Introduction
Ovary is located in the deep pelvic cavity. Ovarian
cancer is one of the three malignant tumors in gynecology, that
accounts for 3% of cancers among women. Although it causes a higher
number of mortalities than any other cancer of the female
reproductive system (1,2), the signs and symptoms of ovarian cancer,
when present, are subtle and vague, which conceals early onset of
the disease making early diagnosis difficult (3). Despite extensive ongoing research on
ovarian cancer, there are presently no good screening tests or
specific tumor markers (4). When
patients exhibit symptoms and seek medical assistance, 70% of them
have already reached an advanced stage of the disease, and their
5-year survival rate is ≤30% (2).
Therefore, it is crucial to identify effective diagnostic and
management strategies for ovarian cancer.
Advances in modern biotechnology have led to
progress in immunological research for the treatment of tumors. It
has been reported that the B7 family, including B7-H1 (also known
as PD-L1, or programmed death-1-ligand 1) and B7-H4 (also known as
B7S1 and B7x), are important co-stimulatory molecules responsible
for T-cell activation (5). Recent
studies have suggested that they may act as negative regulatory
factors in the antitumor immune response of the body (5–7).
The aim of the current study was to investigate the
expression of B7-H1 and B7-H4 in ovarian cancer and their clinical
relevance. To this end, we collected ovarian neoplasm tissues and
relevant clinical characteristics from patients with epithelial
ovarian cancer (EOC) and with ovarian benign neoplasm, and analyzed
the expressions of B7-H1 and B7-H4. The expression level of B7-H1
and B7-H4 as an independent risk factor for EOC recurrence and
death was examined using statistically analyses. Our findings
provide new insights for potential ovarian tumor diagnosis and
targeted immunotherapy.
Materials and methods
Specimens, patient information and
clinical records
Approval for the present study was obtained from the
ethics committee of the Central Hospital of Xuzhou (Jiangsu,
China). Written informed consent for participation in the present
study was obtained from the patients and/or their close
relatives.
Biopsy samples taken from 112 patients with ovarian
cancer were examined. The patients, aged 21–78 years (mean,
55.1±12.5 years), were hospitalized at the Department of Obstetrics
and Gynecology between February 2005 and December 2009. The biopsy
specimens used in the study were collected from the primary tumors.
Of the 112 samples, 93 cases were classified as serous
cystadenocarcinoma, 12 cases as mucinous cystadenocarcinoma, 3
cases as endometrioid adenocarcinoma, and 4 cases as clear cell
carcinoma. The size of these tumors ranged from 1 to 4,000
cm3 (mean, 171.9±423.2 cm3). Tumors were
classified according to the International Federation of Gynecology
and Obstetrics (FIGO) as follows: 26 cases, stage I; 7 cases, stage
II; 72 cases, stage III; and 7 cases, stage IV. In terms of cell
differentiation staging, 9 cases were at stage I, 20 cases at stage
II, 78 cases at stage III, and 5 cases were borderline tumors. In
65 cases, the tumors were located on both sites. In 24 cases, it
was located on the right side only, and in 23 cases, on the left
side only. In 82 cases CA125 was increased, and 85 cases had tumor
metastasis. Another 10 biopsies taken from benign ovarian tumor
patients surgery served as the control.
Patients' inclusion criteria were: i) Post-operative
lifetime ≥3 months, ii) succumbed to ovarian cancer rather than
other diseases, and iii) did not receive any chemotherapy or
radiotherapy before undergoing ovarian biopsy. The patients'
medical records were reviewed, and follow-up was performed by phone
calls and/or clinic visits, over a period of 5–10 years, with the
final follow-up terminating on 31 December 2014. Surgery day was
defined as time 0 for computing survival. Progression-free survival
(PFS) was defined as the duration between time 0 to the day when
patients were diagnosed with tumor recurrence/exacerbation. Overall
survival (OS) was defined as the duration between time 0 to the day
when patients succumbed, underwent truncation or the final
follow-up. PfS and OS were the indices used in the survival
analysis by the Kaplan-Meier method.
