Introduction
Radiotherapy (RT) subsequent to breast-conserving
surgery for the treatment of breast cancer decreases recurrence and
improves survival rate (1). Whole
breast irradiation (WBI) is recommended for all patients who
undergo breast-conserving surgery. Side effects that are commonly
experienced during the acute period include radiation dermatitis,
esophagitis, pharyngitis and nausea. In the subacute phase (2–12
months post-RT), there is a risk of radiation pneumonitis. Late
morbidities occurring >1 year post-RT include arm edema, rib
fractures, brachial plexopathy, secondary malignancies and
long-term cardiac toxicity. Meric et al (2) reported the frequencies of such
complications and stated that of 294 patients that received WBI for
breast cancer, 29 (9.9%) presented with grade 2 or higher
complications at 1 year post-treatment, consisting of arm edema
(n=13), breast skin fibrosis (n=12), a decreased range of motion
(n=4) and pneumonitis (n=2). Treatment-induced secondary
malignancies following RT are rare, and include contralateral
breast cancer and non-breast primary malignancies, such as sarcoma,
lung cancer, esophageal cancer and leukemia (1–4). The risk
of secondary non-breast malignancy is ~1% (3).
Previously, a case of osteosarcoma following RT was
reported by Beck in 1922 (5) and the
development of a soft tissue sarcoma of the breast following RT was
reported by Warren et al in 1936 (6). In 1948, Cahan et al described the
following criteria for the diagnosis of radiation-induced sarcoma
(RIS) (7): A history of RT; an
asymptomatic latency period of several years; an occurrence of
sarcoma within a previously irradiated tissue region; and a
histological confirmation of the sarcomatous nature of the
lesion.
Angiosarcoma is the most common of all RIS, and it
accounts for ~50% of all RIS. In general, sarcomas are classified
according to histological grade, which may indicate the
histological malignancy and distant recurrence rate of the tumor
and mortality prognosis of the patient (8). Patients with RIS have a poorer prognosis
compared with patients with primary sarcoma (9). In addition to poor survival outcomes,
local recurrence rates are also increased in patients with RIS
compared with those with primary sarcomas (10). This may be due to the failure of
complete surgical resection or deficiency of RT as a treatment
(11).
The current study presents a rare case of RIS of the
chest wall 9 years subsequent to RT of the whole breast. In the
present case, the histological type of the tumor was classified as
a low-grade fibromyxoid sarcoma.
Case report
The present patient was a 62-year-old Japanese woman
that was originally diagnosed with non-Hodgkin's lymphoma (NHL) of
the right parotid at in September 1985 (at 43 years of age) at the
University of Tokyo Hospital (Tokyo, Japan). The patient possessed
no other notable personal or familial medical history. The NHL was
a diffuse B-cell lymphoma stage II small-cell type tumor. Following
the diagnosis of NHL in 1985, the patient underwent a resection of
the affected parotid and received chemotherapy. The chemotherapy
regimen administered was two cycles of cisplatin and peplomycin
therapy, which consisted of 100 mg cisplatin (day 1) and 5 mg
peplomycin sulfate (day 1–4), and four cycles of cyclophosphamide,
vincristine and prednisone/prednisolone therapy, which consisted of
800 mg cyclophosphamide (day 1) and 1 mg vincristine (day 1).
Furthermore, the patient received RT, which consisted of 39.6 Gy in
22 fractions administered for 4 weeks, to the whole neck and
supraclavicular region using cobalt-60. The whole neck RT was
performed in lateral 12×14 cm opposed fields and the
supraclavicular RT was treated in the antero-posterior 8×20 cm
opposed fields.
