Introduction
Primary hepatic angiosarcoma (PHA) is a rare
condition difficult to diagnose, particularly if the patient
presents no history of exposure to carcinogens (1). PHA is primarily observed in the elderly,
does not possess any specific tumor markers, and often presents
with nonspecific symptoms, including discomfort or distension of
the abdomen, weight loss and fatigue (2). To date, a limited number of cases of PHA
have been reported in the English literature (3). In order to investigate the diagnosis and
treatment options for PHA, the case of a 64-year-old man who was
initially diagnosed with hydatid cyst and subsequently diagnosed
with a giant PHA in the middle of the liver is described in the
present study.
Case report
A 64-year-old man was admitted to the Department of
General Surgery of The First Affiliated Hospital of Nanchang
University (Nanchang, China) in May 2014 with a space-occupying
lesion of the liver, as revealed by physical examination conducted
~23 days earlier. The patient had not experienced hepatalgia,
nausea, vomiting, hematemesis or hematochezia since the onset of
the lesions. The patient underwent an ultrasound (US; Philips iU22
Ultrasound System; Philips Healthcare, Andover, MA, USA)
examination of the abdomen on May 10, 2014, which revealed the
presence of a cystic mass in the liver. Thus, further examination
was recommended, since the results of the US scan did not discard a
possible hydatid cyst. In consequence, the patient underwent a full
examination for parasites at the Jiangxi Provincial Institute of
Parasitic Diseases (Nanchang, China) on May 16, 2014. The results
of the analyses were negative for toxoplasma enzymes, schistosome
immunity test, and cysticercosis and lung fluke enzyme-linked
immunosorbent assay. Therefore, the patient was readmitted to The
First Affiliated Hospital of Nanchang University for further
treatment. On physical examination, the abdomen was soft, and
tenderness was observed in the whole abdomen, but no rebound
tenderness or palpable mass in the abdomen were detected. The
patient did not present a history of use of intravenous drugs,
tattoos, body piercing, excessive alcohol intake, obesity or
previous work with toxic chemicals. In addition, the patient did
not present a prior history of surgery, medical illnesses or known
allergies, and was not receiving any medication. There was no
significant family history of biliary or liver diseases.
Echocardiography demonstrated the left atrial to be
