Introduction
Carotid body tumor (CBT) is an extra-adrenal
paraganglioma that may also be termed chemodectoma. CBTs originate
from the neural crest tissue in the carotid bifurcation. Usually,
CBT is a solitary occurrence. The majority of cases are considered
to be benign, with only 10–20% demonstrating malignant inclinations
(1). Therefore, malignancy is
uncommon, and distant metastasis is rare. CBTs are generally
presented as unilateral neoplasms that are located in the carotid
bifurcation, without distant metastasis. CBTs account for ~0.03% of
all neoplasm types (2).
Familial forms of CBT account for 6.0–12.5% of all
CBT cases (3). Familial forms are
rarely reported in the literature, and there have been few reports
of cases worldwide since the 1930s (4–12). Due to
the presence of a specific gene mutation in familial forms of CBT,
multifocal lesions and distant metastases are more likely to occur
in familial forms compared with non-familial cases. The present
study reports the case of a patient with bilateral CBT in
association with systemic metastasis. The radiological findings of
a malignant CBT and associated metastases are discussed in
detail.
Case report
On November 1, 2013, a 29-year-old woman was
admitted to the Department of Head and Neck Surgery, Beijing
Tongren Hospital (Capital Medical University, Beijing, China) with
hoarseness of the throat and bilateral neck pain, which had
progressively worsened over seven months. Three months prior to
admittance, the patient developed dysphagia. An ultrasound
examination in Ningxia People's Hospital (Yinchuan, China)
approximately two months prior to admittance revealed neoplasms in
the bilateral neck. The masses were ~4.5×2.5 cm and ~2.0×1.0 cm in
size (right and left side, respectively). The diagnosis was
considered to be bilateral CBTs. Then the patient went to Beijing
Tongren Hospital and was also diagnosed as CBTs. The diagnosis was
considered to be reasonable, as the patient disclosed that 9 family
members had also possessed lumps in the neck region. Surgery was
previously performed on the patient's elder brother, who had also
been confirmed with a diagnosis of CBT.
The vital signs of the patient were normal upon
admission. The only physical findings of importance were restricted
