (H-score range, 0–6). Calpain-1
H-score was dichotomized into low and high immunoreactivity groups
using X-Tile Software (http://medicine.yale.edu/lab/rimm/research/software.aspx),
and correlated with clinicopathological criteria. The expression of
glypican-3 in tumor and cholecystitis cells was manually assessed
as negative (−) or positive (+). A total of
50% of the slides were examined by a second independent assessor
who was blinded to the scores and clinicopathological criteria, and
good concordance existed between the two scorers (single measure
intraclass correlations, >0.8). An average H-score was generated
by calculating the mean of 10 random high-power fields. Average
scores were utilized for analysis due to the relatively small
sample size.
Statistical analysis
The data distribution was assessed using the
Kolmogorov-Smirnov test for goodness of fit. The correlation
between protein expression and clinicopathological characteristics
was analyzed with Pearson's χ2 test of association or
Fisher's exact test in a 2×2 table. Spearman's rank-order
correlations were performed to investigate the associations between
various proteins. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL)
for Windows (Microsoft Corporation, Redmond, WA, USA).
Results
Positive immunohistochemistry results
are observed for calpain-1 and glypican-3
Tissue expression of calpain-1 and glypican-3 was
investigated in patients with GBC and patients with cholecystitis.
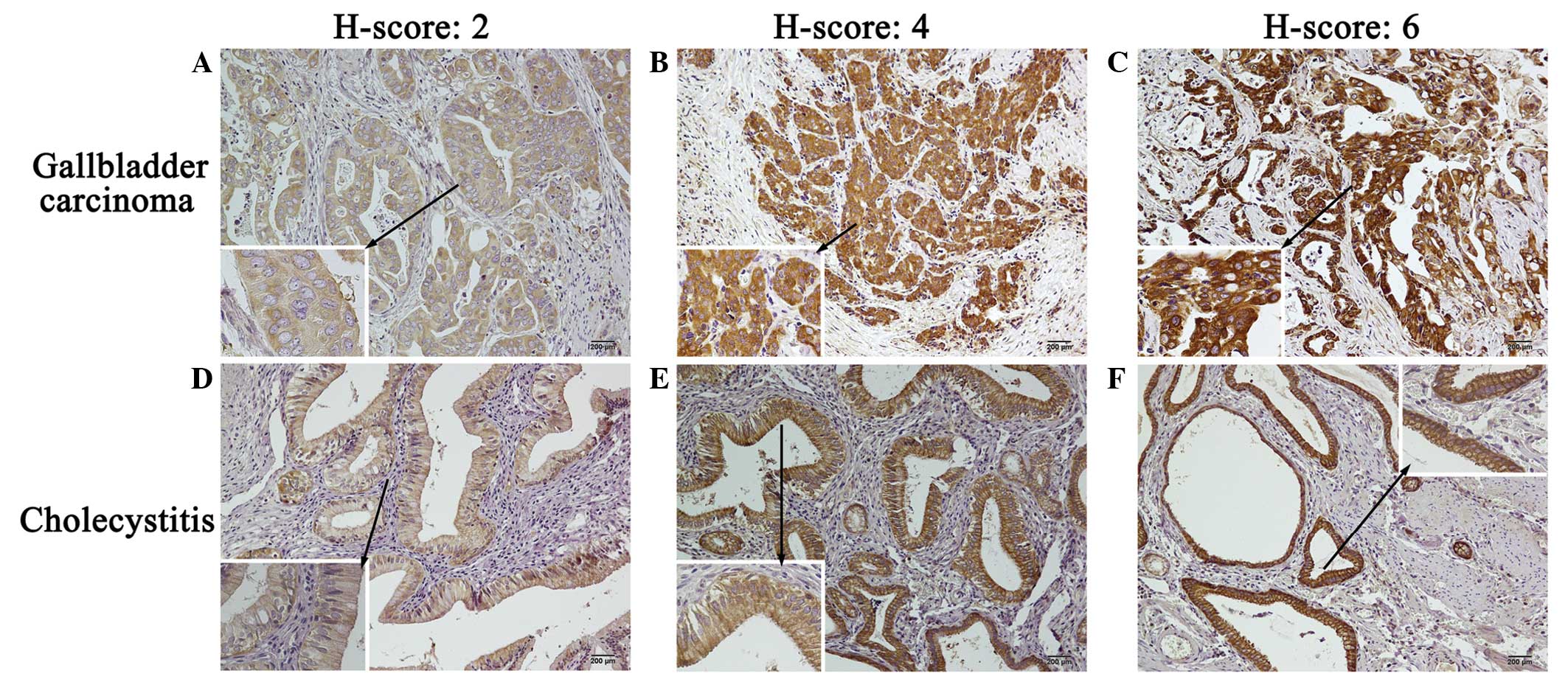
Representative staining patterns of calpain-1 expression in GBC and
cholecystitis tissues are presented in Fig. 1. Cytomembrane and cytoplasmic staining
was observed for calpain-1, with certain granularity and
heterogeneity between adjacent tumor cells and adjacent
cholecystitis cells, varying from weak to strong staining.
