Introduction
Colorectal cancer (CRC) is the third leading cause
of cancer-associated mortality and morbidity in the USA, accounting
for 9% of novel cases and mortalities that affect men and women
(1). At present, the available
diagnostic methods for CRC, including colonoscopy and fecal
occult-blood test, remain unsatisfactory (2), and common premalignant lesions, such as
sessile serrated adenomas, are extremely challenging to identify.
The 5-year survival rate for patients with early stage CRC is ~80%,
following radical surgery (2). In
addition, during the process of CRC cell apoptosis induced by
chemotherapy, tolerant cells often appear and escape treatment.
Therefore, novel effective targets for anti-CRC treatment are
required.
The forkhead box C2 (Foxc2) gene was first
identified in mice during embryogenesis (3). The gene was then observed at high
expression levels in a variety of malignancies, including
esophageal (4), breast (5) and prostate (6) cancers. In addition, there is evidence
that suggests that Foxc2 is associated with the regulation of cell
proliferation, the development of angiogenesis (7) and the metastasis of human tumors
(8–10). Previous studies have established that
Foxc2 plays a predominant role in the modulation of cancer cells,
which resist apoptosis-based tumor surveillance and treatments
(11). Petrova et al reported
that Foxc2 is highly expressed in developing lymphatic vessels and
lymphatic valves (12). However, the
functional role of Foxc2 has not yet been investigated under
physiological and pathological conditions.
A preparatory study revealed that Foxc2 expression
is significantly associated with the progression of CRC in
vitro and in vivo, using reverse
transcription-polymerase chain reaction (RT-PCR) and western blot
analysis. This result is also demonstrated in CRC samples in
paraffin-embedded tissues (13).
Foxc2 was expressed in CRC patients and upregulated in the majority
of these patients. Furthermore, the downregulation of Foxc2 lead to
the apoptosis of cancer cells. Based on these results, the present
study hypothesizes that the ablation of Foxc2 expression may lead
human cancer cells, including CRC cells, to become sensitive to
chemotherapy.
The present study, to the best of our knowledge, is
the first to demonstrate that the morphological alterations
observed in 5-fluorouracil (5-FU)-induced apoptosis are paralleled
by Foxc2 deregulation. These results may have implications for the
design of chemotherapy treatment; the combination of 5-FU treatment
and Foxc2 depletion may lead to an improved treatment strategy for
CRC patients.
Materials and methods
Cell lines
The study was approved by the Ethics Committee of
Southern Medical University (Guangzhou, Guangdong, China). The
human colon carcinoma HCT116 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). These cells
were grown in Gibco RPMI-1640 media (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and supplemented with 10% fetal bovine
serum (GE Healthcare, Life Sciences, Chalfont, UK) and 100 units
Invitrogen penicillin-streptomycin (Thermo Fisher Scientific, Inc.)
at 37°C, with a 5% CO2 atmosphere in a humidified
incubator.
Retroviral infection and reverse
transcription
The Foxc2 expression construct was generated by
cloning PCR-amplified, full-length or deletion mutant human Foxc2
cDNA into a pBabe plasmid (Addgene, Inc., Cambridge, MA, USA). RNA
was extracted from the HCT116 cells using Trizol reagent (Thermo
Fisher Scientific, Inc.), as previously described (14). Foxc2 cDNA was obtained using reverse
transcription, which was performed using the Invitrogen
SuperScript™ First-Strand Synthesis System for RT-PCR (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The human shRNA sequence, as described by Mani et al
(15), was 5′-CCACACGTTTGCAACCCAA-3′.
To knockdown Foxc2, the sequence was cloned into a pSUPER.retro.neo
vector (Oligoengine, Seattle, WA, USA). Retroviral production and
infection were performed, as previously described (16).
Quantitative PCR (qPCR) and western
blot analysis
qPCR and western blot analysis were performed to
confirm the successful infection of the CRC cells. qPCR was
performed using the Applied Biosystems ABI PRISM® 7500 Sequence
Detection System (Thermo Fisher Scientific Inc.) and the iQ™ SYBR®
Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
which contained 5 ng cDNA and 10 pM of each primer. The reference
gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal quantitative control. The methods used were as
previously described (17). The
thermal cycling conditions for the qPCR were as follows:
Denaturation at 95°C for 5 min; 40 cycles of denaturation at 95°C
for 15 sec; annealing at 56.4°C for 20 sec; and extension at 72°C
for 20 sec. Primers were designed using Applied BioSystems Primer
Express version 2.0 software (Thermo Fisher Scientific, Inc.), as
follows: Foxc2 sense, 5′-CTACAGCTACATCGCGCTCATCA-3′ and antisense,
5′-ACTGGTAGATGCCGTTCAAGGTG-3′; Bmi-1 sense,
5′-CTGGTTGCCCATTGACAGC-3′ and antisense,
5′-CAGAAAATGAATGCGAGCCA-3′; and GAPDH sense,
5′-GACTCATGACCACAGTCCATGC-3′ and antisense,
5′-AGAGGCAGGGATGATGTTCTG-3′. qPCR was performed using the Applied
BioSystems SYBR Green I Nucleic Acid Gel Stain (Thermo Fisher
Scientific, Inc.). The data was normalized to the geometric mean of
the reference gene GAPDH, and calculated using the
2−ΔΔCq method (18). For
western blot analysis, an anti-Foxc2 rabbit anti-mouse monoclonal
antibody (dilution, 1:1000; catalog no. A302–383A Bethyl
Laboratories, Inc., Montgomery, TX, USA) was used.