Immunohistochemical staining
Paraffin blocks of the collected ovarian biopsies
were processed into tissue chips by Shanghai Outdo Biotech Co.,
Ltd. (Shanghai, China). The primary antibodies, B7-H4 (animal
origin, rabbit; dilution, PBS; catalog no.: NBP2-30536) and B7-H1
(animal origin, rabbit; dilution, PBS; catalog no.: NBP1-03220)
were purchased from Novus Biologicals, Inc. (Littleton, CO, USA).
The secondary antibody, mouse anti-human polyclonal antibody, was
obtained commercially from Fuzhou Maixin Biotechnology Co., Ltd.
(Fuzhou, China). Immunohistochemical staining was conducted using
the mouse/rabbit EnVision™ detection system. Briefly, after the
paraffin blocks were sliced, dewaxed and hydrated, the sections
were immersed in citrate buffer (10 mmol/l) (MVS-0066, Fuzhou
Maixin Biotechnology Co., Ltd., Fuzhou, China). The sections were
heated in a water bath for 30 min, followed by antigen repair,
after which the sections were cooled down in 3%
H2O2 for 30 min. The sections were then
rinsed with PBS three times, for 5 min each. The primary antibody
(dilution of 1:400) was added, and the sections were kept at 4°C
overnight. PBS was used to replace the primary antibody as the
negative control, after three washes with PBS, 5 min each.
Subsequently, the secondary antibody was added, and the sections
were kept at room temperature (25°C) for 30 min. The remaining
secondary antibody was then rinsed via PBS, and DAB was applied to
develop color. Hematoxylin was used to redye the sections, and 0.1%
hydrochloric acid alcohol was applied to differentiate the stains.
After dehydration with a gradient series of ethanol, the sections
were sealed using neutral resin, and observed under a microscope
(Beijing Boruisi Technology Co., Ltd., Beijing, China).
Immunohistochemical analysis
Representative microscopic images of stained
sections were taken under a microscope, at a magnification of x400.
Immunohistochemical analysis was performed by randomly selecting
five regions of interest (ROI) for each section from the EOC group
and examining the sections at a magnification of x200. The number
of tumor cells with positive staining inside the
cytoplasm/cytomembrane, and the number of total cells presented in
each ROI were manually counted. The percentage of positive counts
was calculated and the mean values were reported. Semi-quantitative
assessment was performed using an immunohistochemical scoring
system which was defined as follows: 0, no positive cells presented
(0%); 1, 1–10% positive cells; 2, 11–50%; 3, 51–80%; and 4,
81–100%. In addition, the strength of positive cell staining was
assessed and scored as follows: 0, negative; 1, weakly positive; 2,
moderately positive; and 3–4, strong positive staining. The
immunohistochemical score of ovarian benign lesions was defined as
the multiplication of the above two parts: (−), 0 point; +, 1–4
points; ++, 5–8 points; and +++, 9–12 points. In the present study,
scores of positive response cells <4 points were defined as weak
or low expression, and scores >4 points signified high
expression.