According to the computational formula of H-scores, the different
intensities of calpain-1 expression (ranging from weak to strong)
corresponded to distinct H-scores (ranging from 2 to 6) in the GBC
(Fig. 1A–C) and cholecystitis
(Fig. 1D–F) tissues. In 100 GBC
tissue samples, calpain-1 exhibited a median H-score of 2.73 and a
standard error of 0.18, while the median H-score observed for
calpain-1 in 30 cholecystitis tissues was 2.47±0.28.
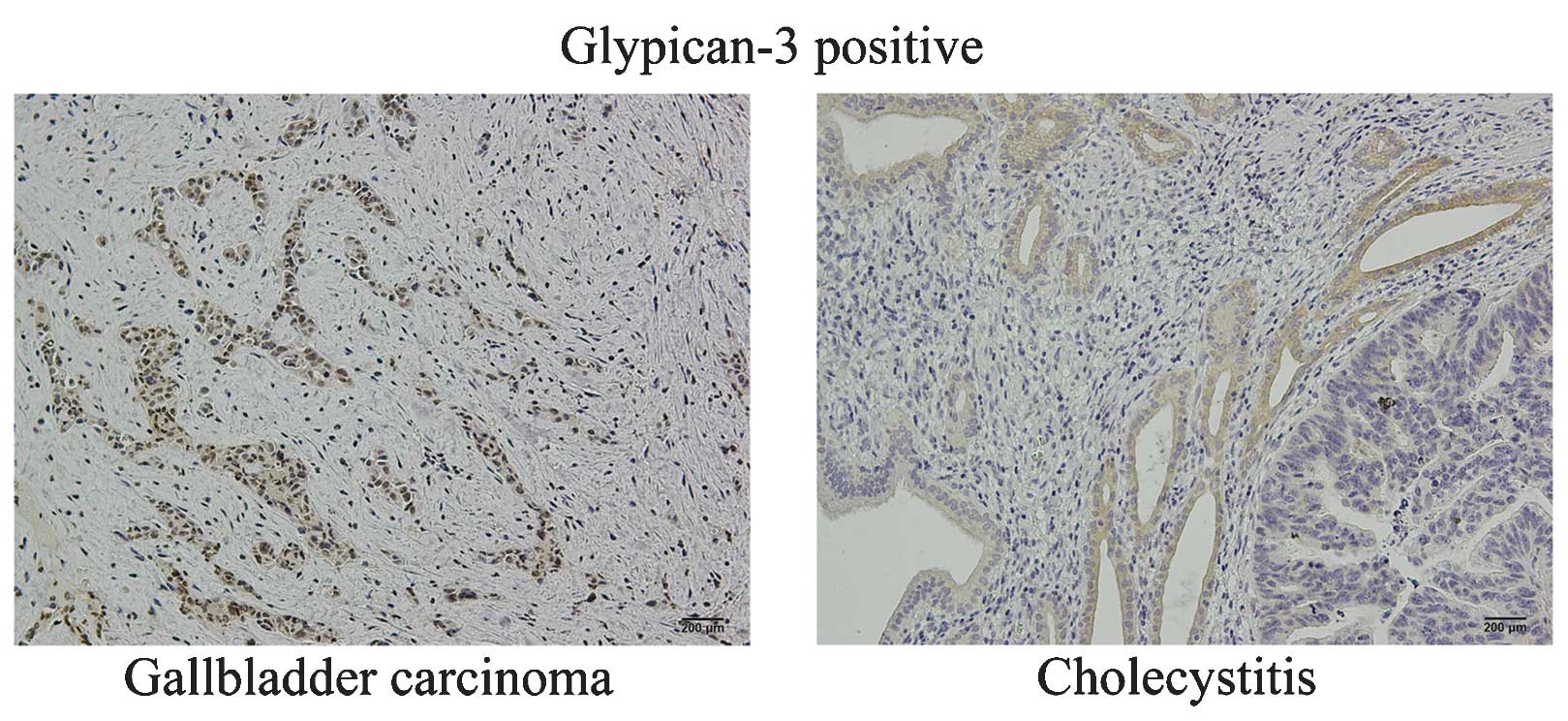
Furthermore, as demonstrated in Fig. 2, glypican-3 expression presented as
cytomembrane and cytoplasmic staining in positively stained GBC and
cholecystitis cells, whereas negative staining was exhibited by a
number of cells. The positive expression rate of glypican-3 was
53.0% (53/100) in GBC tissues and 63.3% (19/30) in cholecystitis
tissues.