Assessment of sensitivity to 5-FU
using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay
Following synchronization for 24 h in a serum-free
medium, a total of 5×103 cells were seeded in 6-well
plates and treated with 0.5, 1, 2 or 4 mg/l 5-FU (Sigma-Aldrich,
Saint Louis, MO, USA) for 48 and 72 h. Cell viability was
determined by an MTT assay. In brief, the cells were seeded on a
96-well plate (103 cells/well), and on the following day
20 µl/well of sterile MTT dye (5 mg/ml; Sigma-Aldrich) was added to
the cells. Following 4 h incubation at 37°C, the medium was
discarded and 150 µl/well of dimethyl sulfoxide (Sigma-Aldrich) was
added. The absorbance was measured at 570 nm. The experiments were
repeated 3 times.
Detecting apoptotic and MAPK and
PI3K/AKT pathway markers in CRC cells by western blot analysis
Western blot analysis was used to observe the
apoptosis of the cells, induced by 5-FU and the depletion of Foxc2,
and to detect mitogen-activated protein kinase (MAPK) and
phosphatidylinositide 3-kinases/protein kinase B (PI3K/AKT)
pathways. The following primary rabbit anti-mouse monoclonal
antibodies were used, at a dilution of 1:1,000 unless otherwise
stated: Anti-extracellular-signal-regulated kinases (ERK; catalog
no. BS3627), anti-phosphorylated (p)-ERK (catalog no. BS4621),
anti-c-Jun N-terminal kinases (JNK; catalog no. BS1544), anti-p-JNK
(catalog no. BS4322), anti-p-P38 (catalog no. BS4766), anti-AKT
(catalog no. BS2987), anti-p-AKT (catalog no. BS4007),
anti-pro-caspase-3 (catalog no. BS1518), anti-cleaved-caspase-3
(catalog no. BS9661S), anti-B cell lymphoma (Bcl)-2 (catalog no.
BS1511) and anti-Bcl-2-associated X protein (Bax; dilution, 1:100;
catalog no. BS2538) (Bioworld Technology, Inc., St. Louis Park, MN,
USA). Bcl-2, Bax and pro-caspase-3 are markers for apoptosis, while
the other antigens are markers for the MAPK and PI3K/AKT pathways.
Western blot analysis was performed as previously described
(17). Briefly, equal amounts of
protein were separated by electrophoresis on a 10% SDS-PAGE gel and
electrotransferred from the gel to a nitrocellulose membrane (Merck
& Co., Inc., Whitehouse Station, NJ, USA). Following blocking
with 5% milk solution (Yili Group Co., Ltd., Neimenggu, China) for
2 h at room temperature, the membranes were incubated with the
primary antibodies at 4°C overnight. The membranes were washed
three times in Tris-buffered saline with Tween-20 (TBS-T; Merck
& Co., Inc.). Anti-α-tubulin rabbit anti-mouse monoclonal
antibody (dilution, 1:2,000; catalog no. KM9007; Tianjin Sungene
Biotech Co., Ltd., Tianjin, China) was used as an internal loading
control. Following washing with TBS-T, the nitrocellulose membrane
was incubated with a secondary antibody against rabbit
immunoglobulin (Ig)G or mouse IgG. The membrane was examined using
an FluorChem FC2 Imaging System (Alpha Innotech, San Leandro, CA,
USA), according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons
between groups for statistical significance were performed using a
two-tailed paired Student's t test. P<0.05 was considered to
indicated a statistically significant difference.