to the neck. On the right side, a mass ~4.5×2.5 cm in size was
palpated over the carotid bifurcation. The mass had a clear margin,
was firm and non-tender and was not easily moved. A mass ~2.5×1.5
cm in size was identified in the II region of the right neck. The
second mass was not as firm as the first, but moved slightly. On
the left side, a somewhat smaller mass ~2.0×1.0 cm in size was also
palpated at the carotid bifurcation. The mass was firm, non-tender
and was not easily moved. No enlarged lymph nodes were identified
in the supraclavicular region.
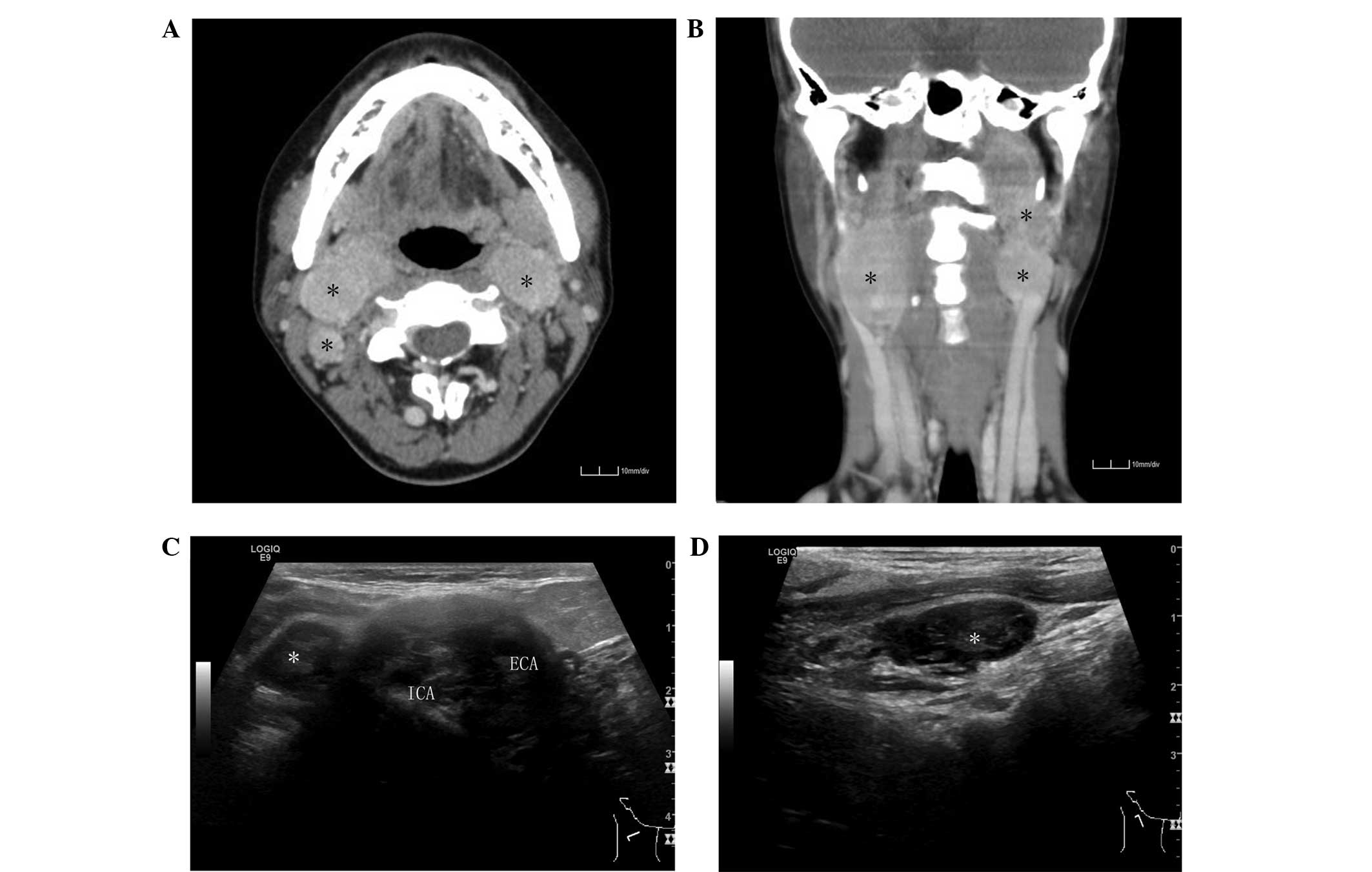
A grayscale ultrasound revealed an inhomogeneous
hypoechoic, well-defined neoplasm in the right neck that spread
across the carotid bifurcation, and the ECA and ICA were encased by
the mass (Fig. 1C). A significantly
enlarged lymph node that was oval in shape and did not exhibit a
normal echogenic hilum was identified in the posterior region of
the neoplasm (Fig. 1D), which
indicated regional lymph node metastasis. A relatively small mass
was also detected in the left carotid bifurcation; however, the
upper margin was not detected clearly due to the deep location of
areas of the lesion. No additional enlarged lymph nodes were
detected in the bilateral neck and supraclavicular region.
Firstly, a computed tomography (CT) scan of the neck
with contrast enhancement and CT angiography was performed
(Fig. 1A and 1B). The CT imaging
revealed a solid, well-defined mass ~4.6×2.3×2.8 cm in size that
was located at the right carotid bifurcation, and surrounded the
external carotid artery (ECA) and the internal carotid artery
(ICA). The mass was well-enhanced following the contrast
enhancement administration. The adjacent internal jugular vein
(IJV) was compressed significantly. An enhanced mass of 2.5×1.4×1.2
cm in size identified in the posterior region of the lesion
indicated an enlarged lymph node. The CT scan also revealed a
similar neoplasm at the left carotid bifurcation that infiltrated
into the left parapharyngeal space and extended to the base of the
skull. The ECA was displaced anteromedially, the ICA was displaced
posterolaterally and the adjacent IJV was compressed. No enhanced
lymph nodes of >1.0 cm in the short diameter were identified in
the region.