Differential distribution of calpain-1
and glypican-3 expression is observed in patients with GBC and
patients with cholecystitis
H-score cut-offs were as follows: 0, negative
expression; 1–3, low expression; and 4–6, high expression. All 100
patients with GBC and 30 patients with cholecystitis presented
positive expression of calpain-1, thus exhibiting a 100.0% positive
expression rate for this protein. Of the 100 patients with GBC, 32
exhibited high expression levels of calpain-1 and 68 exhibited low
expression levels, resulting in a high expression rate of 32.0%. Of
the 30 patients with cholecystitis, 2 exhibited high expression
levels of calpain-1 and 28 exhibited low expression levels.
Therefore the high expression rate for this protein was 6.7%.
Pearson's χ2 test demonstrated that the high expression
rate of calpain-1 was significantly increased in patients with GBC,
compared with patients with cholecystitis (χ2=7.668;
P=0.006; Table II).
 | Table II.Expression distribution of calpain-1
and glypican-3 in patients with GBC and patients with
cholecystitis. |
Table II.
Expression distribution of calpain-1
and glypican-3 in patients with GBC and patients with
cholecystitis.
|
|
Calpain-1a | Glypican-3 |
|---|
|
|
|
|
|---|
| Expression
distribution | Low, n (%) |
| High, n (%) | Negative, n
(%) |
| Positive, n
(%) |
|---|
| GBC (n=100) | 68 (68.0) |
| 32 (32.0) | 47 (47.0) |
| 53 (53.0) |
| Cholecystitis
(n=30) | 28 (93.3) |
| 2 (6.7) | 11 (36.7) |
| 19 (63.3) |
|
χ2/P-valueb |
| 7.668/0.006 |
|
| 0.997/0.318 |
|
Glypican-3 expression was manually assessed as
negative (−) or positive (+). Of the 100
patients with GBC, 53 presented positive glypican-3 expression and
47 demonstrated negative expression; therefore the positive
expression rate was 53.0%. Of the 30 patients with cholecystitis,
19 demonstrated positive glypican-3 expression and 11 exhibited
negative expression, therefore the positive expression rate was
63.3%. Pearson's χ2 test indicated no significant
difference in the positive expression rate of glypican-3 between
patients with GBC and patients with cholecystitis
(χ2=0.997; P=0.318; Table
II).
No significant associations exist
between calpain-1 and glypican-3 expression and various
clinicopathological variables in patients with GBC
The correlation of calpain-1 expression with
clinicopathological variables in patients with GBC was analyzed
using Fisher's exact (2×2) test, as shown in Table III. With regard to gender, 21 female
patients presented high expression levels of calpain-1 and 36
demonstrated low expression, while 11 male patients exhibited high
expression levels of calpain-1 and 32 demonstrated low expression.