Results
Establishment of Foxc2 small hairpin
(sh) RNA stable cell lines
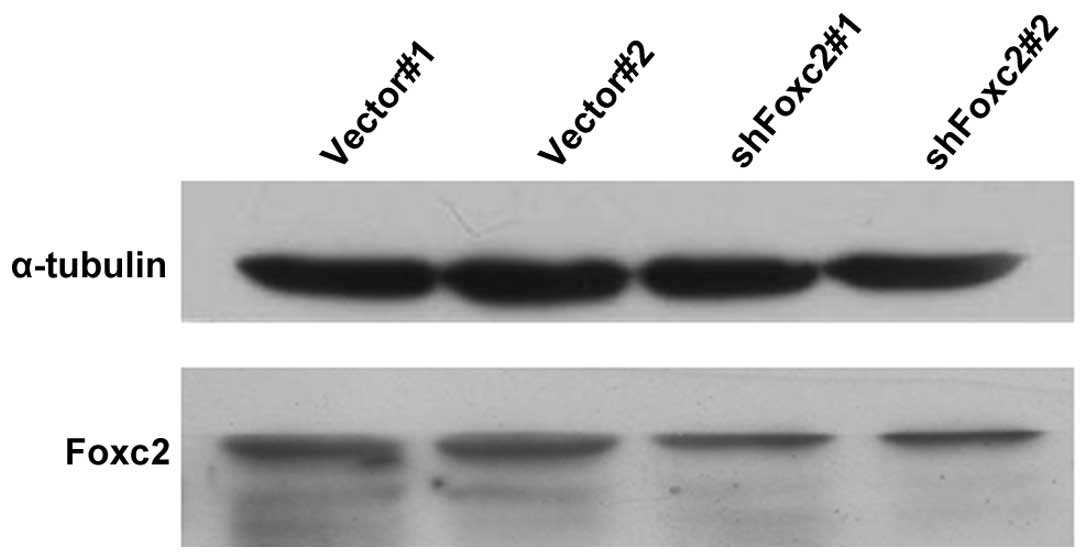
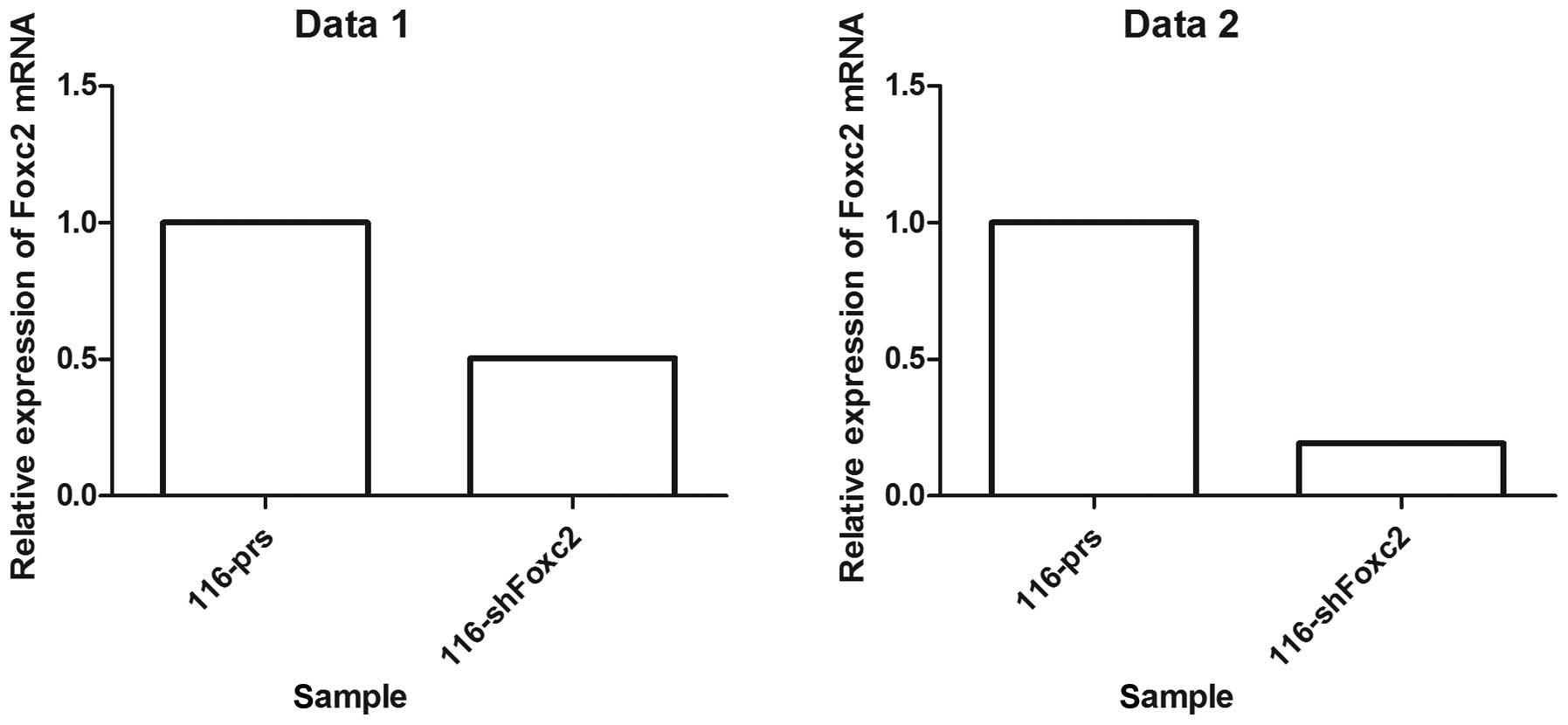
Foxc2-shRNA stable cell lines were established to
study the role of Foxc2 in CRC cell survival. Western blot analysis
and RT-qPCR revealed that protein and mRNA expression of Foxc2 was
markedly downregulated in HCT116-shFoxc2 cells, in contrast to
those in HCT116-vector cells (Figs. 1
and 2).