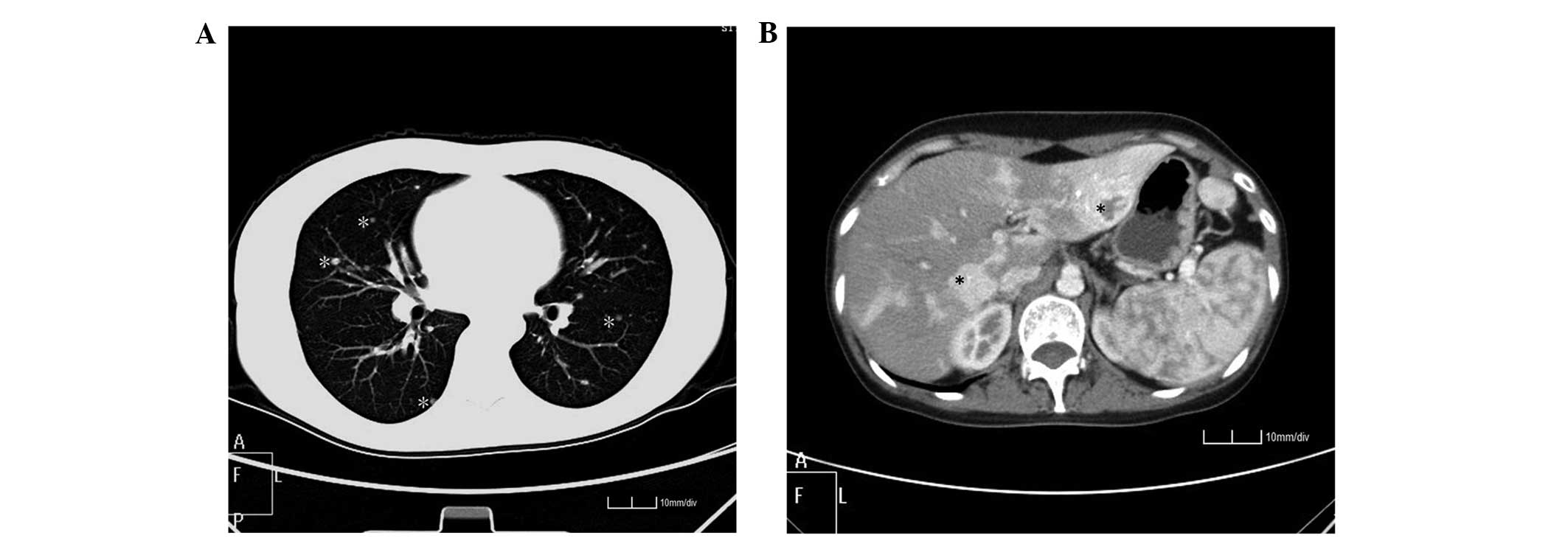
Following the aforementioned examinations, the
diagnosis of malignant CBT was suspected. Therefore a systemic
review of the patient was essential. Additional non-enhanced CT
examinations revealed multiple nodules scattered in the bilateral
lung field (Fig. 2A). An enhanced CT
scan revealed numerous nodules of various sizes within the liver
and heterogeneous significant enhancement at the arterial phase
(Fig. 2B). The central region of the
lesion in the left hepatic lobe remained unenhanced in the scanning
time and the low density within the nodules indicated a central
necrosis of the malignancy.