Fisher's exact (2×2) test indicated no significant difference
(χ2=1.428; P=0.232). With regard to age, 20 patients
presented high expression levels of calpain-1 and 41 demonstrated
low expression levels in the ≥60 years group, while 12 patients
exhibited high expression levels of calpain-1 and 27 demonstrated
low expression levels in the <60 years group. Fisher's exact
test indicated no significant difference (χ2=0.045;
P=0.833). With regard to the degree of tumor differentiation, 22
patients presented high expression levels of calpain-1 and 51
demonstrated low expression levels in the poor and moderate
differentiation group, while 10 patients exhibited high expression
and 17 low expression in the well-differentiated group. Fisher's
exact test indicated no significant difference
(χ2=0.431; P=0.511). With regard to TNM classification,
8 patients presented high expression levels of calpain-1 and 9
demonstrated low expression for stages I+II, while 24 patients
exhibited high expression levels of calpain-1 and 59 demonstrated
low expression for stages III+IV. Fisher's exact test indicated no
significant difference (χ2=2.134; P=0.144). The results
of the present study suggested that the expression of calpain-1 had
no significant correlation with gender, age, tumor differentiation
degree and TNM classification in patients with GBC.
 | Table III.Correlations between calpain-1 and
glypican-3 expression and clinicopathologic variables in 100
patients with GBC. |
Table III.
Correlations between calpain-1 and
glypican-3 expression and clinicopathologic variables in 100
patients with GBC.
|
| Calpain-1
expression | Glypican-3
expression |
|---|
|
|
|
|
|---|
| GBC (n=100) | Low | High |
χ2/P-valuea | Negative | Positive |
χ2/P-valuea |
|---|
| Gender |
|
| 1.428/0.232 |
|
| 1.903/0.168 |
|
Male | 32 | 11 |
| 21 | 22 |
|
|
Female | 36 | 21 |
| 26 | 31 |
|
| Age, years |
|
| 0.045/0.833 |
|
| 0.018/0.892 |
|
|
<60 | 27 | 12 |
| 18 | 21 |
|
|
≥60 | 41 | 20 |
| 29 | 32 |
|
| Degree of
differentiation |
|
| 0.431/0.511 |
|
| 1.474/0.225 |
| Poor
and moderate | 51 | 22 |
| 37 | 36 |
|
|
Well | 17 | 10 |
| 10 | 17 |
|
Tumor-node-metastasis classification |
|
| 2.134/0.144 |
|
| 1.127/0.288 |
|
I+II | 9 | 8 |
| 6 | 11 |
|
|
III+IV | 59 | 24 |
| 41 | 42 |
|
The correlation of glypican-3 expression with
clinicopathological variables in the patients with GBC presented a
similar pattern to that mentioned above for calpain-1 expression
(Table III). With regard to gender,
31 female patients presented positive glypican-3 expression and 26
negative, while 22 male patients demonstrated positive expression
and 21 negative. Fisher's exact test indicated no significant
difference (χ2=1.903; P=0.168). With regard to age, 32
patients presented positive glypican-3 expression and 29 negative
in the ≥60 years group, while 21 patients demonstrated positive
expression and 18 negative in the <60 years group. Fisher's
exact test indicated no significant difference
(χ2=0.018; P=0.892). With regard to tumor
differentiation, 36 patients presented positive glypican-3
expression and 37 negative in the poor and moderate differentiation
group, while 17 patients were positive and 10 were negative in the
well-differentiated group. Fisher's exact test indicated no
significant difference (χ2=1.474; P=0.225). With regard
to TNM classification, 11 patients presented positive glypican-3
expression and 6 negative for stages I+II, while 42 patients were
positive and 41 were negative for stages III+IV. Fisher's exact
test indicated no significant difference (χ2=1.127;
P=0.288). The results of the present study suggested that the
expression of glypican-3 had no significant correlation with
gender, age, tumor differentiation degree and TNM classification in
patients with GBC.
Varying correlations are observed
between calpain-1 and glypican-3 expression in patients with GBC
and patients with cholecystitis
As presented in Table
IV, in the GBC group, 29 patients presented high expression of
calpain-1 and positive expression of glypican-3, 44 patients
demonstrated low expression of calpain-1 and negative expression of
glypican-3, 3 patients demonstrated independent high expression of
calpain-1 and 24 patients demonstrated independent positive
expression of glypican-3. Spearman's rank-order correlations
indicated that the expression of calpain-1 and glypican-3 in
patients with GBC presented a significantly positive correlation
(r=0.517; P<0.01).
 | Table IV.Correlation between calpain-1 and
glypican-3 expression in 100 patients with GBC and 30 patients with
cholecystitis. |
Table IV.