enlarged (41 mm), and the ejection fraction of the ventriculus
sinister to be 64%. Abdominal magnetic resonance imaging (MRI;
Magnetom® Trio Tim 3.0 MRI; Siemens AG, Munich, Germany) revealed a
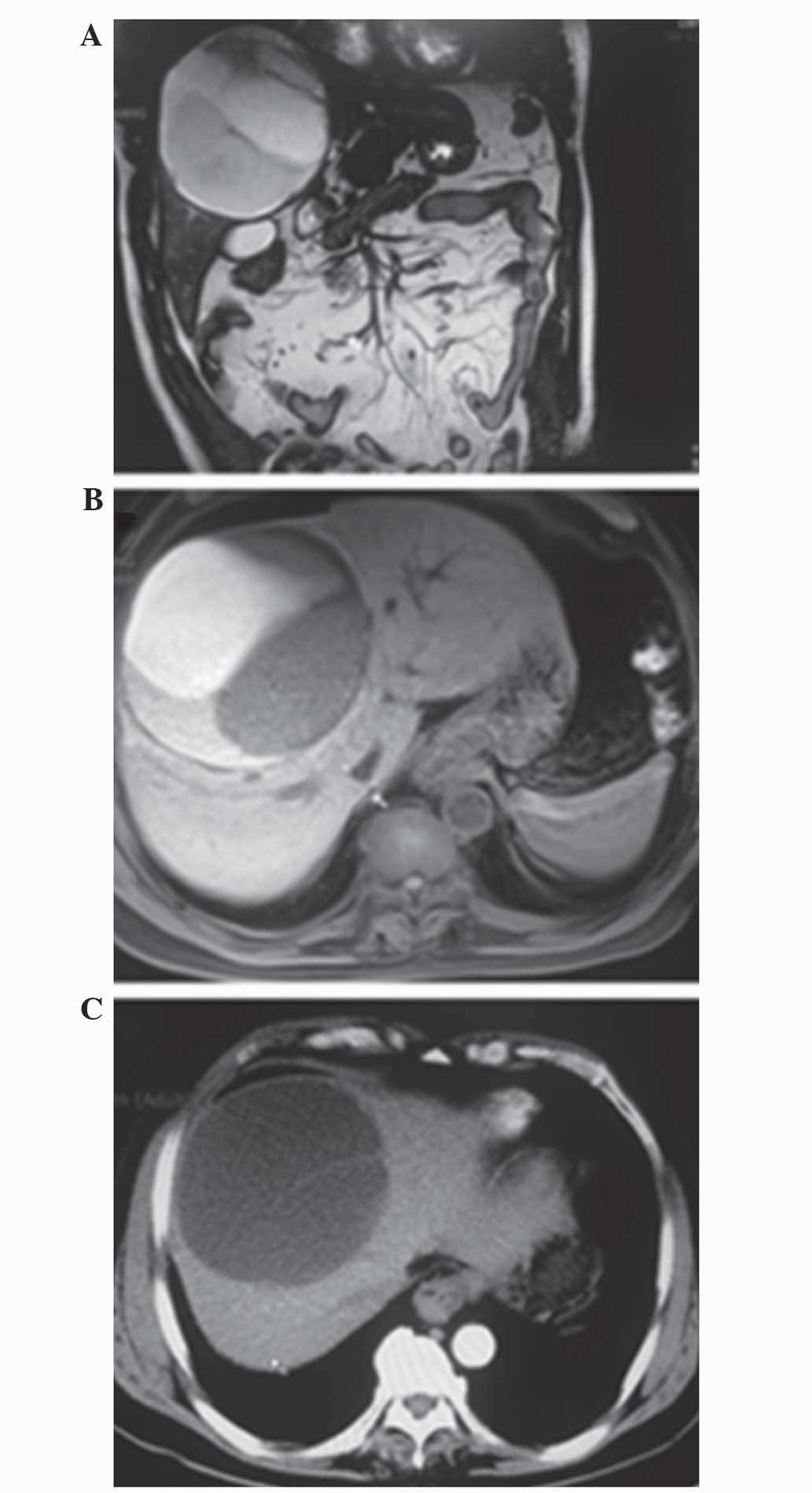
multiple cystic space-occupying lesion, measuring ~10.3×12.0 cm,
which originated from the middle of liver (Fig. 1A). On T2-weighted imaging (WI), the
tumor was characterized by a medium-high intensity signal, which
was clearly delineated from the surrounding liver tissue with
internal septal structures separating the fluid-filled spaces
(Fig. 1B), while on T1-WI, a low
intensity signal was apparent within the cystic spaces (Fig. 1C).
The results of the laboratory tests were within the
normal limits. The following blood tests conducted on the patient
were all normal: Prothrombin time, 10.6 sec (normal range, 9.8–12.1
sec); prothrombin time ratio, 0.91 (normal range, 0.85–1.15);
international normalized ratio, 94% (normal range, 70–130%);
activated partial thromboplastin time, 24.2 sec (normal range,
22.7–31.8 sec); and thrombin time, 10.6 sec (normal range,
14.0–21.0 sec). Urine tests revealed that pH and urine specific
gravity were normal, 6.0 (normal range, 5.0–8.0) and 1.020 (normal
range, 1.010–1.035), respectively. There was no protein, glucose,
ketones, urobilinogen, bilirubin, leukocytes, occult blood or
vitamin C in the urine. The patient was positive for hepatitis B
surface antigen (HBsAg, >250 IU/ml; normal range, 0–0.050
IU/ml), negative for hepatitis B surface antibody (HBsAb, 0.590
mIU/ml; normal range, 0–10 mIU/ml), negative for hepatitis B
envelope antigen (HBeAg, 0.074 PEI U/ml; normal range, 0–0.18 PEI
U/ml), negative for hepatitis B envelope antibody (0.020 S/CO;
normal range, >1 S/CO) and positive for hepatitis B core
antibody (13.010 S/CO; normal range, 0–1 S/CO). In addition, the
patient's leukocyte, erythrocyte and platelet levels were analyzed
and were 6.26×109 cells/l (normal range,
3.97–9.15×109 cells/l), 3.42×1012 cells/l
(normal range, 4.09–5.71×1012 cells/l) and
98×109 cells/l (normal range, 85–303×109
cells/l), respectively. The levels of serum carcinoembryonic
antigen (1.60 ng/ml; normal range, 0–6.5 ng/ml), carbohydrate
antigen (CA)-199 (13.71 U/ml; normal range, 0–27.00 U/ml), CA-125
(10.35 U/ml; normal range, 0–35.00 U/ml) and α-fetoprotein (AFP;
2.23 ng/ml; normal range, 0–7.00 ng/ml) were also normal. On the
basis of these findings, the patient was diagnosed with liver
lesions.