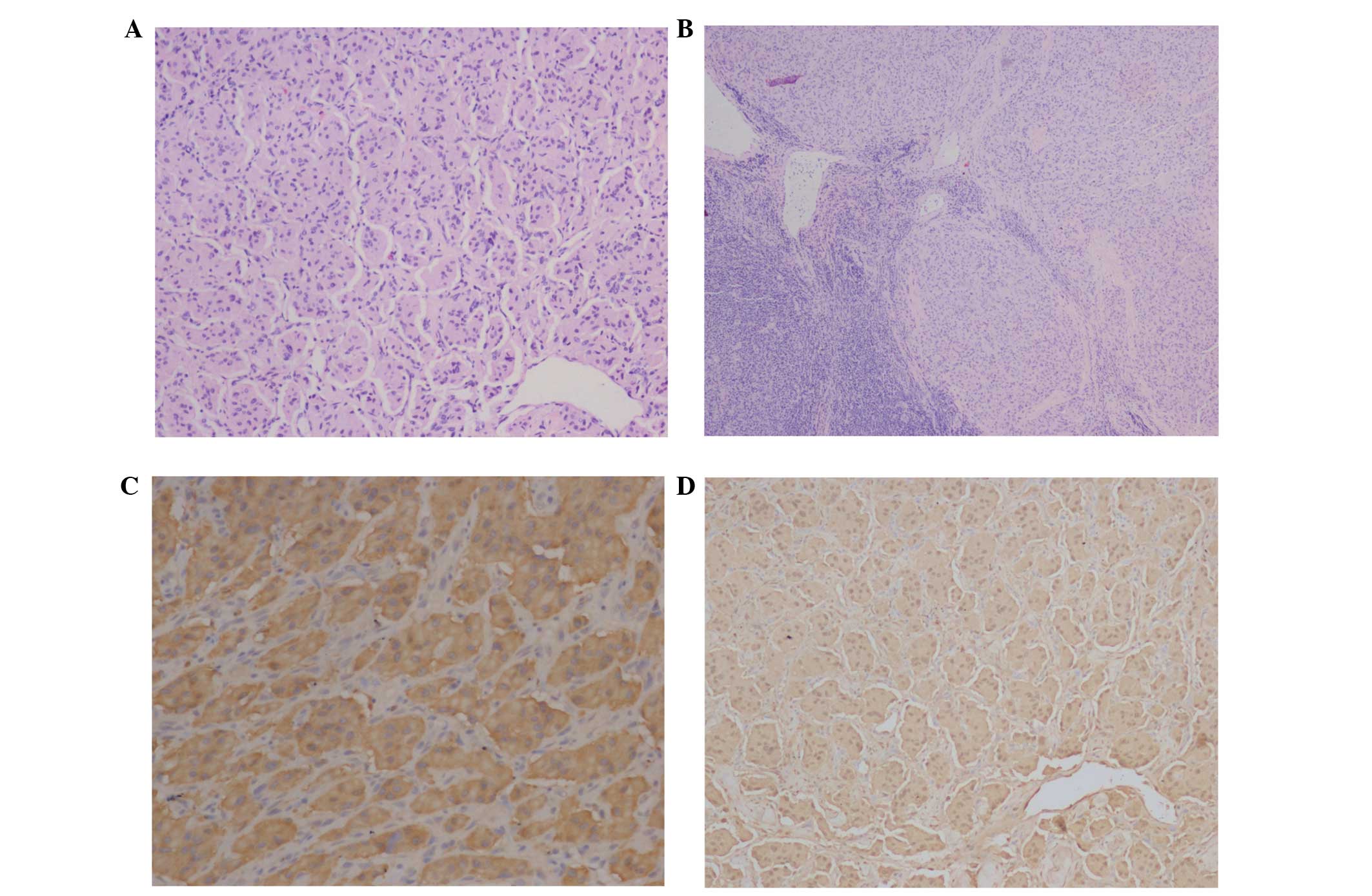
Three lymph nodes from the II region of the right
neck were resected for pathological examination in order to guide
the selection of chemotherapy. Upon microscopic examination, the
tumor cells exhibited the typical Zellballen growth pattern,
including nuclear pleomorphisms and mitoses. The cell nests were
separated by large epithelial cells of the blood sinusoid.
Immunohistochemically, the diagnosis of the neurologically
originating neoplasm was confirmed by the expression of
chromogranin, synaptophysin and neuron specific enolase (NSE)
(Figs. 3A–3D).
Combined with the radiological findings, the
pathological examination established a diagnosis of bilateral CBT
with regional lymph node, pulmonary and hepatic metastases.