Correlation between calpain-1 and
glypican-3 expression in 100 patients with GBC and 30 patients with
cholecystitis.
| Patients | Calpain-1 | Glypican-3
(+), n | Glypican-3
(−), n |
r/P-valuea |
|---|
| GBC (n=100) | High | 29 | 3 |
0.517/<0.01 |
|
| Low | 24 | 44 |
|
| Cholecystitis
(n=30) | High | 1 | 18 |
−0.856/<0.01 |
|
| Low | 10 | 1 |
|
In the cholecystitis group, 1 patient presented high
expression of calpain-1 and positive expression of glypican-3, 1
patient demonstrated low expression of calpain-1 and negative
expression of glypican-3, 18 patients demonstrated independent high
expression of calpain-1 and 10 patients demonstrated independent
positive expression of glypican-3. Spearman's correlation analysis
indicated that the expression of calpain-1 and glypican-3 in
patients with cholecystitis presented a significantly negative
correlation (r=-0.856; P<0.01).
Discussion
GBC always results in advanced disease with invasion
of adjacent organs or distant metastases at the time of
presentation, primarily attributed to its relatively low incidence
and unclear symptomatology, thereby leading to poor prognosis and
reduced survival rates (24).
Previous reports have demonstrated that several risk factors
including age, parity, gender, obesity and ethnicity may be
associated with GBC (24,25). The initiation and development of GBC
may be due to a wide range of etiologies, including infectious and
environmental exposure to chemical carcinogens, mechanical
obstruction via gallstones, autoimmune disease, polyps, adenomas
and anatomical variations such as pancreaticobiliary malfunction
(24,25). Although significant progress has been
made to identify potential prognostic biomarkers for GBC, this
disease remains an uncommon and challenging malignancy with an
overall poor prognosis (25).
Carbohydrate antigen 19-9 and carcinoembryonic
antigen are the most commonly used clinical biomarkers in GBC
(26). However, they are frequently
increased in the advanced stages of the disease with a low
specificity, and therefore, they are not generally used
independently for GBC prognosis (25). Previous studies have demonstrated that
calpain-1 may be involved in the progression of certain types of
cancer, including breast cancer (8)
and renal cell carcinoma (10).
Furthermore, a number of reports have indicated that glypican-3
expression may be involved in various types of cancer, including
HCC (16), melanoma (17), ovarian clear cell carcinoma (18) and others (20,21).
However, to the best of our knowledge, the expression of calpain-1
and glypican-3 in GBC has not been investigated to date.
In the present study, the expression of calpain-1
and glypican-3 was detected in 100 patients with GBC and 30
patients with cholecystitis by immunohistochemistry, and the
correlations between calpain-1 and glypican-3 expression and the
clinicopathological characteristics of the patients were analyzed.
It was identified that all patients presented positive expression
of calpain-1. Notably, the high expression rate of calpain-1 in
patients with GBC was markedly increased compared with that
observed in patients with cholecystitis. Furthermore, the
expression of calpain-1 and glypican-3 had no significant
correlation with gender, age, degree of tumor differentiation and
TNM classification in patients with GBC. Notably, calpain-1 and
glypican-3 expression presented a significantly positive
correlation in patients with GBC, but a significantly negative
correlation in patients with cholecystitis.
The results of the present study are interesting in
the light of previous studies highlighting the role of calpain-1 in
the progression of cancer (8,27,28).