In May 1995, when the patient was 53 years of age, a
small lump in the left breast was identified at the University of
Tokyo Hospital, and a surgeon was consulted. A 2.5 cm diameter
nodule with dimpling in the upper-outer region of the left breast
was identified. A clinical examination resulted in the diagnosis of
primary breast carcinoma (cT2N0M0; stage IIA). On 4 October 1995,
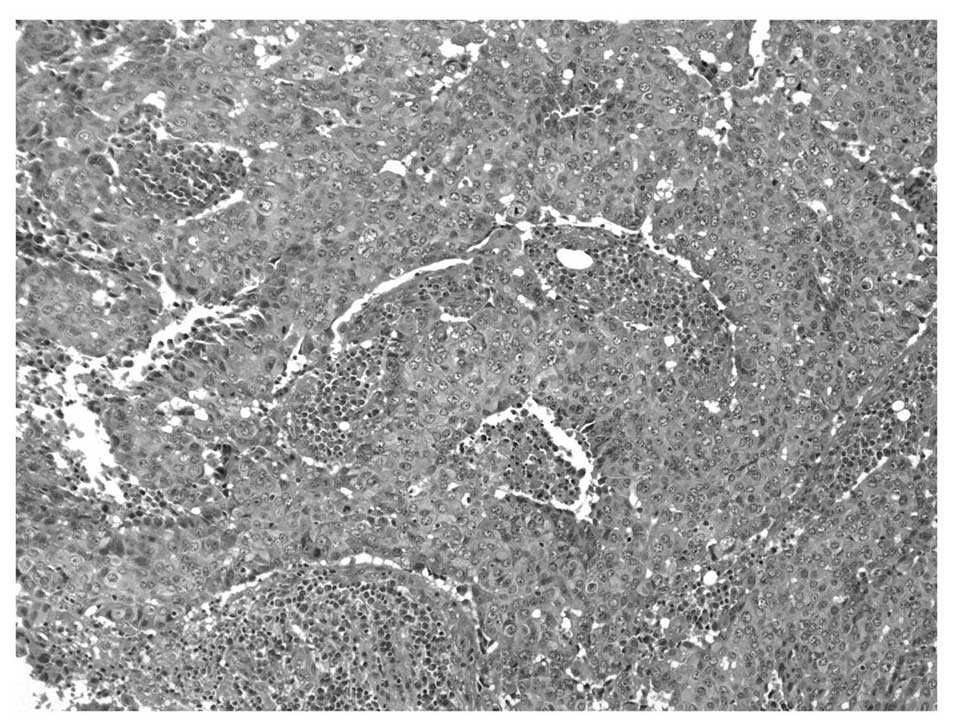
the patient underwent a tumor excision. Pathological examination
revealed an invasive ductal carcinoma of a solid tubular type
(Fig. 1). No immunohistological
examinations (for example, human epidermal growth factor receptor
2, estrogen receptor and progesterone receptor tests) were
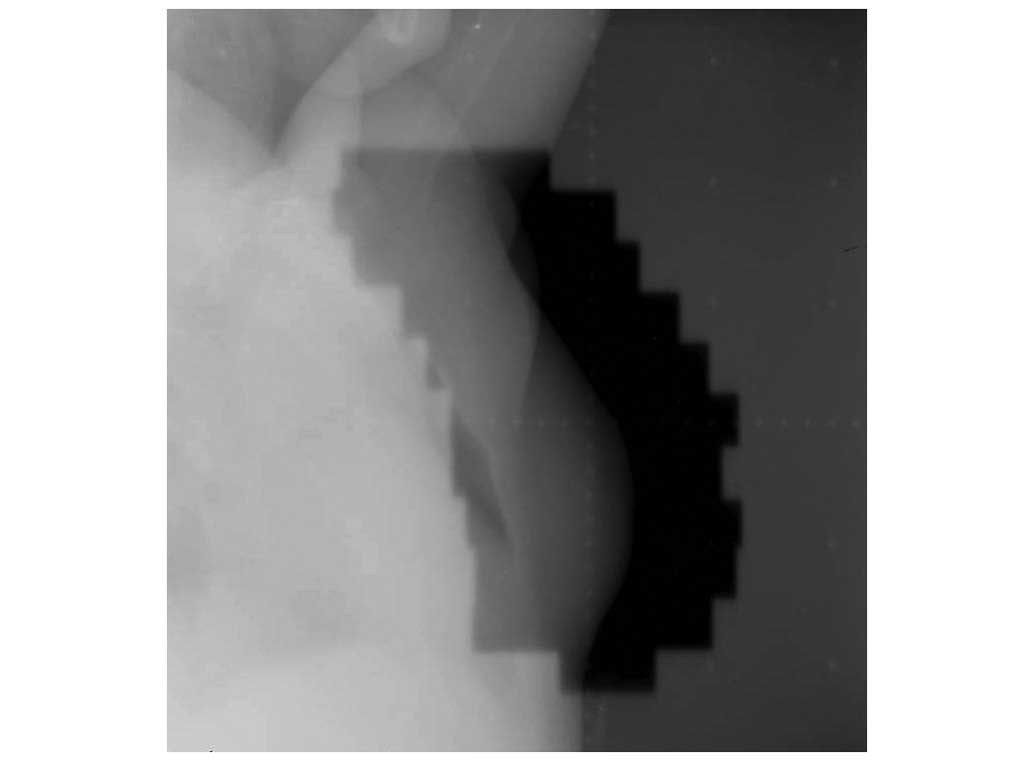
performed. Post-operatively, the patient received RT, which was
delivered as 6 MV photons to the whole left breast using a
tangential irradiation technique. The whole breast region was
210×115 mm in size (Fig. 2). The
whole breast received 46 Gy radiation in 23 fractions (2 Gy per