Next, the patient underwent an exploratory
laparotomy with complex liver resection and cholecystectomy through
a right back ‘L’ incision. A large cyst-solitary mass located on
the middle of the liver was detected, and subsequently excised. On
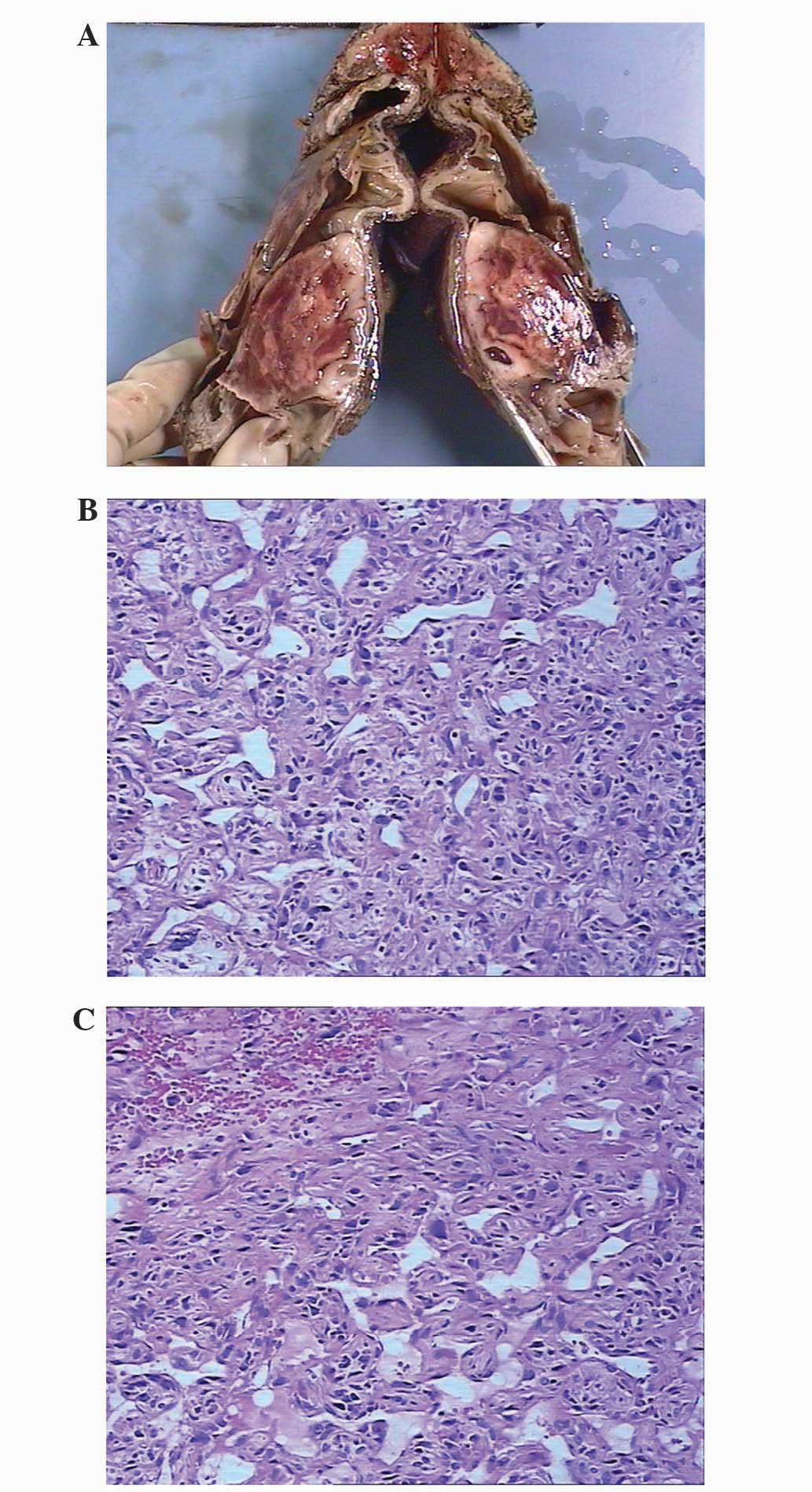
macroscopic examination, the resected liver specimen was observed
to contain a cystic-appearing mass measuring 10.5×10.0×4.0 cm, and
a solid-appearing mass measuring 8.0×5.0×3.5 cm (Fig. 2A). The thickness of the cyst wall was
0.2–0.5 cm, and the gallbladder displayed chronic inflammation.
Microscopically, the lumen was disordered and the vascular lumens
anatomosed each other. Blood tumor-like vessels were present in
certain areas, and lining cells of different sizes were observed,
alongside abundant tumor cells that were located between the
vessels. The cells contained abundant cytoplasm and oval or
irregular nuclei. A number of tumor giant cells with unusual
appearance and multiple nuclei were detected using hematoxylin and
eosin (H&E; Nanchang Rain Experiment Equipment Co., Ltd.,
Nanchang, China) staining (Fig. 2B and
