Introduction
Epithelial ovarian cancer (EOC) is one of the most
common types of cancer among females and the most lethal
gynecological malignancy (1). Due to
a lack of obvious symptoms to allow for its early detection, EOC is
usually diagnosed after the disease has already advanced to a late
stage. By this time, treatments are limited or ineffective.
Therefore, understanding the mechanisms of EOC initiation and
progression is essential for its early diagnosis.
Epithelial-mesenchymal transition (EMT) is a complex
and reversible process during which cellular phenotype, function
and the expression of a large number of molecules are changed
(2,3).
EMT is connected not only to tumor invasion and metastasis but also
to the early stages of carcinogenesis in epithelial malignancy
(4,5).
During classical EMT, epithelial markers, including E-cadherin,
cytokeratins, ZO-1 and claudins, are downregulated, while
mesenchymal markers, including vimentin, N-cadherin, fibronectin
and MUC1, are upregulated. Among the epithelial markers, loss of
E-cadherin is considered to be a hallmark of EMT. The disruption of
E-cadherin-mediated intercellular adhesion is the initiating
process in EMT, and this disruption plays a role in malignant
transformation and tumor progression in a number of carcinomas
(6,7).
The expression of E-cadherin is regulated by various transcription
factors, including Snail, Slug, Twist, ZEB1 and ZEB2. These
transcription factors bind the E-box sequence in the promoter
region of CDH1 and repress E-cadherin expression (8).
Several studies have indicated that
epithelial-mesenchymal interconversions are involved in the
development and progression of ovarian cancer (9,10).
However, the ovarian surface epithelium (OSE) is unique in that it
has characteristics of epithelial and mesenchymal cells, and it
alters its state of differentiation between epithelial and stromal
phenotypes in response to environmental factors (11,12).
Mesenchymal-epithelial transition (MET) occurs during the formation
of inclusion cysts from OSE. The inclusion cysts then gain
epithelial characteristics and may be the origin of EOC (13,14). EMT
occurs during the development of ovarian tumors (15). Based on previous studies, we
hypothesize that EMT and MET are dynamically regulated during the
development and progression of ovarian cancer. The expression of
EMT markers, including E-cadherin and vimentin, may also vary
during this dynamic regulation. This may help explain
inconsistencies in the expression of E-cadherin.
Transcription factors are considered to have a key
role in the induction of EMT. The Snail and Twist families of
transcription factors have been demonstrated to regulate E-cadherin
expression and are associated with tumor progression in ovarian
cancer (16–18). Studies into the ZEB family in ovarian
cancer are relatively few, and the role of the ZEB family in
ovarian cancer is currently unknown (12).
In this study, we analyzed the expression of
E-cadherin and vimentin in various ovarian tissues and assessed the
roles of EMT and MET in the development and progression of ovarian
tumors. We also examined the regulatory effect of the ZEB family of
proteins on E-cadherin, as well as the association of ZEB1, ZEB2,
vimentin and E-cadherin with clinical parameters.
Materials and methods
Patients
A total of 72 formalin-fixed paraffin-embedded
ovarian tissues and metastatic tissues were obtained from the
Department of Pathology at the First and Third Affiliated Hospitals
of Harbin Medical University, China, between 2009 and 2011. The
samples included 10 normal ovarian samples, 12 benign epithelial
ovarian tumors, 8 borderline epithelial ovarian tumors and 31
epithelial ovarian cancers. Additionally, 11 metastatic lesions
were obtained from the above 31 cancer cases. The normal ovarian
samples were obtained from patients who had received an ovariotomy
due to endometrial cancer or cervical carcinoma. The diagnoses for
all samples were confirmed by at least two pathologists. None of
the patients received any therapy prior to surgery, and all patient
samples had complete clinical information. All patients gave their
informed consent prior to their inclusion in the study. The study
was approved by the ethics committee of Harbin Medical
University.
Immunohistochemistry
Immunohistochemistry was performed on 5-µm-thick
paraffin-embedded tissue sections for all samples. Briefly, the
slides were deparaffinized in xylene and rehydrated with a series
of graded ethanol solutions. Endogenous peroxidase was blocked by
3% H2O2 at room temperature for 15 min.