fraction) for 4 weeks and 6 days, followed by a cone down boost of
14 Gy in 7 fractions (2 Gy per fraction), therefore a total dose of
60 Gy in 30 fractions was administered. RT was completed on 25
November 1995. The patient tolerated the therapy well; however, the
patient suffered from grade 2 dermatitis (12). The patient did not receive adjuvant
chemotherapy or hormonal therapy.
Overall, the patient remained active and clinically
stable until August 2004, 9 years subsequent to the
breast-conserving therapy, when the patient observed a small lump
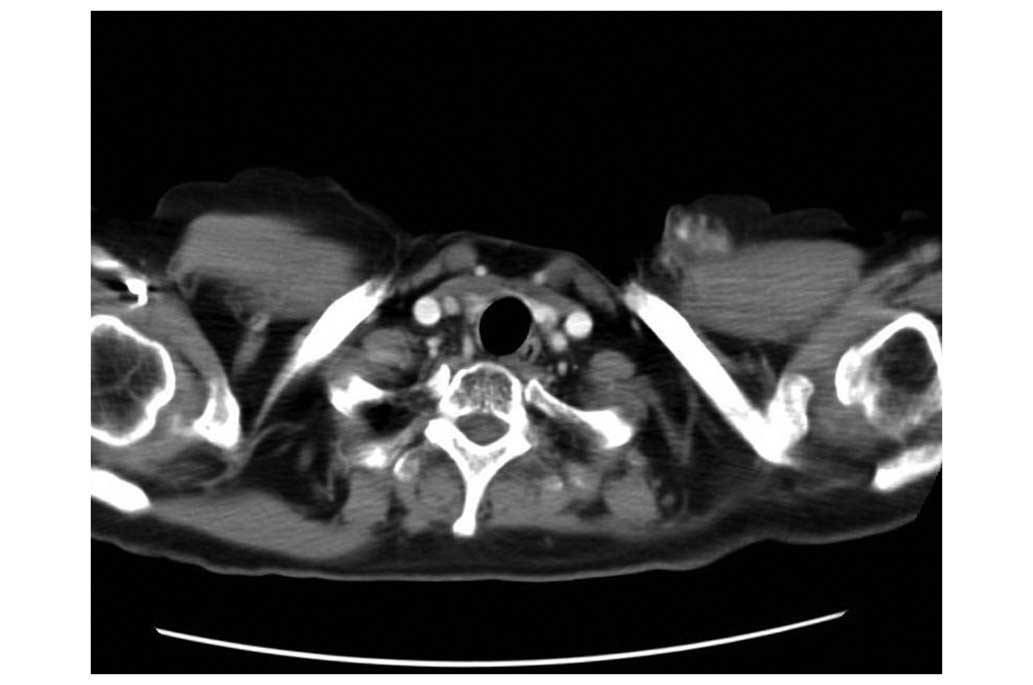
in the left chest wall. On 1 November 2004, a chest computed
tomography scan revealed the presence of a tumor mass. A 2.5 cm
diameter nodule anterior to the insertion of the left
sternocleidomastoid muscle was identified (Fig. 3). A biopsy confirmed that the tumor
consisted of atypical spindle cells. On 13 January 2005, the
patient underwent excision of the tumor and pectoralis major fascia
and a biopsy of the left axillary lymph nodes was performed.