Ablation of Foxc2 expression leads to
human colon cancer HCT116 cell lines becoming sensitized to
5-FU
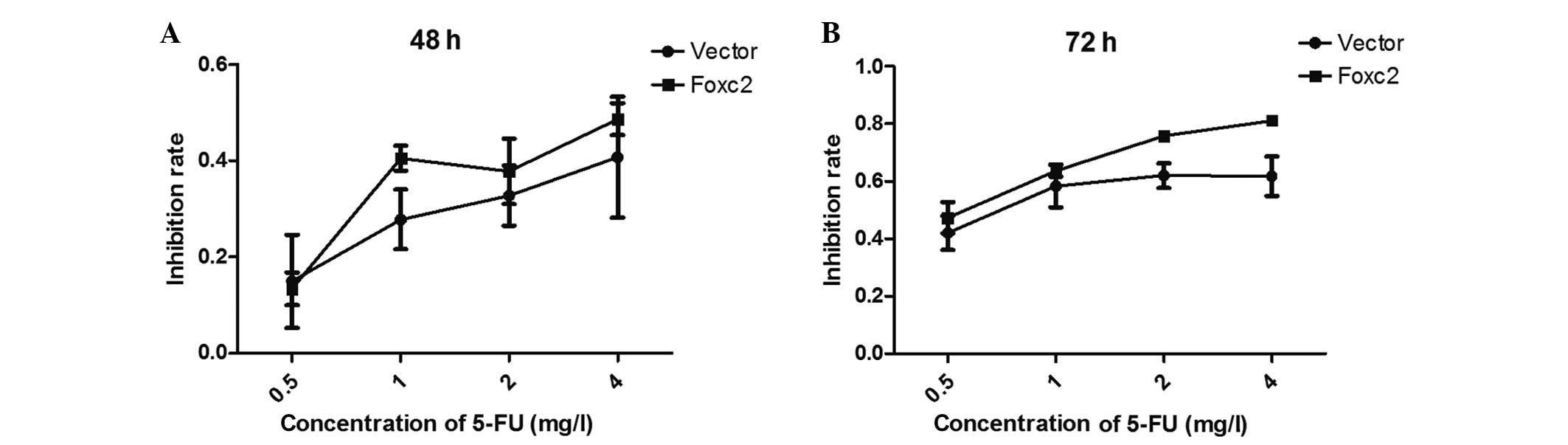
An MTT assay was performed to examine the effect of
5-FU on the survival rate of Foxc2-knockdown cells. The cells were
treated with 5-FU for 48 and 72 h, and it was demonstrated that
downregulation of Foxc2 significantly decreased the growth rate of
HCT116 cells compared with vector cells. In detail, the present
study identified that the viability of the HCT116-Foxc2/RNA
interference cells were decreased compared with HCT116-vector cells
(control). Following treatment with 5-FU for 48 h, the half maximal
inhibitory concentration (IC50) of 5-FU in the
HCT116-vector and HCT116-shFoxc2 cells were 6.3919 mg/l and 5.2965
mg/l (P>0.05), respectively. Following treatment for 72 h, the
IC50 decreased to 1.8839 and 1.1238 mg/l, respectively
(P<0.05; Fig. 3; Table I). The results suggest that following
a depletion of Foxc2 all the cells become sensitized to 5-FU,
particularly following a 72-h treatment time.
 | Table I.Data obtained from the MMT assay,
which examined the effect of 5-fluorouracil on the survival of
Foxc2 knockdown cells. |
Table I.
Data obtained from the MMT assay,
which examined the effect of 5-fluorouracil on the survival of
Foxc2 knockdown cells.