Due to the family history of head and neck tumors,
early onset and malignant bilateral nature of the disease, a
genetic analysis was performed to identify mutations in the
succinate dehydrogenase (SDH) B, C and D genes. Among the patient's
31 family members, 12 were identified as expressing the SDHD mutant
gene, accounting for 38.7% (12/31) of the family.
Written informed consent was obtained from the
patient for the publication of this article and any accompanying
images.
Discussion
Carotid body tumors (CBTs) are rare neoplasms and a
type of extra-adrenal paraganglioma. CBTs are often diagnosed using
the location, clinical symptoms and imaging findings of the tumor
(13). The majority of CBTs are
benign; however, certain lesions may demonstrate malignant
inclinations and behavior. In addition, there has been considerable
debate associated with the definition of malignancy in CBTs. Lack
et al used histological findings, consisting of central
necrosis of clusters, invasion of vascular spaces and mitoses, to
define a malignant CBT (14).
However, other studies considered that pathological examinations do
not allow the differentiation between benign and malignant tumors.
Only the presence of regional lymph nodes or distant metastasis may
indicate malignancy (3,13,15–17).
Therefore, malignant CBTs are usually diagnosed using the
development of local recurrence, regional lymph node metastasis or
the presence of distant metastasis.
Previously, studies have stressed the importance of
an accurate list of anamnestic information in order to detect the
presence of familial chemodectoma. The present study demonstrates
that it is useful to consider the analysis of SDH genes. The
analysis may detect gene mutations, which may be important for
starting a familial genetic counseling process that may allow the
early diagnosis of relatives. In the present study, only two of the
patient's family members underwent surgery or biopsy; however, all
relatives underwent a gene analysis and mutation of the SDHD gene
was detected. In familial cases, hereditary CBT genes code for
subunits B, C or D of succinate dehydrogenase, a mitochondrial
enzyme (11). Certain genetic
mutations are transmittable to offspring in the familial form of
CBT (4,5), and patients with the SDHD gene mutation
are more likely to develop head and neck paragangliomas and
multifocal tumors, such as bilateral CBTs (18). Among the 31 family members of the
present patient, 13 expressed the SDHD gene mutation; however, 9
had developed bilateral CBTs, accounting for 29.0% (9/31) of all
family members. The incidence in the present study was slightly
increased compared with the study conducted by Rush (25.9%; 7/27)
(19). Of the three types of genetic
mutation, SDHC gene carriers are seldom associated with malignancy
and this mutation usually occurs as an isolated mutation (11). However, patients with the SDHD or SDHB
genetic mutation are more likely to develop CBTs at a relatively
early age (18,20,21). All 9
family members with CBT identified masses in the neck during their
thirties and forties. The 3 members without masses were young in
age, at ~3, 4 and 7 years old. Predicting the development of CBTs
in the 3 children may be challenging. However, the incidence of
CBTs is increased in the familial form compared with sporadic
cases, which elucidates the requirement for extra vigilance in
order to enable the early detection of disease (21). A previous study reported that the
incidence of malignancy is decreased in mutated SDHD gene carriers
compared with mutated SDHB gene carriers, affecting 0/34 and 11/32
family members, respectively (18).
The only family member to demonstrate malignant CBT in the
SDHD-positive family members reported in the present study is a
rare case.