Macroscopically, the resected tumor measured 7×7×2 cm in size and
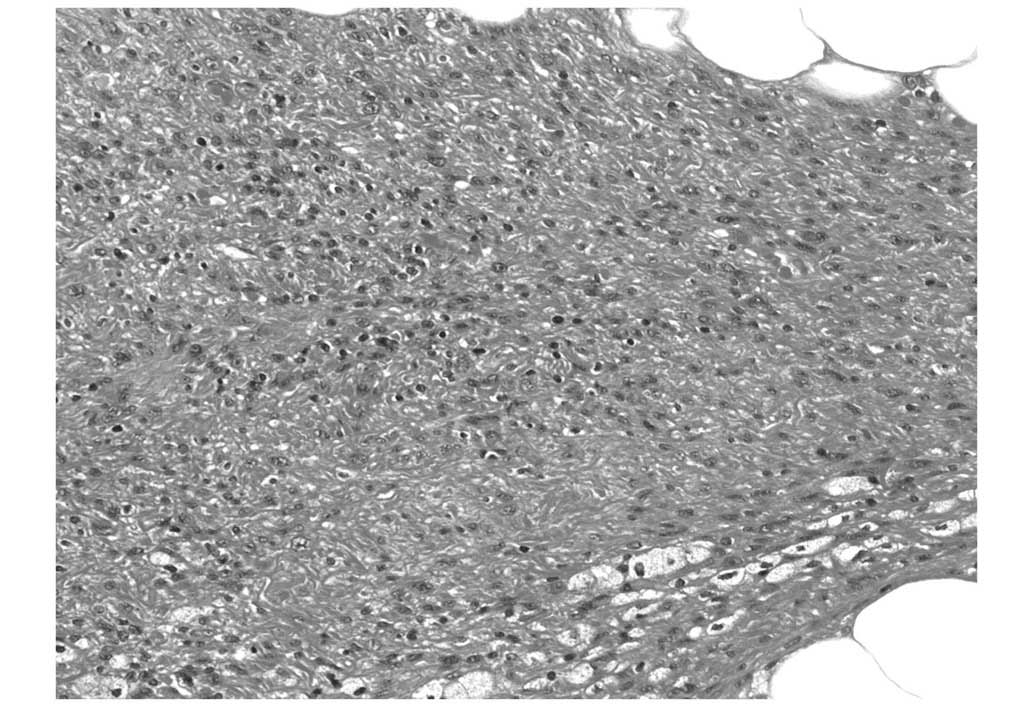
was tan and poorly-marginated. Microscopically, the lesion
consisted of atypical spindle cells with myxoid stroma that were
mainly in the subcutaneous fatty tissue, which invaded the dermis
(Fig. 4). Immunohistologically, the
tumor cells were positive for vimentin, a type III intermediate
filament protein that is often used as a sarcoma marker (4). In total, 50% of the tumor cells
expressed molecular immunology borstel-1, but the tumor did not
express other markers, including S-100 protein, desmin, mouse
monoclonal muscle actin antibody HHF-35, sulfotransderase 1A-4,
cluster of differentiation (CD)-31, CD34 and lymphatic endothelium
monoclonal antibody D2–40. Pathologists concluded that the tumor
was a low-grade fibromyxoid sarcoma. Pathology identified malignant
cells within the surgical margin. In total, 5 lymph nodes that
contained no malignant cells were removed. Since the tumor
developed from tissue that had been previously irradiated, it was
considered to be RT-induced. Although additional surgery and
chemotherapy were advised, the patient declined.
The patient remained clinically stable for the
following 4 years. In 2008, the sarcoma recurred locally and the
patient underwent multiple surgeries for mass reduction. In
September 2011, the patient developed uncontrolled bleeding from
the recurrent mass. The patient succumbed to the sarcoma on 19
October 2011, 18 years subsequent to whole breast RT, and 9 years
subsequent to the initial symptoms of the sarcoma.