|
| HCT116-vector,
control | HCT116-Foxc2/RNAi
cells |
|---|
|
|
|
|
|---|
| Variable | Mean | SD | Mean | SD |
|---|
| Data set 1 |
|
|
|
|
| 0.5 | 0.149116600 | 0.096946720 | 0.133288900 | 0.034042870 |
| 1 | 0.277738500 | 0.062077910 | 0.404865100 | 0.025722880 |
| 2 | 0.327444000 | 0.062361180 | 0.377874000 | 0.067800570 |
| 4 | 0.407302700 | 0.126097800 | 0.486171300 | 0.033669970 |
| Data set 2 |
|
|
|
|
| 0.5 | 0.420335700 | 0.059749480 | 0.473311200 | 0.054933290 |
| 1 | 0.583677900 | 0.074168170 | 0.637435200 | 0.021126600 |
| 2 | 0.620165400 | 0.042991280 | 0.757917100 | 0.014314910 |
| 4 | 0.618219400 | 0.068660390 | 0.811879100 | 0.009069179 |
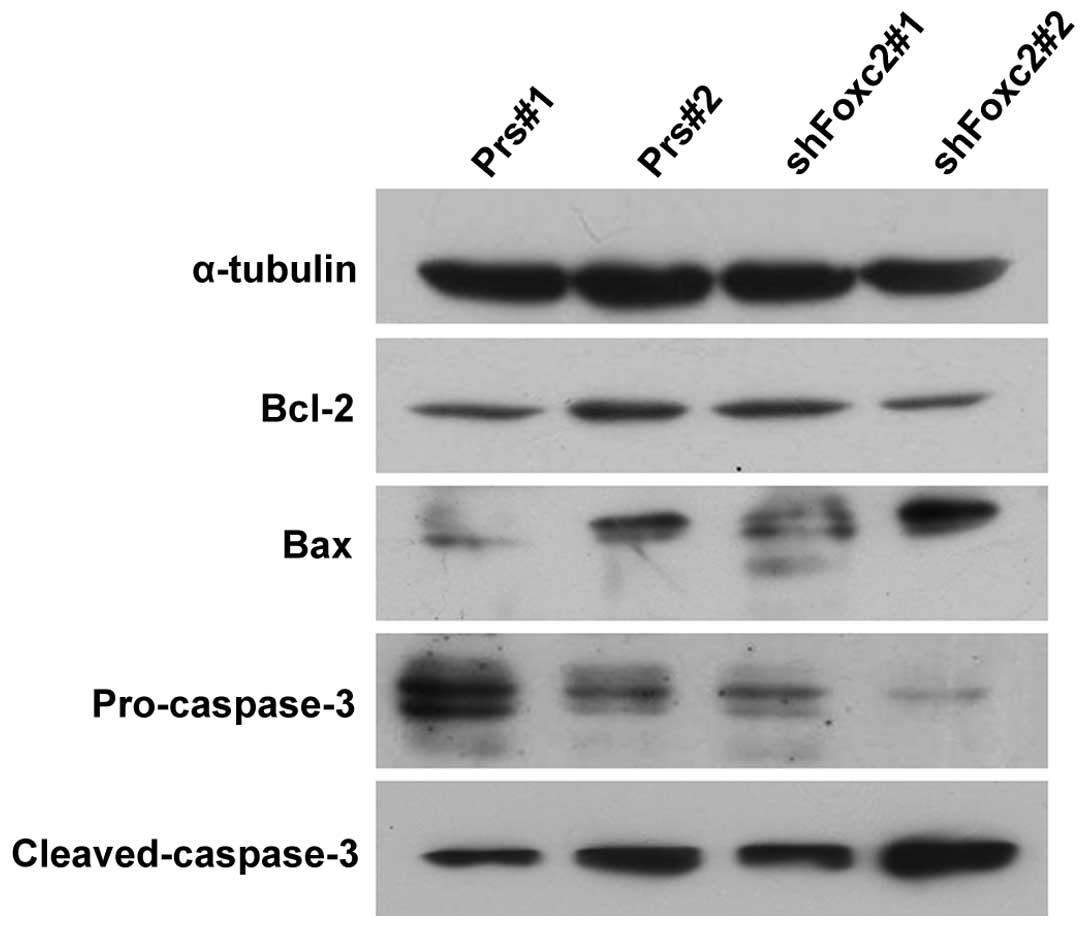
Foxc2 aids cells in resisting
apoptosis and regulating the expression levels of Bcl-2, Bax and
caspase-3
Due to the unsatisfactory clinical therapies
available, as a result of the resistance of cancer cells to
apoptosis, the present study investigated whether Foxc2 enhanced
the anti-apoptotic activity of CRC cells. To investigate the
mechanism by which apoptosis is upregulated when treated with 5-FU
and a knockdown of Foxc2, the expression levels of the apoptosis
regulators Bcl-2, Bax, pro-caspase-3 and cleaved-caspase-3 were
observed in HCT116-shFoxc2 and HCT116-vector cells. Western blot
analysis revealed a downregulation of Bcl-2 and pro-caspase-3 in
Foxc2-knockdown cells, while Bax and cleaved-caspase-3 were
upregulated (Fig. 4).
MAPK and PI3K/AKT pathways are
essential for the sensitization effect of Foxc2 to 5-FU
treatment
The PI3K/AKT signaling pathway is an important
survival pathway in numerous cellular systems and the activation of
this pathway is required to prevent cell apoptosis (19,20). To
investigate whether the depletion of Foxc2 enhances the apoptosis
of cells through the PI3K/AKT pathways, the present study analyzed
p-AKT and total AKT levels. As revealed in Fig. 5, p-AKT was decreased in HCT116-shFoxc2
cells, but the total AKT levels were not altered. The result
confirms that the PI3K/AKT pathway plays an important role in the
sensitization effect of Foxc2 to 5-FU treatment.