Antigen retrieval was performed using a microwave treatment at 95°C
for 15 min in citrate buffer (pH 6.0). After washing three times
with phosphate-buffered saline (PBS) for 3 min each, the sections
were treated with 5% bovine serum albumin for 10 min at room
temperature to block nonspecific reactions. The sections were then
incubated with primary antibody against E-cadherin (ZSGB Bio,
Beijing, China; 1:50), vimentin (ZSGB Bio; 1:50), ZEB1 (Biorbyt,
Cambridge, UK; 1:100) or ZEB2 (Sigma-Aldrich, St. Louis, MO, USA;
1:100) overnight at 4°C. The bound antibodies were detected using a
streptavidin-biotin peroxidase kit (ZSGB Bio), and the final
staining was completed with DAB (ZSGB Bio). Negative controls were
created by replacing the primary antibodies with PBS. The positive
controls were samples that had previously been demonstrated to
express high levels of the protein being tested.
Evaluation of immunohistochemistry results was
carried out by a pathologist who was blinded to the clinical
information of the patients. The immunohistochemical expression was
scored for intensity and extent. Staining intensity was quantified
as follows: negative (0), weak (1),
moderate (2) or strong (3). Staining extent was scored according to
the percentage of positive cells: none (0), <25% (1), 25–50% (2),
50–75% (3) or >75% (4). The final immunohistochemical score was
then calculated as the intensity score multiplied by the extent
score.
Statistical analysis
All data are presented as the means ± standard
deviation. The two groups were compared using Student's t-test. The
Pearson correlation test was performed to determine associations
between antibody staining patterns. P<0.05 was considered to
indicate a statistically significant difference.
Results
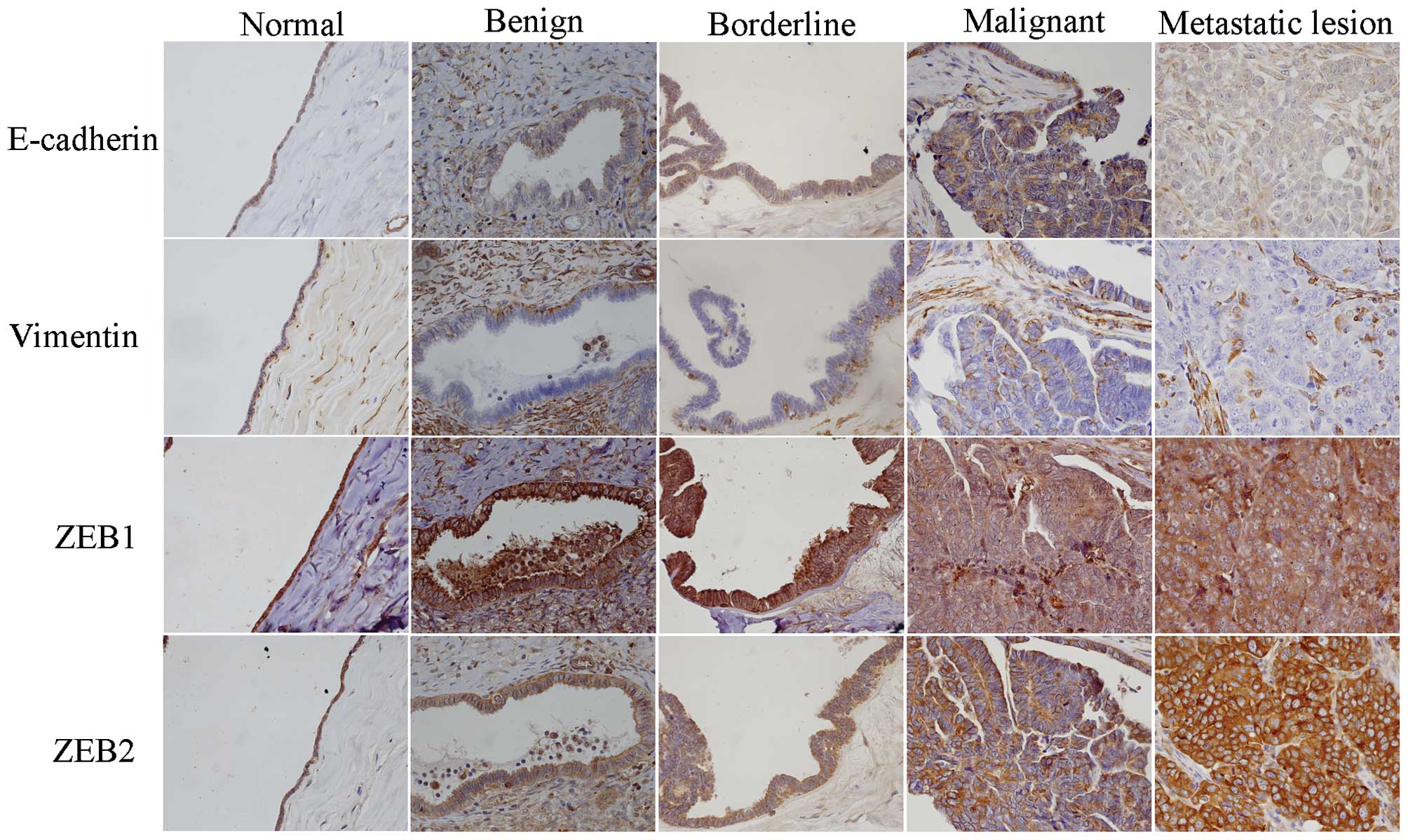
Expression of E-cadherin, vimentin,
ZEB1 and ZEB2 in ovarian tissues
According to the results of immunohistochemical
staining (Fig. 1 and Table I), E-cadherin was almost negative in
normal ovarian epithelium and was positive in ovarian neoplastic