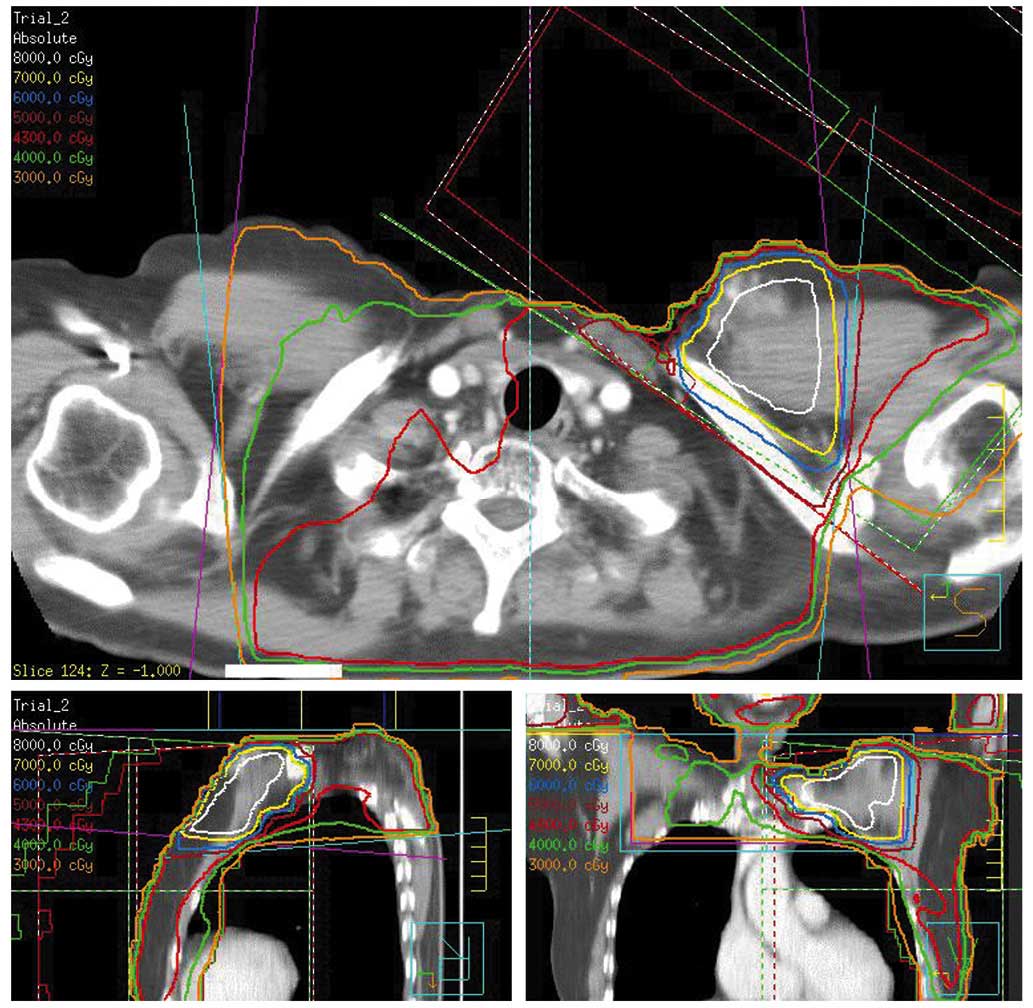
The present study retrospectively reconstructed the
RT field of the RT the patient received for the treatment of NHL
and invasive ductal carcinoma of a solid tubular type, observed 9
years later, using the Pinnacle 3 three-dimensional radiation
therapy system (Hitachi Medical Corporation, Tokyo, Japan; Fig. 5).
Discussion
RT for the treatment of breast cancer increases the
risk of developing all types of soft tissue sarcoma, particularly
rare angiosarcoma (13,14). Although women that undergo RT for
breast cancer demonstrate an increased risk of in-field sarcomas,
the absolute magnitude of risk for a post-irradiation sarcoma is
small (15). In 274,572 cases of
primary breast cancer identified from the Surveillance,
Epidemiology and End Results database, the 15-year cumulative
incidence rates for any sarcoma in women that received and did not
receive RT were 3.2 and 2.3 per 1,000 women, respectively (15).