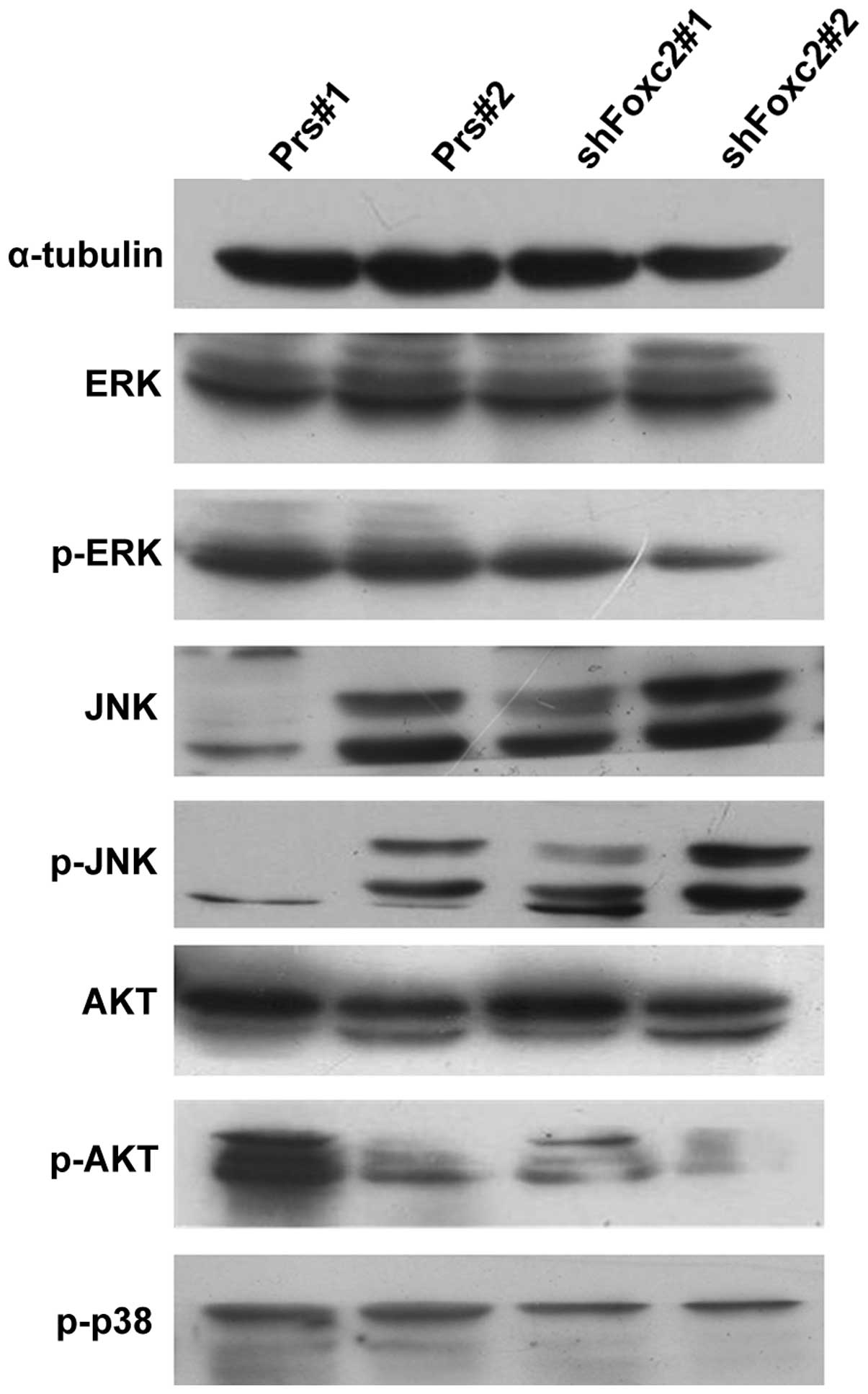 | Figure 5.Foxc2 activates the mitogen-activated
protein kinase and phosphatidylinositide 3-kinases/protein kinase B
signaling pathways. The protein levels of the indicated proteins
were determined by western blot analysis. Foxc2, forkhead box C2
gene; 5-FU, 5-fluorouracil; ERK, extracellular-signal-regulated
kinases; p, phosphorylated; JNK, c-Jun N-terminal kinases; AKT,
protein kinase B; Prs#1, human colon cancer HCT116 cells without
treatment of 5-FU; Prs#2, human colon cancer HCT116 cells with 2
mg/l 5-FU; shFoxc2#1, Foxc2 short hairpin RNA stable cell lines
without treatment of 5-FU; shFoxc2#2, Foxc2 short hairpin RNA
stable cell lines with 2 mg/l 5-FU. |
In addition, in the MAPK pathway, the present study
observed that although the total levels of ERK and JNK were not
significantly altered subsequent to treatment with 5-FU, p-ERK did
markedly decrease with the downregulation of Foxc2. By contrast,
the levels of p-JNK increased, indicating Foxc2-mediated activation
may occur via the MAPK pathway.
Discussion
Previous studies have demonstrated that Foxc2 is
vital in tumor metastasis and metabolism (21). The present study revealed that the
development of CRC is associated with a high expression of Foxc2,
and a downregulation of Foxc2 enhanced the apoptotic rate of CRC
cells. These findings indicate that Foxc2 may act as a potential
diagnostic marker in CRC, and a specific inhibitor of Foxc2 may be
a novel strategy for the treatment of CRC patients.