cells. E-cadherin was localized on the cell membranes and/or in the
cytoplasm. Membranous expression of E-cadherin was significant in
benign tumors and was reduced in borderline tumors. The majority of
ovarian cancer tissues expressed low levels of membranous
E-cadherin, and almost all metastatic lesions were negative.
Cytoplasmic expression of E-cadherin was gradually increased in
benign tumors, borderline tumors and ovarian cancers, although
there were no significant differences between them. Notably,
cytoplasmic E-cadherin expression was markedly reduced in
metastatic lesions.
 | Table I.Immunohistochemical scores of
E-cadherin, vimentin and ZEB in ovarian tissues. |
Table I.
Immunohistochemical scores of
E-cadherin, vimentin and ZEB in ovarian tissues.
| Variable | Normal | Benign | Borderline | Malignant | Metastatic
lesions |
|---|
| E-cadherin-C | 0.90±0.99 |
4.42±1.93b | 6.63±3.25 | 8.29±3.37 |
1.91±0.83b |
| E-cadherin-M | 0.80±0.79 |
7.58±2.97b |
5.00±1.85a |
1.61±1.17b |
0.27±0.47b |
| Vimentin | 3.20±1.87 |
1.33±1.97a | 2.88±1.64 |
4.74±2.31a |
8.00±2.10b |
| ZEB1 | 8.60±2.07 | 8.67±2.77 | 7.75±2.43 | 7.48±2.67 | 7.73±2.53 |
| ZEB2 | 8.10±1.85 |
4.25±1.48b |
6.64±1.92a | 8.29±2.44 | 9.09±2.70 |
Normal epithelium tissues were positive for vimentin
expression, but almost all benign tumors were negative. The
expression level of vimentin was increased in borderline tumors and
ovarian cancer tissues and significantly increased in metastatic
lesions. Additionally, positive expression of vimentin was mainly
localized around the cancer nest in the primary lesion,
particularly in the cells which had detached from the cancer nest
and migrated into the stroma.
ZEB1 and ZEB2 were expressed mainly in the cytoplasm
of the normal epithelium and tumor cells. There was no difference
in the expression of ZEB1 among the various types of ovarian
tissues, although the expression of ZEB2 was higher in normal
ovarian tissues, reduced in benign tumors and increased
progressively in borderline tumors, ovarian cancer and metastatic
lesions.