Radiological findings are important in diagnosing
CBT. Usually, CT and computed tomographic angiography (CTA) scans,
magnetic resonance (MR) and magnetic resonance angiography (MRA)
imaging, conventional ultrasounds, color Doppler ultrasounds and
carotid conventional angiography (CA) are used (22). The CT images best revealed the shape,
size, margin, blood supply and adjacent infiltrations of the tumor
in the present case. On CT images, a carotid tumor is identified as
a well-defined soft tissue mass with a homogeneous enhancement that
is located within the carotid sheath. Larger tumors are frequently
inhomogeneous due to necrotic and hemorrhagic regions (23). The ECA is usually displaced
anteromedially and the ICA is typically displaced posterolaterally,
which strongly indicates a diagnosis of CBT (24). These features were characteristically
identified in the images of the present study. Fritzsche et
al regarded signs such as increased tumor weight, confluent
necrosis and the presence of vascular or extensive local invasion
as indications of malignant CBTs (25). However, these signs were not always
present in malignant cases. In the present study, confluent
necrosis and vascular invasion was not identified. The majority of
the tumor regions were clear; however, the size of the tumor and
local invasion may be indications of malignancy. Potential
indicators to better diagnose a malignant CBT according to the
present study may include: i) The surrounding of the ECA and ICA by
the tumor and ii) the upwards infiltration of the lesion reaching
up to the base of the skull, which indicates a neurological tumor
with an infiltrative growth pattern along the nerve; and iii) the
presence of enlarged and significantly enhanced regional lymph
nodes and multiple pulmonary and hepatic lesions, which support
metastasis. Additionally, Serra et al evaluated
metalloproteinase (MMP) levels in the plasma, using the
enzyme-linked immunosorbent assay (ELISA) test, and in tissue
samples, using western blot analysis (26). The previous study reported that
patients with malignant CBTs showed significantly increased levels
(P<0.01) of MMP-1, MMP-2 and MMP-3 compared with patients with
benign CBTs. This finding may provide another key point of
differentiation between benign and malignant CBTs.
An ultrasound is a rapid, convenient and
non-invasive measure that may be used to detect the margin,
vascularity and invasion of a mass, and any regional lymph node
metastasis. Ultrasounds are more useful for screening familial
cases and follow-up procedures. In total, 31 members of the
patient's family were scanned using an ultrasound in the present
study, 9 of which were identified as having unilateral or bilateral
CBT. The possible diagnosis of CBT may be anticipated when a solid
mass is detected at the carotid bifurcation. A Doppler analysis of
the mass is useful to evaluate intratumor blood flow and is
valuable in differentiating chemodectomas from other solid,
non-hypervascular masses (27).
Doppler analysis may reveal the association between the tumors and
carotid artery clearly. Doppler imaging is also sufficient for the
primary diagnosis of CBT as it may reveal abundant blood flow,
which is characterized as an intense blush of the tumor (28). Contrast ultrasonography may also aid
the evaluation of the blood supply to the tumor (29). Therefore, the ultrasound is a suitable
technique for the identification of a CBT. However, ultrasounds are
also unable to determine whether the CBT is benign or malignant.
The possibility of a malignant CBT may only be considered if
significant vascular infiltration, regional lymph node invasion or
distant metastasis are present. Ultrasounds are also limited due to
an inability to identify deeply located lesions (27). In the present study, the ultrasound
failed to identify the margin of the left lesion that infiltrated
into the left parapharyngeal space, which may have led to the
neglect of the broad extent of the mass, if no additional CT or MR
scans had been used.
Microscopic features may not predict the biological
behavior of CBTs. According a previous study, malignant CBTs may
present with a typical Zellballen growth pattern, necrosis and
vascular invasion (30). A highly
proliferative and broadly infiltrative growth pattern, necrosis and
vascular or perineural invasion were also reported in other cases
of malignant CBT (25). The present
study reported the pathological features of the lymph node
metastasis of the CBT rather than the features of the tumor due to
the high risk associated with fine-needle aspiration biopsy or
surgery (31). Obtaining histological
confirmation of distant metastases may also be challenging;
however, in the present study, the Zellballen growth pattern of the
lymph node metastasis was quite similar to that of CBT.
Additionally, the immunohistochemical examination aided the
diagnosis of a neuroendocrine-originating tumor, which was
indicated by a strong cytoplasmic reactivity to synaptophysin and
NSE. Future studies should note that the aforementioned diagnostic
features may also be detected in benign CBTs. Nuclear pleomorphisms
and mitoses may provide additional evidence of a malignant
mass.