Resisting the apoptosis of cells is a hallmark of
the majority of cancers (22). At
present, the elimination of the resistance to apoptosis is an
anticancer therapy (23). Activation
of apoptotic pathways is currently considered a vital step in the
development of tumors (24,25). Therefore, the identification of the
mechanism underlying the apoptotic pathway is important. Common
stresses inducing apoptosis are imbalances in signaling pathways,
resulting in altered levels of oncogene signaling. Alternatively,
tumors may increase the expression of anti-apoptotic regulators,
including Bcl-2 and Bcl-extra large, and survival signals, such as
insulin-like growth factor 1, by downregulating pro-apoptotic
factors, including Bax, Bcl-2 and cleaved-caspase-3, or by eluding
the extrinsic ligand-induced death pathway (26).
The present study demonstrated that Foxc2 is vital
in creating an anti-apoptotic environment in CRC cells that is
relatively insensitive to chemotherapy. The increased expression of
Foxc2 in CRC cells enhances the resistance to apoptosis induced by
5-FU, a common chemotherapeutic drug used in alimentary canal
neoplasm. By contrast, a knockdown of Foxc2 markedly enhanced the
sensitivity of the cells to 5-FU; therefore, allowing the cells to
undergo apoptosis. The present study concludes that the activity of
Foxc2 is associated with the increased survival rate of CRC cells,
when 5-FU is used for therapy. Therefore, Foxc2 may be a potential
target for chemotherapy.
MAPK and PI3K/AKT signaling pathways are frequently
involved in the promotion of proliferation of cells (20,27,28), while
inhibition of the MAPK pathway suppresses the proliferation of
cells by induction of cell apoptosis through caspase activation
(29). The activation of several
proteins, including ERK, JNK and AKT, is regulated by the MARK and
PI3K/AKT pathways (30–32). The present study investigated whether
PI3K and MAPKs are critical in the Foxc2 inhibition of apoptosis,
and demonstrated that several MAPK-regulated proteins were
upregulated in Foxc2-overexpressing CRC cells and downregulated in
Foxc2-inhibited CRC cells. Since Foxc2 is a transcriptional gene,
it may not affect the MAPK and PI3K/AKT pathways through
phosphorylation directly, and it is evident that the molecular
mechanism of how Foxc2 affected the pathway requires additional
investigation. Furthermore, several other questions require
resolution; the potential role of Foxc2 in CRC cells remains
unclear, and the involvement of other signaling pathways in the
anti-apoptosis of CRC should be determined, although the present
study doubts the involvment of additional pathways. Consequently,
additional details concerning the function of Foxc2 require
investigation.
In conclusion, the present study demonstrates the
methods behind the combination of Foxc2 depletion and 5-FU
treatment. A knockdown of Foxc2 induces cancer cells to become more
sensitive to 5-FU, and a depletion of Foxc2 enhances 5-FU-induced
apoptosis. Furthermore, the present study demonstrated that the
MAPK and PI3K/AKT pathways are critical for the development of
Foxc2. In the present study, a knockdown of Foxc2 inhibits AKT
activation and regulates the expression of Bcl-2 and Bax. In
addition, p-ERK markedly decreased with the downregulation of Foxc2
under the treatment with 5-FU. Overall, the present study reveals
that a combination of Foxc2 depletion and 5-FU treatment may be a
potential clinical therapy for CRC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30901791, 81172055
and 81071735), Major Projects of the National Natural Science
Foundation of China (grant no. 81090422), State Key Program of the
National Natural Science Foundation of China (grant no. U1201226),
National Basic Research Program of China (The 973 Program; grant
nos. 2010CB529402 and 2010CB529403), Guangdong Provincial Natural
Science Foundation of China (grant no. S2012010009643), Zhu Jiang
Science and Technology New Star Foundation in Guangzhou City (grant
nos. 201212200052 and 2012J2200044), Science and Technology
Innovation Foundation of Guangdong Higher Education (grant no.
CXZD1016) and Key Program of the National Natural Science
Foundation of Guangdong (grant no. 2010B031500012).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hendon SE and DiPalma JA: U.S. practices
for colon cancer screening. Keio J Med. 54:179–183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miura N, Wanaka A, Tohyama M and Tanaka K:
MFH-1, a new member of the fork head domain family, is expressed in
developing mesenchyme. FEBS Lett. 326:171–176. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishida N, Mimori K, Yokobori T, Sudo T,
Tanaka F, Shibata K, Ishii H, Doki Y and Mori M: FOXC2 is a novel
prognostic factor in human esophageal squamous cell carcinoma. Ann
Surg Oncol. 18:535–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hollier BG, Tinnirello AA, Werden SJ,
Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV,
Battula VL, et al: FOXC2 expression links epithelial-mesenchymal
transition and stem cell properties in breast cancer. Cancer Res.