Correlation between E-cadherin and
ZEB2 in ovarian tissues
Membranous E-cadherin expression was significantly
negatively correlated with that of ZEB2 during the progression of
ovarian cancer, with a correlation coefficient of −0.514. However,
the expression of cytoplasmic E-cadherin was not associated with
that of ZEB2. There was no correlation between E-cadherin and
ZEB1.
Correlation of E-cadherin, vimentin
and ZEB with clinical pathological parameters in ovarian
cancer
The correlation of E-cadherin, vimentin, ZEB1 and
ZEB2 with clinical pathological parameters was analyzed in 31
patients with ovarian cancer. As shown in Table II, the expression of cytoplasmic
E-cadherin was higher in patients with FIGO stage III/IV ovarian
cancer than in those with FIGO stage I/II ovarian cancer
(P<0.01). ZEB1 and ZEB2 were also more highly expressed in
patients with FIGO stage III/IV cancer (P<0.01 and P<0.05,
respectively). Additionally, the expression of ZEB1 was associated
with ascitic fluid volume, such that patients with more ascitic
fluid had increased expression of ZEB1 (P<0.05).
 | Table II.Correlation of E-cadherin, vimentin
and ZEB with clinical pathological parameters in ovarian
cancer. |
Table II.
Correlation of E-cadherin, vimentin
and ZEB with clinical pathological parameters in ovarian
cancer.
| Variable | E-cadherin-C | E-cadherin-M | Vimentin | ZEB1 | ZEB2 |
|---|
| Age |
|
|
|
|
|
| ≤50
years | 8.59±3.02 | 1.65±1.22 | 4.24±2.05 | 7.41±2.87 | 8.47±2.87 |
| >50
years | 7.93±3.83 | 1.57±1.16 | 5.36±2.53 | 7.57±2.50 | 8.07±1.86 |
| CA125 |
|
|
|
|
|
| ≤35
U/ml | 8.0±3.74 | 1.25±0.96 | 5.50±1.00 | 5.50±3.00 | 7.50±1.73 |
| >35
U/ml | 8.33±3.39 | 1.67±1.21 | 4.63±2.44 | 7.78±2.55 | 8.41±2.53 |
| Ascitic fluid
volume |
|
|
|
|
|
| ≤100
ml | 7.18±3.22 | 1.73±1.19 | 4.27±2.49 |
6.18±2.75a | 8.09±2.84 |
| >100
ml | 8.90±3.37 | 1.55±1.19 | 5.00±2.22 | 8.20±2.40 | 8.40±2.26 |
| Residual tumor |
|
|
|
|
|
| ≤2
cm | 8.30±3.43 | 1.80±0.63 | 4.90±2.51 | 7.20±1.03 | 8.80±2.66 |
| >2
cm | 8.29±3.42 | 1.52±1.36 | 4.67±2.27 | 7.62±3.19 | 8.05±2.36 |
| FIGO stage |
|
|
|
|
|
|
I–II |
6.53±2.65b | 1.71±1.26 | 5.00±2.42 |
5.88±1.93b |
7.41±2.29a |
|
III–IV | 10.43±2.93 | 1.50±1.09 | 4.43±2.21 | 9.43±2.10 | 9.36±2.24 |
| Tumor grade |
|
|
|
|
|
| High or
moderate | 7.60±3.74 | 1.80±1.21 | 4.53±2.88 | 7.67±2.87 | 8.73±2.87 |
|
Low | 8.94±2.95 | 1.44±1.15 | 4.94±1.69 | 7.31±2.55 | 7.88±1.96 |
| Histological
type |
|
|
|
|
|
|
Serous | 8.77±3.52 | 1.64±0.90 | 4.73±2.19 | 7.68±2.68 | 8.68±2.40 |
|
Mucinous | 7.11±2.80 | 1.56±1.74 | 4.78±2.73 | 7.00±2.74 | 7.33±2.40 |
Discussion
Epithelial-mesenchymal interconversions are involved
in the carcinogenesis and progression of ovarian cancer. However,
the expression of EMT markers in the normal and neoplastic ovary
are complex and do not fully follow the typical EMT model. Normal
OSE expresses little or no E-cadherin, while OSE cells that line
the wall of inclusion cysts are typically positive for E-cadherin
(14). E-cadherin expression in OSE
was also reported to vary with different locations within the ovary
and with cell shape (19). The
literature describing E-cadherin expression in ovarian cancer is
inconsistent. Certain studies indicate that E-cadherin expression
is reduced in primary ovarian cancer and is re-expressed in ovarian
cancer effusions at a higher level (20). Other studies indicate that primary
ovarian cancer expresses E-cadherin, and its expression is reduced
in advanced tumors (21). Further
studies revealed that E-cadherin expression is increased in
metastatic ovarian lesions compared with the primary lesion
(22).