In patients with primary breast sarcoma,
angiosarcoma, fibrosarcoma and pleomorphic cell sarcoma occur at a
frequency of ~24% for each type of sarcoma, followed by fibromyxoid
sarcoma at 12% (16). However,
angiosarcoma accounts for ~50% of all RIS. The patient in the
present study possessed a low-grade fibromyxoid sarcoma tumor,
which is an uncommon type of RIS. Low-grade fibromyxoid sarcoma,
also termed Evans tumor, was first reported by Evans in 1987
(17). Evans presented 2 cases where
the pathological features appeared to be benign or possessed
low-grade atypical cytology at the first presentation, but local
and metastatic recurrences occurred. In 1993, Evans reported 12
additional cases, which were similar to the previous 2 cases
(18).
In an analysis of the Swedish Cancer Registry,
Karlsson et al identified that the amount of radiation
energy used for the treatment of breast cancer was associated with
the risk of developing a non-angiosarcoma secondary soft-tissue
sarcoma, whereas upper extremity edema was the only risk factor
associated with the development of angiosarcoma (19). Karlsson et al revealed that the
risk for the development of sarcoma, other than angiosarcoma,
increased linearly with an integral dose of 150–200 J and reached a
plateau at higher energies. The odds ratio was 2.4 (95% confidence
interval, 1.4–4.2) for energy of 50 J, which is equivalent to the
RT received by the breast following breast-conserving surgery. In
an additional study, which analyzed >6,500 women with breast
cancer, the risk of developing sarcoma, including malignant fibrous
histiocytoma, fibrosarcoma and angiosarcoma, increased with a
higher radiation dose, regardless of edema (20). RIS incidence is considered to be a
function of the RT dose (21,22). The majority of RIS studies following
breast irradiation have been concerned with doses of 60–80 Gy, with
a minimal dose of 10 Gy in standard fractionations (23).
In the current study, the whole neck and bilateral
supraclavicular region of the patient had been irradiated for the
treatment of NHL. The present study retrospectively reconstructed
the RT field of that treatment, using the Pinnacle 3
three-dimensional radiation therapy system. The reconstructed dose
distribution indicated that there may have been an overlapping
region of supraclavicular irradiation in 1986 and whole breast
irradiation in 1995, and that the sarcoma of the patient possibly
developed from this overlapping irradiated region. In this region
the total biological effective dose (α/β=10) was estimated to be
almost 95 Gy, which may have augmented the risk of the patient
developing sarcoma. In addition, the patient possessed a history of
chemotherapy; cyclophosphamide has been hypothesized to be a risk
factor for the development of a secondary cancer (24).
Overall, the present study reported the case of a
low-grade fibromyxoid sarcoma in a 62 year old woman, which
developed 9 years subsequent to whole breast irradiation for
carcinoma of the left breast, and 18 years following chemotherapy
and RT for NHL. The patient succumbed to the tumor 25 years
subsequent to RT for NHL, 18 years subsequent to whole breast RT
and 9 years subsequent to the initial symptoms of the sarcoma.
The present study indicates the importance of
considering the RT history of a patient, due to the potential
development of RIS, including the development of a low-grade
fibromyxoma, which possesses a poor prognosis.
References
|
1
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J,
et al: Effect of radiotherapy after breast-conserving surgery on
10-year recurrence and 15-year breast cancer death: Meta-analysis
of individual patient data for 10,801 women in 17 randomised
trials. Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meric F, Buchholz TA, Mirza NQ, Vlastos G,
Ames FC, Ross MI, Pollock RE, Singletary SE, Feig BW, Kuerer HM, et
al: Long-term complications associated with breast-conservation
surgery and radiotherapy. Ann Surg Oncol. 9:543–549. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galper S, Gelman R, Recht A, Silver B,
Kohli A, Wong JS, Van Buren T, Baldini EH and Harris JR: Second
nonbreast malignancies after conservative surgery and radiation
therapy for early-stage breast cancer. Int J Radiat Oncol Biol
Phys. 52:406–414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toyoshima M, Okamura C, Niikura H, Ito K
and Yaegashi N: Epithelioid leiomyosarcoma of the uterine cervix: a
case report and review of the literature. Gynecol Oncol.