C; magnification, x200; Olympus BX41; Olympus Corporation,
Tokyo, Japan). Immunohistochemistry demonstrated the tumor cells to
be cytokeratin− (mouse monoclonal; catalog no.,
MAB-0671), epithelial membrane antigen− (mouse
monoclonal; catalog no., Kit-0011), vimentin+
(Vim+; rabbit monoclonal; catalog no., RMA-0547),
hepatocyte paraffin 1− (mouse monoclonal; catalog no.,
MAB-0249), glycine− (rabbit polyclonal; catalog no.,
ab9442; Abcam, Cambridge, UK) smooth muscle actin+
(SMA+; mouse monoclonal; catalog no., Kit-0006)
desmin− (mouse monoclonal; catalog no., Kit-0023),
factor (F) VIII+ (rabbit polyclonal; catalog no.,
RB-0070), actin+ (mouse monoclonal; catalog no.,
Kit-0032), cluster of differentiation (CD)31+ (mouse
monoclonal; catalog no., MAB-0031), CD34+ (mouse
monoclonal; catalog no., Kit-0004) in the vessels, and
Ki-67− (~60% locally; mouse monoclonal; catalog no.,
Kit-0005). All antibodies were purchased from Fuzhou Maixin
Biotech., Co., Ltd. (Fuzhou, China) unless otherwise stated, and
were ready to use. The dilution of Glycine (ab 9442) was 1:1,000.
The surgical margins were negative, and a final diagnosis of PHA
was established.