EMT plays a significant role during late invasion
and metastasis (15). We detected the
two classic EMT markers, E-cadherin and vimentin, in normal and
ovarian tumors. Our results revealed that membranous and
cytoplasmic E-cadherin were expressed at low levels in normal OSE.
Additionally, membranous E-cadherin expression was higher in benign
ovarian tumors, decreased in borderline and malignant tumors, and
almost non-existent in metastatic lesions. The expression profile
of vimentin was opposite to that of membranous E-cadherin. These
findings indicate that epithelial-mesenchymal interconversions are
dynamic during the development and progression of ovarian tumors.
Furthermore, as OSE is the site of frequent metaplastic and
dysplastic changes and is considered to be the origin of tumor
formation, a theory which is supported by certain authors, MET may
in fact occur here first (14,19).
Traditionally, E-cadherin is regarded as a significant component of
cell-to-cell adherens junctions, and most investigators evaluate
the expression of E-cadherin on the membranes of different tumor
cells. However, E-cadherin is also observed in the cytoplasm and
nucleus of tumor cells. Voutilainen et al (23) reported that strong cytoplasmic
expression of E-cadherin was present in 9% of EOC cases. In our
study, we noted that cytoplasmic E-cadherin was progressively
increased in benign, borderline and malignant ovarian tumors and
significantly decreased in metastatic lesions. It has been reported
that E-cadherin in the cytoplasm or nucleus may participate in
specific signaling networks to promote tumor progression (24). It may also act as a regulator of gene
transcription by modulating the activity of several signaling
pathways (25,26). Our results also support the emerging
correlation between cytoplasmic E-cadherin and tumor progression.
However, the exact function of cytoplasmic E-cadherin remains
unclear. One intriguing question is why cytoplasmic E-cadherin is
significantly decreased in metastatic lesions.
A number of studies have indicated that E-cadherin,
the hallmark of EMT, is mainly regulated by Snail, Twist, ZEB2/SIP1
and other transcription factors (16,27).
Yoshida et al (16) observed
that the expression of Snail, Slug, ZEB2/SIP1 and Twist increased
progressively in benign, borderline and malignant tumors. Among
these molecules, the expression of Snail was significantly
negatively correlated with E-cadherin expression. Nuclear Snail
expression was demonstrated to be correlated with tumor
progression, but was not associated with clinicopathological
factors or prognosis (18). In recent
years, Twist has been recognized as having a central role in EMT
(28), and data from clinical studies
suggest a prognostic role for Twist (17). The ZEB family includes ZEB1 and
ZEB2/SIP1. The clinical role of ZEB1 and ZEB2 in ovarian carcinoma
is currently not well established. Our results revealed that ZEB1
and ZEB2 are expressed at high levels in ovarian tissue. ZEB1
expression did not change among the various types of ovarian
tissues, but ZEB2 expression was higher in OSE, decreased in benign
ovarian tumors, and increased progressively in borderline tumors,
malignant tumors and metastatic lesions. In contrast to our data,
ZEB1 mRNA levels were previously reported to be significantly
higher in metastases compared with primary carcinomas and effusions
(29). A similar expression profile
for ZEB2 was observed in another study (16). However, ZEB2 mRNA expression levels
were significantly higher in effusions compared with primary tumors
and solid metastases, and ZEB2 was revealed to be a main regulator
of E-cadherin in effusions (26).