73:1981–1992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Heul-Nieuwenhuijsen L, Dits NF and
Jenster G: Gene expression of forkhead transcription factors in the
normal and diseased human prostate. BJU Int. 103:1574–1580. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papanicolaou KN, Izumiya Y and Walsh K:
Forkhead transcription factors and cardiovascular biology. Circ
Res. 102:16–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano H, Leboeuf JP, Novitskiy SV, Seo S,
Zaja-Milatovic S, Dikov MM and Kume T: The Foxc2 transcription
factor regulates tumor angiogenesis. Biochem Biophys Res Commun.
392:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kume T: Foxc2 transcription factor: A
newly described regulator of angiogenesis. Trends Cardiovasc Med.
18:224–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe T, Kobunai T, Yamamoto Y, Matsuda
K, Ishihara S, Nozawa K, Iinuma H, Kanazawa T, Tanaka T, Konishi T,
et al: Gene expression of mesenchyme forkhead 1 (FOXC2)
significantly correlates with the degree of lymph node metastasis
in colorectal cancer. Int Surg. 96:207–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Subramanya RD, Coda AB and Sinha AA:
Transcriptional profiling in alopecia areata defines immune and
cell cycle control related genes within disease-specific
signatures. Genomics. 96:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petrova TV, Karpanen T, Norrmén C, Mellor
R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P,
Ylä-Herttuala S, et al: Defective valves and abnormal mural cell
recruitment underlie lymphatic vascular failure in lymphedema
distichiasis. Nat Med. 10:974–981. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui YM, Jiang D, Zhang SH, Wu P, Ye YP,
Chen CM, Tang N, Liang L, Li TT, Qi L, et al: FOXC2 promotes
colorectal cancer proliferation through inhibition of FOXO3a and
activation of MAPK and AKT signaling pathways. Cancer Lett.
353:87–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Likhite N and Warawdekar UM: A unique
method for isolation and solubilization of proteins after
extraction of RNA from tumor tissue using trizol. J Biomol Tech.
22:37–44. 2011.PubMed/NCBI
|
|
15
|
Mani SA, Yang J, Brooks M, Schwaninger G,
Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL and Weinberg
RA: Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis
and is associated with aggressive basal-like breast cancers. Proc
Natl Acad Sci USA. 104:10069–10074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao WT, Wang X, Xu LH, Kong QL, Yu CP, Li
MZ, Shi L, Zeng MS and Song LB: Centromere protein H is a novel
prognostic marker for human nonsmall cell lung cancer progression
and overall patient survival. Cancer. 115:1507–1517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón P: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watanabe A, Suzuki H, Yokobori T, Altan B,
Kubo N, Araki K, Wada S, Mochida Y, Sasaki S, Kashiwabara K, et al:
Forkhead box protein C2 contributes to invasion and metastasis of
extrahepatic cholangiocarcinoma, resulting in a poor prognosis.
Cancer Sci. 104:1427–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu JJ, Lin M, Yu JY, Liu B and Bao JK:
Targeting apoptotic and autophagic pathways for cancer
therapeutics. Cancer Lett. 300:105–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denicourt C and Dowdy SF: Medicine.
Targeting apoptotic pathways in cancer cells. Science.
305:1411–1413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hougardy BM, Maduro JH, van der Zee AG,
Willemse PH, de Jong S and de Vries EG: Clinical potential of
inhibitors of survival pathways and activators of apoptotic
pathways in treatment of cervical cancer: Changing the apoptotic
balance. Lancet Oncol. 6:589–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rinn JL, Wang JK, Allen N, Brugmann SA,
Mikels AJ, Liu H, Ridky TW, Stadler HS, Nusse R, Helms JA and Chang
HY: A dermal HOX transcriptional program regulates site-specific
epidermal fate. Genes Dev. 22:303–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leonis MA, Thobe MN and Waltz SE:
Ron-receptor tyrosine kinase in tumorigenesis and metastasis.
Future Oncol. 3:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuchiya T, Tsuno NH, Asakage M, Yamada J,
Yoneyama S, Okaji Y, Sasaki S, Kitayama J, Osada T, Takahashi K and
Nagawa H: Apoptosis induction by p38 MAPK inhibitor in human colon
cancer cells. Hepatogastroenterology. 55:930–935. 2008.PubMed/NCBI
|
|
30
|
Mao JD, Wu P, Huang JX, Wu J and Yang G:
Role of ERK-MAPK signaling pathway in pentagastrin-regulated growth
of large intestinal carcinoma. World J Gastroenterol.
20:12542–12550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esmaeili MA, Farimani MM and Kiaei M:
Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling
pathways, ROS-mediated pathway and mitochondrial dysfunction in
hepatoblastoma cancer (HepG2) cells. Mol Cell Biochem. 397:17–31.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen JY, Zhang L, Zhang H, Su L and Qin
LP: Triggering of p38 MAPK and JNK signaling is important for
oleanolic acid-induced apoptosis via the mitochondrial death
pathway in hypertrophic scar fibroblasts. Phytother Res.
28:1468–1478. 2014. View
Article : Google Scholar : PubMed/NCBI
|