The second afternoon ~50 h following surgery, the
patient experienced abdominal distension and vomited yellow bile
fluid-like liquid. In addition, the patient experienced dizziness
when rising. The blood pressure was 126/83 mmHg, and the laboratory
tests revealed levels of white blood cells = 14.4×109
cells/l, hemoglobin = 92 g/l, percentage of neutrophilic
granulocyte = 88%, alanine transaminase = 596 U/l and aspartate
aminotransferase = 572 U/l. Chest X-ray (Kodak Direct View DR5100;
Kodak, Rochester, NY, USA) detected two patchy areas of high
density in the lung field, suggestive of infection, in addition to
elevation of the right diaphragm, presence of a liquid-gas shadow
surrounding the liver and intestinal pneumatosis. In consequence,
the patient received treatment to protect the liver, including
administration of enema with glycerin and local application of
mirabilite on the abdomen. That evening, at 11:30 p.m. (60 h
following surgery), the patient was walking in the ward aided by
other individuals when the patient suddenly lost consciousness. The
vital signs of the patient were undetectable, and his pulmonary
artery pulsatility and respiratory capacity disappeared. In
consequence, cardiopulmonary resuscitation was immediately
initiated, and the patient was administered Ringer's lactate
solution and dicarbonate to expand the circulating blood volume and
correct acidosis, dopamine to increase blood pressure, and
adrenaline, lidocaine and atropine to promote cardiac
resuscitation.
Following 80 min, the patient did not exhibit
noticeable cardiac rhythm or signs of breathing. Therefore, the
patient was announced deceased.
Discussion
Angiosarcomas are malignant neoplasms of
endothelial-type cells that line the walls of blood vessels, and
account for 2–3% of all soft tissue sarcomas in adults. The primary
sites for this type of tumor include skin, breast, soft tissues,
bone and viscera (4). Among these,
skin and breast are the most common sites for primary angiosarcomas
(4). By contrast, PHAs are rare, and
account for <5% of all angiosarcomas (4,5) and 1.8%
of all hepatic malignancies (6).
Accurate diagnosis of PHAs is difficult, since the majority of
cases of PHA do not exhibit any obvious risk factors, and the
common symptoms of PHA, including abdominal pain, fatigue and
weight loss, are nonspecific (2). In
the present study, the case of a 64-year-old man who presented a
giant PHA located in the middle of the liver is reported.
The etiology of PHA remains unclear, although
previous case-control studies have indicated that ~1/3 of all cases
of PHA appear to be caused by exposure to environmental
carcinogens, including thorium dioxide, arsenical insecticides or
polyvinyl chloride. Exposure to these chemicals is rare, and the
etiology of PHA remains unknown (7).
The diagnosis of PHA requires pathological analysis
(8). Epithelioid hemangioendothelioma
(EHE), a rare and usually low-grade malignant tumor, should be
considered in the differential diagnosis of PHA (3). Compared with EHE, PHA displays limited
stromal tissue and elongated or round tumoral endothelial cells
with severe nuclear atypia and frequent mitoses, which grow along
dilated sinusoids separated by surviving atrophic or hyperplastic
hepatocytes (9,10). Tumor markers such as CD31, CD34,
podoplanin and FVIII-related antigen are often used in combination
for the immunohistochemical diagnosis of angiosarcomas, since loss
of expression of ≥1 of these markers has been reported in 40% of
tumors. In particular, CD31 and FVIII-related antigen has been
suggested to be the most sensitive of the aforementioned
combinations, since 90% of the cases of PHA previously studied were
observed to express one of these two markers (11). In the present case report,
immunohistochemical analysis demonstrated the tumor to be
CD31+, FVIII+, CD34+,
Vim+ and SMA+.
The diagnosis of PHA is difficult, particularly if
the patient does not present a history of exposure to carcinogens,
since PHA is not characterized by any specific tumor marker
(12). Morphologically, PHA may
appear as multiple nodules, dominant masses, or a diffusely
infiltrating lesion, and its appearance may vary slightly in
computed tomography (CT) and MRI (13), being more complex if the patients are
affected by intratumoral hemorrhage, cirrhosis or exposure to toxic
substances (3). Selective hepatic
angiography in combination with dual-phase spiral CT may aid the
diagnosis of PHA and facilitate the analysis of potential
complications, since it may provide sufficient information about
the main blood vessels in the liver and tumor (14,15). In
the present case, MRI revealed a multiple cystic space-occupying
lesion, which measured ~10.3×12.0 cm and originated from the middle
of the liver. On T1-WI, a low intensity signal was detected within
the cystic spaces, while on T2-WI, the tumor exhibited a
medium-high intensity signal, clearly delineated from the
surrounding liver tissue.