Additionally, our results revealed that ZEB1 and ZEB2 were mainly
located in the cytoplasm, which contradicts the results of most
other studies. In order to confirm the specificity of antibodies,
we analyzed ZEB1 and ZEB2 in brain tissues according to the manual,
and the staining was nuclear. ZEB1 and ZEB2 are expressed in brain
tissues and may, therefore, be used as a positive control.
Therefore, our results are credible. Gamba et al (30) also reported nuclear and cytoplasmic
staining for ZEB2 in invasive micropapillary carcinoma, and
suggested that cytoplasmic ZEB2 might be a significant factor in
the early stages of malignancy and predicts a poor overall survival
rate. Li et al (31) also
demonstrated cytoplasmic staining of ZEB1 and ZEB2 in HCC cells and
adjacent non-tumoral liver cells.
A correlation analysis revealed that membranous
E-cadherin was significantly negatively correlated with ZEB2
expression during the progression of ovarian cancer. Our results
suggest that ZEB2 is involved in the regulation of
epithelial-mesenchymal interconversions in ovarian cells.
In summary, the expression profiles of membranous
E-cadherin and vimentin indicated that dynamic
epithelial-mesenchymal interconversions occur during the
development and progression of ovarian cancer. ZEB2, but not ZEB1,
participated in the regulation of E-cadherin expression during this
process. These results provide a molecular basis for studying the
pathogenesis of ovarian cancer and exploring these potentially
valuable therapeutic targets further.
Acknowledgements
This study was supported by the Heilongjiang
Provincial Health Bureau (grant no. 2012–518), the National Natural
Science Foundation of China (grant nos. 81302061 and 81241092) and
the 973 Program for an earlier research project (grant no.
2012CB526705).
Glossary
Abbreviations
Abbreviations:
|
EOC
|
epithelial ovarian cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
OSE
|
ovarian surface epithelium
|
|
MET
|
mesenchymal-epithelial transition
|
|
PBS
|
phosphate-buffered saline
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010.PubMed/NCBI
|
|
3
|
Le Bras GF, Taubenslag KJ and Andl CD: The
regulation of cell-cell adhesion during epithelial-mesenchymal
transition, motility and tumor progression. Cell Adh Migr.
6:365–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Wever O, Pauwels P, De Craene B, Sabbah
M, Emami S, Redeuilh G, Gespach C, Bracke M and Berx P: Molecular
and pathological signatures of epithelial-mesenchymal transitions
at the cancer invasion front. Histochem Cell Biol. 130:481–494.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansieau S, Bastid J, Doreau A, Morel AP,
Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S,
et al: Induction of EMT by twist proteins as a collateral effect of
tumor-promoting inactivation of premature senescence. Cancer Cell.
14:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev
Cancer. 4:118–132. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pećina-Slaus N: Tumor suppressor gene
E-cadherin and its role in normal and malignant cells. Cancer Cell
Int. 3:172003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponnusamy MP, Lakshmanan I, Jain M, Das S,
Chakraborty S, Dey P and Batra SK: MUC4 mucin-induced epithelial to
mesenchymal transition: A novel mechanism for metastasis of human
ovarian cancer cells. Oncogene. 29:5741–5754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurrey NK, K A and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed N, Thompson EW and Quinn MA:
Epithelial mesenchymal interconversions in normal ovarian surface
epithelium and ovarian carcinomas: an exception to the norm. J Cell
Physiol. 213:581–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davidson B, Tropé CG and Reich R:
Epithelial-mesenchymal transition in ovarian carcinoma. Front
Oncol. 2:332012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scully RE: Pathology of ovarian cancer
precursors. J Cell Biochem Suppl. 23:208–218. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okamoto S, Okamoto A, Nikaido T, Saito M,
Takao M, Yanaihara N, Takakura S, Ochiai K and Tanaka T:
Mesenchymal to epithelial transition in the human ovarian surface
epithelium focusing on inclusion cysts. Oncol Rep. 21:1209–1214.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lili LN, Matyunina LV, Walker LD, Wells
SL, Benigno BB and McDonald JF: Molecular profiling supports the
role of epithelial-to-mesenchymal transition (EMT) in ovarian
cancer metastasis. J Ovarian Res. 6:492013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida J, Horiuchi A, Kikuchi N, Hayashi
A, Osada R, Ohira S, Shiozawa T and Konishi I: Changes in the
expression of E-cadherin repressors, Snail, Slug, SIP1 and Twist,
in the development and progression of ovarian carcinoma: the
important role of Snail in ovarian tumorigenesis and progression.