Kulkarni et al (27)
investigated the role of calpain-1 in trastuzumab-treated human
epidermal growth factor receptor 2 (HER2)+ breast cancer
in vitro. The authors demonstrated that calpain-1 was
activated following trastuzumab treatment, and subsequently cleaved
HER2, thus disrupting signaling, while trastuzumab-resistant cells
were dependent on calpain-1 activity for survival (27). Storr et al (8) reported that calpain-1 expression was
associated with relapse-free survival, and proposed that calpain-1
expression may be a useful biomarker for the prediction of
relapse-free survival in patients with breast cancer treated with
adjuvant trastuzumab and chemotherapy. An additional study by Storr
et al (28) demonstrated that
the expression of calpain-1 and calpastatin were associated with
various clinicopathological features, including tumor grade and
estrogen receptor expression, which was verified in an independent
cohort of patients. Furthermore, Storr et al (29) investigated the protein expression
levels of calpastatin and calpain-1, −2 and −9 in surgically
excised gastroesophageal adenocarcinomas derived from patients
treated with neoadjuvant chemotherapy and in tumors that had not
been previously exposed to chemotherapy, and identified that
expression of the calpain system was associated with poor clinical
outcomes. Therefore, the authors proposed that calpain-1, calpain-2
and calpastatin may be clinically relevant prognostic biomarkers in
gastroesophageal adenocarcinoma (29). In addition, Storr et al
(30) indicated that the expression
of these proteins was significantly associated with carcinoma of
the pancreas, bile duct and ampulla, and influenced the progression
of disease.
The potential mechanisms by which the calpain family
participates in cancer progression are associated with a number of
interrelated signaling pathways (31). Integrin engagement is able to induce
focal adhesion kinase (FAK) phosphorylation, resulting in
extracellular signal-regulated kinase activation of calpain-1 to
cleave FAK, which subsequently enhances cell motility (32). FAK, like phosphatidylinositol
(3,4,5)-trisphosphate 3-phosphatase, may be
dephosphorylated by phosphatase and tensin homolog, which is
indicative of signaling pathway overlap (32). These interrelated signaling pathways
synergistically contribute to the progression of various types of
cancer (31,33,34). In
the present study, all patients with GBC presented positive
expression of calpain-1, suggesting that calpain-1 expression may
be associated with GBC. However, the expression of calpain-1 had no
significant correlation with gender, age, degree of tumor
differentiation and TNM classification in these patients, which
suggested that calpain-1 may be a potentially useful biomarker for
GBC prognosis. Notably, the results of the present study
demonstrated that the high expression rate of calpain-1 in patients
with GBC was markedly increased compared with that observed in
patients with cholecystitis (32.0 vs. 6.7%; P=0.006). Thus, it may
be speculated that the expression of calpain-1 is associated with
progression from cholecystitis to GBC.
Glypican-3 is a cell surface protein that is highly
expressed in certain types of human cancer, including HCC and
melanoma (17,35). It is associated with cell
proliferation and survival, possibly due to its interaction with
insulin-like growth factor (IGF) 2 (11). Song et al (36) mated glypican-3 knockout mice with
insulin receptor substrate 1 nullizygous mice, and demonstrated
that glypican-3 regulated organism growth independently of IGF
signaling. Notably, glypican-3 knockout mice exhibited changes in
Wnt signaling (36). Glypican-3 is
able to form a complex with Wnt molecules, thereby promoting cancer
growth by stimulation of canonical Wnt signaling (12), and is also able to regulate
developmental growth via interaction with the Hedgehog signaling
pathway (13). In addition,
glypican-3 is able to act as a negative regulator of Hedgehog
signaling during mammalian development (13). A number of studies have indicated that
mutated glypican-3 lacking the GPI anchor domain was able to block
Wnt signaling and inhibit the growth of Wnt-dependent tumors
(14,15). Previous studies have reported that
glypican-3 expression is associated with various types of cancer,
including HCC (16), melanoma
(17), ovarian clear cell carcinoma
(18), YST (19), neuroblastoma, hepatoblastoma, Wilms'
tumor and others (20,21). The present study demonstrated that 53
patients with GBC presented positive expression of glypican-3
(53.0%), which suggested that glypican-3 expression may be
associated with GBC.
The combined detection of multiple molecular markers
is able to enhance the specificity and sensitivity of tumor
prognostic assessment, and has become a potentially effective
method for the prediction of tumor prognosis (37,38). The
present study identified a significantly positive correlation
between the expression of calpain-1 and glypican-3 in patients with
GBC, and a significantly negative correlation in patients with
cholecystitis via Spearman's correlation analysis. Thus, it may be
speculated that the combined detection of calpain-1 and glypican-3
may be beneficial for the prognostic assessment of GBC.
Identification and validation of additional patient studies on
various human populations at risk of developing GBC, which include
clinicopathological variables and prognostic values, is required in
order to provide further insight into calpain-1 and glypican-3
detection as a potential prognostic biomarker for GBC.