In conclusion, familial cases of bilateral CBTs are
rare. Families with members that possess the SDHD gene mutation may
demonstrate a significantly increased incidence of multifocal
lesions. Radiological images are complementary techniques that may
be used to evaluate the extent of lesions. Pathological features
and immunohistochemical examinations may be used as diagnostic
tools for the identification of NSEs. Malignant CBTs may present
the following features: i) The ECA and ICA are surrounded by the
tumor; ii) the upwards infiltration of the lesion reaches the skull
base, which may indicate a neurological tumor, with an infiltrative
growth pattern along the nerve; and iii) metastasis supported by
enlarged and significantly enhanced regional lymph nodes and
multiple pulmonary and hepatic lesions. An ultrasound may reveal
the association between the tumors and carotid artery clearly.
However, with the exception of the ELISA and western blot analysis
of the MMP level, CT imaging and pathological examinations do not
aid the differentiation between benign and malignant tumors if
infiltration or local/distant metastases are not exhibited. Extra
vigilance is required in order to enable the early detection of
CBTs in the familial setting.
Acknowledgements
This work was supported by the project of the China
National Natural Fund Director Fund, ‘Research of influence factors
and pathogenesis mechanism of SDHD truncated mutation R38X in the
head and neck familial paraganglioma’ (grant no., 81470123), the
Beijing Municipal Science and Technology Commission ‘Leading
Talent’ Project No. (2015) 160 from the Beijing Scholars Program
(grant no., 141107001514002) and the China Scholarship Council
(grant no., 201508110233).
References
|
1
|
Andersen KF, Altaf R, Krarup-Hansen A,
Kromann-Andersen B, Horn T, Christensen NJ and Hendel HW: Malignant
pheochromocytomas and paragangliomas - the importance of a
multidisciplinary approach. Cancer Treat Rev. 37:111–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JH, Barich F, Karnell LH, Robinson RA,
Zhen WK, Gantz BJ and Hoffman HT: American College of Surgeons
Commission on Cancer; American Cancer Society: National Cancer Data
Base report on malignant paragangliomas of the head and neck.
Cancer. 94:730–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patetsios P, Gable DR, Garrett WV, Lamont
JP, Kuhn JA, Shutze WP, Kourlis H, Grimsley B, Pearl GJ, Smith BL,
et al: Management of carotid body paragangliomas and review of a
30-year experience. Ann Vasc Surg. 16:331–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Young AL, Baysal BE, Deb A and Young WF
Jr: Familial malignant catecholamine-secreting paraganglioma with
prolonged survival associated with mutation in the succinate
dehydrogenase B gene. J Clin Endocrinol Metab. 87:4101–4105. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammer S, Jansen JC, van der
Kleij-Corssmit EP, Hes FJ and Kruit MC: Case of spontaneous
regression of carotid body tumor in a SDHD mutant: A discussion on
potential mechanisms based on a review of the literature. World J
Surg Oncol. 10:2182012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taylor H: Bilateral carotid body tumour.
Proc R Soc Med. 58:173–175. 1965.PubMed/NCBI
|
|
7
|
Sugarbaker EV, Chretien PB and Jacobs JB:
Bilateral familial carotid body tumors: Report of a patient with an
occult contralateral tumor and postoperative hypertension. Ann
Surg. 174:242–247. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewison EF and Weinberg T: Carotid Body
Tumors. A case report of bilateral carotid body tumors with an
unusual family incidence. Surgery. 27:4371950.
|
|
9
|
Chase WH: Familial and bilateral tumors of
the carotid body. J Path Bact. 36:1–12. 1933. View Article : Google Scholar
|
|
10
|
Wilson H: Carotid body tumors. Familial
and bilateral. Ann Surg. 171:843–848. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hall TC, Renwick P and Stafford ND:
Recurrent familial malignant carotid body tumour presenting with
lymph node metastasis: Case report, and review of diagnosis and
management of familial carotid body tumours. J Laryngol Otol.