The long-term survival in patients with PHA is poor,
due to the rapid progression of the disease, its high recurrence
rate, and its resistance to traditional chemo- and radiotherapies
(16–18). Currently, no formal guidelines exist
for the treatment of PHA (19). PHA
is a rare type of angiosarcoma, and is associated with poorer
prognosis than other types of angiosarcomas. Patients with PHA do
not exhibit any specific symptoms or signs, although spontaneous
PHA with intraperitoneal hemorrhage is common, and may be fatal
(3). Thus, the pathological diagnosis
of PHA is essential. However, CT or US-guided fine-needle
aspiration biopsy are dangerous and non-diagnostic procedures.
There are various treatment regimens for patients
with PHA. However, due to the high recurrence rate and poor
post-transplant survival rate of patients, liver transplantation is
no longer provided (20). Kim et
al (8) reported that a
combination of chemotherapy resulted in an improved outcome for 2
out of 4 patients, suggesting the potential usefulness of
palliative chemotherapy to improve the survival rate of patients.
The initial first-line chemotherapy for 3 out of the 4 patients was
5-fluorouracil and carboplatin in combination with transhepatic
artery infusion of doxorubicin. Following 2 cycles of treatment 2
patients succumbed to progressive disease (PD). The 3rd patient
received a second palliative ifosfamide/doxorubicin (IA) regimen
chemotherapy composed of intravenous ifosfamide infusion every 3
weeks, accompanied by doxorubicin infusion via hepatic artery every
5 weeks for 5 cycles. Subsequently, the patient received 6
additional cycles of chemotherapy with ifosfamide and doxorubicin
intravenously. The patient survived with stable disease for 16
months following diagnosis. The 4th patient was treated with IA
regimen as the first-line chemotherapy and had stable disease for 4
months. Following 5 cycles, the patient developed PD and was
administered with paclitaxel, which was replaced subsequent to the
first cycle with bevacizumab. Finally, the patient succumbed to PD
following the first cycle of bevacizumab, with an overall survival
time of 9 months. Even though 2 patients succumbed <3 months
following diagnosis, the remaining 2 patients received second or
third lines of chemotherapy regimens and survived for 16 and 9
months, respectively (8).
Currently, the best treatment option for PHA is
partial surgical resection of the liver to remove the tumor
(3,21). Zhou et al (22) reported that out of 6 patients with PHA
with solitary masses, 3 patients underwent right hepatectomy (2
patients survived for >1 year; 1 patient succumbed to disease
perioperatively), 1 patient underwent extended right hepatectomy
(survived for 6 months) and 2 patients underwent left hepatectomy
(1 patient survived for 10 months; 1 patient was alive without
recurrence 29 months later). In the present case report, the
patient was initially diagnosed with hydatid cyst, and underwent an
exploratory laparotomy with complex liver resection and
cholecystectomy. Subsequently, the patient was diagnosed with a
giant PHA in the middle of the liver, but suddenly passed away
following surgery. Acute myocardial infarction was considered to be
the direct cause of mortality, since the left atrial was enlarged
and the blood pressure was high prior to the anesthesia. However,
the exact cause of mortality remains unknown, since autopsy was
refused by the patient's family.
In conclusion, in those cases of PHA where the liver
tumors are supplied by multiple blood vessels, the possibility of
antidiastole should be considered, particularly if the patient
presents a long history of exposure to chemicals, no history of
hepatitis or cirrhosis, and the immunohistochemistry results for
tumor markers such as AFP are negative.
Acknowledgements
The authors would like to thank Mr. Hua Qiu (The
First Affiliated Hospital of Nanchang University) for his important
contribution to the manuscript.