In conclusion, the present study demonstrated that
calpain-1 expression was associated with GBC, and may be a useful
potential biomarker for the assessment of prognosis in patients
with GBC. Notably, the expression of calpain-1 may be associated
with progression from cholecystitis to GBC. Furthermore, combined
detection of calpain-1 and glypican-3 may be beneficial for the
assessment of prognosis in patients with GBC.
Acknowledgements
The present study was supported by the National
Science and Technology Major Projects of the Chinese Ministry of
Science and Technology (Beijing, China) (grant no.
2012ZX10002-017), the National Natural Science Foundation of China
for Innovative Research Group of China (Beijing, China) (grant no.
81421062) and the Medical and Health Science and Techonology
Project of Zhejiang Province (Hangzhou, China) (grant no.
2014KYA210).
References
|
1
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henson DE, Albores-Saavedra J and Corle D:
Carcinoma of the gallbladder. Histologic types, stage of disease,
grade, and survival rates. Cancer. 70:1493–1497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Åndrén-Sandberg A and Deng Y: Aspects on
gallbladder cancer in 2014. Curr Opin Gastroenterol. 30:326–331.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheth S, Bedford A and Chopra S: Primary
gallbladder cancer: Recognition of risk factors and the role of
prophylactic cholecystectomy. Am J Gastroenterol. 95:1402–1410.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Misra S, Chaturvedi A, Misra NC and Sharma
ID: Carcinoma of the gallbladder. Lancet Oncol. 4:167–176. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benyamin Y: The structural basis of
calpain behavior. FEBS J. 273:3413–3414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Storr SJ, Woolston CM, Barros FF, Green
AR, Shehata M, Chan SY, Ellis IO and Martin SG: Calpain-1
expression is associated with relapse-free survival in breast
cancer patients treated with trastuzumab following adjuvant
chemotherapy. Int J Cancer. 129:1773–1780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang Y and Wang KK: The calpain family
and human disease. Trends Mol Med. 7:355–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamei M, Webb GC, Young IG and Campbell
HD: SOLH, a human homologue of the Drosophila melanogaster
small optic lobes gene is a member of the calpain and zinc-finger
gene families and maps to human chromosome 16p13.3 near CATM
(cataract with microphthalmia). Genomics. 51:197–206. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho M and Kim H: Glypican-3: A new target
for cancer immunotherapy. Eur J Cancer. 47:333–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capurro MI, Xiang YY, Lobe C and Filmus J:
Glypican-3 promotes the growth of hepatocellular carcinoma by
stimulating canonical Wnt signaling. Cancer Res. 65:6245–6254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Capurro MI, Xu P, Shi W, Li F, Jia A and
Filmus J: Glypican-3 inhibits Hedgehog signaling during development
by competing with patched for Hedgehog binding. Dev Cell.
14:700–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zittermann SI, Capurro MI, Shi W and
Filmus J: Soluble glypican 3 inhibits the growth of hepatocellular
carcinoma in vitro and in vivo. Int J Cancer.
126:1291–1301. 2010.PubMed/NCBI
|
|
15
|
Feng M, Kim H, Phung Y and Ho M:
Recombinant soluble glypican 3 protein inhibits the growth of
hepatocellular carcinoma in vitro. Int J Cancer.
128:2246–2247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu HC, Cheng W and Lai PL: Cloning and
expression of a developmentally regulated transcript MXR7 in
hepatocellular carcinoma: Biological significance and
temporospatial distribution. Cancer Res. 57:5179–5184.
1997.PubMed/NCBI
|
|
17
|
Nakatsura T, Kageshita T, Ito S, Wakamatsu
K, Monji M, Ikuta Y, Senju S, Ono T and Nishimura Y: Identification
of glypican-3 as a novel tumor marker for melanoma. Clin Cancer
Res. 10:6612–6621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stadlmann S, Gueth U, Baumhoer D, Moch H,
Terracciano L and Singer G: Glypican-3 expression in primary and
recurrent ovarian carcinomas. Int J Gynecol Pathol. 26:341–344.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zynger DL, Dimov ND, Luan C, Teh BT and
Yang XJ: Glypican 3: A novel marker in testicular germ cell tumors.