124:1344–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grufferman S, Gillman MW, Pasternak LR,
Peterson CL and Young WG Jr: Familial carotid body tumors: Case
report and epidemiologic review. Cancer. 46:2116–2122. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishijima H, Asakage T and Sugasawa M:
Malignant carotid body tumor with systemic metastases. Ann Otol
Rhinol Laryngol. 120:381–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lack EE, Cubilla AL and Woodruff JM:
Paragangliomas of the head and neck region. A pathologic study of
tumors from 71 patients. Hum Pathol. 10:191–218. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lau D, La Marca F, Camelo-Piragua S and
Park P: Metastatic paraganglioma of the spine: Case report and
review of the literature. Clin Neurol Neurosurg. 115:1571–1574.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhofer G, Bornstein SR, Brouwers FM,
Cheung NK, Dahia PL, de Krijger RR, Giordano TJ, Greene LA,
Goldstein DS, Lehnert H, et al: Malignant pheochromocytoma: Current
status and initiatives for future progress. Endocr Relat Cancer.
11:423–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldstein RE, O'Neill JA Jr, Holcomb GW
III, Morgan WM III, Neblett WW III, Oates JA, Brown N, Nadeau J,
Smith B, Page DL, et al: Clinical experience over 48 years with
pheochromocytoma. Ann Surg. 229:755–764. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neumann HP, Pawlu C, Peczkowska M, Bausch
B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA,
et al: European-American Paraganglioma Study Group: Distinct
clinical features of paraganglioma syndromes associated with SDHB
and SDHD gene mutations. JAMA. 292:943–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rush BF Jr: Familial bilateral carotid
body tumors. Ann Surg. 157:633–636. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Havekes B, Corssmit EP, Jansen JC, van der
Mey AG, Vriends AH and Romijn JA: Malignant paragangliomas
associated with mutations in the succinate dehydrogenase D gene. J
Clin Endocrinol Metab. 92:1245–1248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peck BW, Rich TA, Jimenez C and Kupferman
ME: A novel SDHB mutation associated with hereditary head and neck
paraganglioma. Laryngoscope. 121:2572–2575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pacheco-Ojeda LA and Martínez-Viteri MA:
Preoperative imaging diagnosis of carotid body tumors. Int Surg.
95:242–246. 2010.PubMed/NCBI
|
|
23
|
Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS,
Kang EY, Kim KA and Lee NJ: Extraadrenal paragangliomas of the
body: Imaging features. AJR Am J Roentgenol. 187:492–504. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alkadhi H, Schuknecht B, Stoeckli SJ and
Valavanis A: Evaluation of topography and vascularization of
cervical paragangliomas by magnetic resonance imaging and color
duplex sonography. Neuroradiology. 44:83–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fritzsche FR, Bode PK, Koch S and
Frauenfelder T: Radiological and pathological findings of a
metastatic composite paraganglioma with neuroblastoma in a man: A
case report. J Med Case Reports. 4:3742010. View Article : Google Scholar
|
|
26
|
Serra R, Grande R, Gallelli L, Rende P,
Scarcello E, Buffone G, Caliò FG, Gasbarro V, Amato B and de
Franciscis S: Carotid body paragangliomas and matrix
metalloproteinases. Ann Vasc Surg. 28:1665–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Derchi LE, Serafini G, Rabbia C, De
Albertis P, Solbiati L, Candiani F, Musante F, Bertoglio C and
Rizzatto G: Carotid body tumors: US evaluation. Radiology.
182:457–459. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arslan H, Unal O, Kutluhan A and Sakarya
ME: Power Doppler scanning in the diagnosis of carotid body tumors.
J Ultrasound Med. 19:367–370. 2000.PubMed/NCBI
|
|
29
|
Giannoni MF, Irace L, Vicenzini E, Massa
R, Gossetti B and Benedetti-Valentini F: Carotid body tumors:
Advantages of contrast ultrasound investigation. J Neuroimaging.
19:388–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu JK, Sameshima T, Gottfried ON,
Couldwell WT and Fukushima T: The combined transmastoid retro- and
infralabyrinthine transjugular transcondylar transtubercular high
cervical approach for resection of glomus jugulare tumors.
Neurosurgery. 59(Suppl 1): S115–S125. 2006.
|
|
31
|
Rosa M and Sahoo S: Bilateral carotid body
tumor: The role of fine-needle aspiration biopsy in the
preoperative diagnosis. Diagn Cytopathol. 36:178–180. 2008.
View Article : Google Scholar : PubMed/NCBI
|