References
|
1
|
Maluf D, Cotterell A, Clark B, et al:
Hepatic angiosarcoma and liver transplantation: Case report and
literature review. Transplant Proc. 37:2195–2199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Locker GY, Doroshow JH, Zwelling LA and
Chabner BA: The clinical features of hepatic angiosarcoma: A report
of four cases and a review of the English literature. Medicine
(Baltimore). 58:48–64. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng YW, Zhang XW, Zhang JL, et al:
Primary hepatic angiosarcoma and potential treatment options. J
Gastroenterol Hepatol. 29:906–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lahat G, Dhuka AR, Hallevi H, et al:
Angiosarcoma: Clinical and molecular insights. Ann Surg.
251:1098–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fayette J, Martin E, Piperno-Neumann S, et
al: Angiosarcomas, a heterogeneous group of sarcomas with specific
behavior depending on primary site: A retrospective study of 161
cases. Ann Oncol. 18:2030–2036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alrenga DP: Primary angiosarcoma of the
liver. Review article. Int Surg. 60:198–203. 1975.PubMed/NCBI
|
|
7
|
Thomas LB and Popper H: Pathology of
angiosarcoma of the liver among vinyl chloride-polyvinyl chloride
workers. Ann N Y Acad Sci. 246(1 Toxicity of V): 268–277. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HR, Rha SY, Cheon SH, et al: Clinical
features and treatment outcomes of advanced stage primary hepatic
angiosarcoma. Ann Oncol. 20:780–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bioulac-Sage P, Laumonier H, Laurent C,
Blanc JF and Balabaud C: Benign and malignant vascular tumors of
the liver in adults. Semin Liver Dis. 28:302–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho NH, Lee KG and Jeong MG: Cytologic
evaluation of primary malignant vascular tumors of the liver. One
case each of angiosarcoma and epithelioid hemangioendothelioma.
Acta Cytol. 41:1468–1476. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao P, Lahat G, Arnold C, et al:
Angiosarcoma: A tissue microarray study with diagnostic
implications. Am J Dermatopathol. 35:432–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZB, Yuan J, Chen W and Wei LX:
Transcription factor ERG is a specific and sensitive diagnostic
marker for hepatic angiosarcoma. World J Gastroenterol.
20:3672–3679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koyama T, Fletcher JG, Johnson CD, et al:
Primary hepatic angiosarcoma: Findings at CT and MR imaging.
Radiology. 222:667–673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rademaker J, Widjaja A and Galanski M:
Hepatic hemangiosarcoma: Imaging findings and differential
diagnosis. Eur Radiol. 10:129–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park YS, Kim JH, Kim KW, et al: Primary
hepatic angiosarcoma: Imaging findings and palliative treatment
with transcatheter arterial chemoembolization or embolization. Clin
Radiol. 64:779–785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maddox JC and Evans HL: Angiosarcoma of
skin and soft tissue: A study of forty-four cases. Cancer.
48:1907–1921. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Almogy G, Lieberman S, Gips M, et al:
Clinical outcomes of surgical resections for primary liver sarcoma
in adults: Results from a single centre. Eur J Surg Oncol.
30:421–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holden CA, Spittle MF and Jones EW:
Angiosarcoma of the face and scalp, prognosis and treatment.
Cancer. 59:1046–1057. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chien CY, Hwang CC, Yeh CN, et al: Liver
angiosarcoma, a rare liver malignancy, presented with
intraabdominal bleeding due to rupture - a case report. World J
Surg Oncol. 10:232012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orlando G, Adam R, Mirza D, et al: Hepatic
hemangiosarcoma: An absolute contraindication to liver
transplantation - the European Liver Transplant Registry
experience. Transplantation. 95:872–877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Timaran CH, Grandas OH and Bell JL:
Hepatic angiosarcoma: Long-term survival after complete surgical
removal. Am Surg. 66:1153–1157. 2000.PubMed/NCBI
|
|
22
|
Zhou YM, Li B, Yin ZM, et al: Results of
hepatic resection for primary hepatic angiosarcoma in adults. Med
Sci Monit. 16:CR61–CR66. 2010.PubMed/NCBI
|