97:957–960. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beck A: The question of whether
radiation-induced sarcoma contributes to the pathogenesis of
sarcoma. Munch Med Wochenschr. 69:623–625. 1922.(In German).
|
|
6
|
Warren S and Sommer GN: Fibrosarcoma of
the soft parts with special reference to recurrence and metastasis.
Arch Surg. 33:425–450. 1936. View Article : Google Scholar
|
|
7
|
Cahan WG, Woodard HQ, Higinbotham NL,
Stewart FW and Coley BL: Sarcoma arising in irradiated bone: Report
of eleven cases 1948. Cancer. 82:8–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Berrington Gonzalez A, Curtis RE,
Gilbert E, Berg CD, Smith SA, Stovall M and Ron E: Second solid
cancers after radiotherapy for breast cancer in SEER cancer
registries. Br J Cancer. 102:220–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zagars GK, Ballo MT, Pisters PW, Pollock
RE, Patel SR, Benjamin RS and Evans HL: Prognostic factors for
patients with localized soft-tissue sarcoma treated with
conservation surgery and radiation therapy: An analysis of 1225
patients. Cancer. 97:2530–2543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gladdy RA, Qin LX, Moraco N, Edgar MA,
Antonescu CR, Alektiar KM, Brennan MF and Singer S: Do
radiation-associated soft tissue sarcomas have the same prognosis
as sporadic soft tissue sarcomas? J Clin Oncol. 28:2064–2069. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bjerkehagen B, Småstuen MC, Hall KS,
Skjeldal S, Smeland S and Fosså SD: Why do patients with
radiation-induced sarcomas have a poor sarcoma-related survival? Br
J Cancer. 106:297–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou
C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, et al:
Angiosarcoma: Clinical and molecular insights. Ann Surg.
251:1098–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap J, Chuba PJ, Thomas R, Aref A, Lucas
D, Severson RK and Hamre M: Sarcoma as a second malignancy after
treatment for breast cancer. Int J Radiat Oncol Biol Phys.
52:1231–1237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adem C, Reynolds C, Ingle JN and
Nascimento AG: Primary breast sarcoma: Clinicopathologic series
from the Mayo Clinic and review of the literature. Br J Cancer.
91:237–241. 2004.PubMed/NCBI
|
|
17
|
Evans HL: Low-grade fibromyxoid sarcoma. A
report of two metastasizing neoplasms having a deceptively benign
appearance. Am J Clin Pathol. 88:615–619. 1987.PubMed/NCBI
|
|
18
|
Evans HL: Low-grade fibromyxoid sarcoma. A
report of 12 cases. Am J Surg Pathol. 17:595–600. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karlsson P, Holmberg E, Samuelsson A,
Johansson KA and Wallgren A: Soft tissue sarcoma after treatment
for breast cancer - a Swedish population-based study. Eur J Cancer.
34:2068–2075. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rubino C, Shamsaldin A, Lê MG, Labbé M,
Guinebretière JM, Chavaudra J and de Vathaire F: Radiation dose and
risk of soft tissue and bone sarcoma after breast cancer treatment.
Breast Cancer Res Treat. 89:277–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wijnmaalen A, van Ooijen B, van Geel BN,
Henzen-Logmans SC and Treurniet-Donker AD: Angiosarcoma of the
breast following lumpectomy, axillary lymph node dissection, and
radiotherapy for primary breast cancer: Three case reports and a
review of the literature. Int J Radiat Oncol Biol Phys. 26:135–139.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Monroe AT, Feigenberg SJ and Mendenhall
NP: Angiosarcoma after breast-conserving therapy. Cancer.
97:1832–1840. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirova YM, Vilcoq JR, Asselain B,
Sastre-Garau X and Fourquet A: Radiation-induced sarcomas after
radiotherapy for breast carcinoma: A large-scale single-institution
review. Cancer. 104:856–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Philibert D and Cattran D: Remission of
proteinuria in primary glomerulonephritis: We know the goal but do
we know the price? Nat Clin Pract Nephrol. 4:550–559. 2008.
View Article : Google Scholar : PubMed/NCBI
|