Am J Surg Pathol. 30:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baumhoer D, Tornillo L, Stadlmann S,
Roncalli M, Diamantis EK and Terracciano LM: Glypican 3 expression
in human nonneoplastic, preneoplastic, and neoplastic tissues: A
tissue microarray analysis of 4,387 tissue samples. Am J Clin
Pathol. 129:899–906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saikali Z and Sinnett D: Expression of
glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 89:418–422.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM: Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics: REporting recommendations for
tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol.
2:416–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edge SB, Byrd DR, Compton CC, et al:
Gallbladder. AJCC Cancer Staging Manual (7th). (New York, NY).
Springer. 211–217. 2010.
|
|
24
|
Boutros C, Gary M, Baldwin K and
Somasundar P: Gallbladder cancer: Past, present and an uncertain
future. Surg Oncol. 21:e183–e191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srivastava K, Srivastava A and Mittal B:
Potential biomarkers in gallbladder cancer: Present status and
future directions. Biomarkers. 18:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP,
Li Z, Jiang XQ and Peng ZH: Combined detection tumor markers for
diagnosis and prognosis of gallbladder cancer. World J
Gastroenterol. 20:4085–4092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kulkarni S, Reddy KB, Esteva FJ, Moore HC,
Budd GT and Tubbs RR: Calpain regulates sensitivity to trastuzumab
and survival in HER2-positive breast cancer. Oncogene.
29:1339–1350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Storr SJ, Lee KW, Woolston CM, Safuan S,
Green AR, Macmillan RD, Benhasouna A, Parr T, Ellis IO and Martin
SG: Calpain system protein expression in basal-like and
triple-negative invasive breast cancer. Ann Oncol. 23:2289–2296.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Storr SJ, Pu X, Davis J, Lobo D,
Reece-Smith AM, Parsons SL, Madhusudan S and Martin SG: Expression
of the calpain system is associated with poor clinical outcome in
gastro-oesophageal adenocarcinomas. J Gastroenterol. 48:1213–1221.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Storr SJ, Zaitoun AM, Arora A, Durrant LG,
Lobo DN, Madhusudan S and Martin SG: Calpain system protein
expression in carcinomas of the pancreas, bile duct and ampulla.
BMC Cancer. 12:5112012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baudry M, Chou MM and Bi X: Targeting
calpain in synaptic plasticity. Expert Opin Ther Targets.
17:579–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sawhney RS, Cookson MM, Omar Y, Hauser J
and Brattain MG: Integrin alpha2-mediated ERK and calpain
activation play a critical role in cell adhesion and motility via
focal adhesion kinase signaling: Identification of a novel
signaling pathway. J Biol Chem. 281:8497–8510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Storr SJ, Thompson N, Pu X, Zhang Y and
Martin SG: Calpain in breast cancer: Role in disease progression
and treatment response. Pathobiology. 82:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moretti D, Del Bello B, Allavena G and
Maellaro P: Calpains and cancer: Friends or enemies? Arch Biochem
Biophys. 564:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan Z, Chen C, Long H, Lei C, Tang G, Li
L, Feng J and Chen F: Overexpression of GPC3 inhibits
hepatocellular carcinoma cell proliferation and invasion through
induction of apoptosis. Mol Med Rep. 7:969–974. 2013.PubMed/NCBI
|
|
36
|
Song HH, Shi W, Xiang YY and Filmus J: The
loss of glypican-3 induces alterations in Wnt signaling. J Biol
Chem. 280:2116–2125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Field MA, Cho V, Andrews TD and Goodnow
CC: Reliably detecting clinically important variants requires both
combined variant calls and optimized filtering strategies. PLoS
One. 10:e01431992015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Earl J, Garcia-Nieto S, Martinez-Avila JC,
Montans J, Sanjuanbenito A, Rodríguez-Garrote M, Lisa E, Mendía E,
Lobo E, Malats N, et al: Circulating tumor cells (Ctc) and kras
mutant circulating free Dna (cfdna) detection in peripheral blood
as biomarkers in patients diagnosed with exocrine pancreatic
cancer. BMC Cancer. 15:7972015. View Article : Google Scholar : PubMed/NCBI
|