Med Mol Morphol. 42:82–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosono S, Kajiyama H, Terauchi M, Shibata
K, Ino K, Nawa A and Kikkawa F: Expression of Twist increases the
risk for recurrence and for poor survival in epithelial ovarian
carcinoma patients. Br J Cancer. 96:314–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tuhkanen H, Soini Y, Kosma VM, Anttila M,
Sironen R, Hämäläinen K, Kukkonen L, Virtanen I and Mannermaa A:
Nuclear expression of Snail1 in borderline and malignant epithelial
ovarian tumours is associated with tumour progression. BMC Cancer.
9:2892009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maines-Bandiera SL and Auersperg N:
Increased E-cadherin expression in ovarian surface epithelium: an
early step in metaplasia and dysplasia? Int J Gynecol Pathol.
16:250–255. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davidson B, Berner A, Nesland JM, Risberg
B, Berner HS, Tropè CG, Kristensen GB, Bryne M and Florenes Ann V:
E-cadherin and alpha-, beta- and gamma-catenin protein expression
is up-regulated in ovarian carcinoma cells in serous effusions. J
Pathol. 192:460–469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sundfeldt K: Cell-cell adhesion in the
normal ovary and ovarian tumors of epithelial origin; an exception
to the rule. Mol Cell Endocrinol. 202:89–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davidson B: Ovarian carcinoma and serous
effusions. Changing views regarding tumor progression and review of
current literature. Anal Cell Pathol. 23:107–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voutilainen KA, Anttila MA, Sillanpää SM,
Ropponen KM, Saarikoski SV, Juhola MT and Kosma VM: Prognostic
significance of E-cadherin-catenin complex in epithelial ovarian
cancer. J Clin Pathol. 59:460–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez FJ, Lewis-Tuffin LJ and
Anastasiadis PZ: E-cadherin's dark side: possible role in tumor
progression. Biochim Biophys Acta. 1826:23–31. 2012.PubMed/NCBI
|
|
25
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du W, Liu X, Fan G, Zhao X, Sun Y, Wang T,
Zhao R, Wang G, Zhao C, Zhu Y, et al: From cell membrane to the
nucleus: an emerging role of E-cadherin in gene transcriptional
regulation. J Cell Mol Med. 18:1712–1719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elloul S, Silins I, Tropé CG, Benshushan
A, Davidson B and Reich R: Expression of E-cadherin transcriptional
regulators in ovarian carcinoma. Virchows Arch. 449:520–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vernon AE and LaBonne C: Tumor metastasis:
a new twist on epithelial-mesenchymal transitions. Curr Biol.
14:R719–R721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elloul S, Vaksman O, Stavnes HT, Trope CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gamba CO, Campos LC, Negreiros-Lima GL,
Maciel-Lima K, Sousa LP, Estrela-Lima A, Ferreira E and Cassali GD:
ZEB2 and ZEB1 expression in a spontaneous canine model of invasive
micropapillary carcinoma of the mammary gland. Res Vet Sci.
97:554–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li YM, Xu SC, Li J, Han KQ, Pi HF, Zheng
L, Zuo GH, Huang XB, Li HY, Zhao HZ, et al: Epithelial-mesenchymal
transition markers expressed in circulating tumor cells in
hepatocellular carcinoma patients with different stages of disease.
Cell Death Dis. 4:e8312013. View Article : Google Scholar : PubMed/NCBI
|