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software package (SPSS, Inc., Chicago, IL, USA). Numerical data
were presented by means ± standard deviation. The χ2
test was applied to compare a high and low expression in the B7-H1
and B7-H4 groups. The log-rank test and Kaplan-Meier survival curve
method were used for survival analysis. COX model analysis was
performed to examine the correlations of the expression of B7-H1
and B7-H4 with multiple factors including patients' age, grade of
cell differentiation, level of CA125, tumor size, metastatic
status, and FIGO staging. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical
characteristics
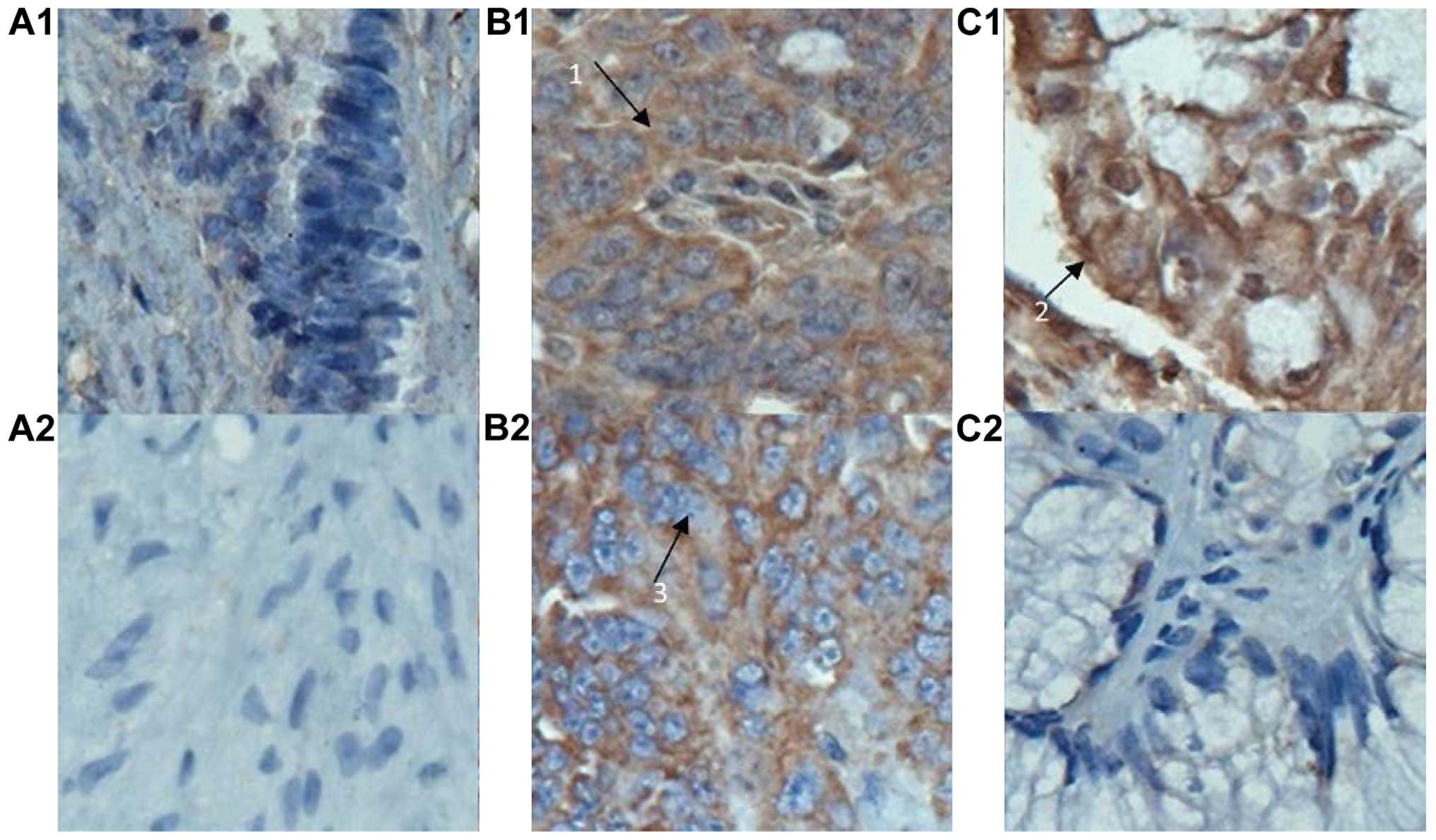
A positive expression of B7-H1 and B7-H4 was
evidenced in 55.4% (62/112) and 37.5% (42/112) of cases,
respectively, in collected ovarian carcinoma tissues, and their
coinciding expression rate was 31.3% (35/112). A positive
expression was located in the cytoplasm and/or cytomembrane in
tumor cells, shown as brown particles or block mass (Fig. 1). By contrast, a low or negative
expression of B7-H1 and B7-H4 was found in the 10 cases with benign
ovarian cyst.
Association of B7-H1 and B7-H4
expression with patient clinical characteristics
Our study examined the association of B7-H1 and
B7-H4 expression with the patient clinical characteristics,
including age, histological type, tissue differential degree, FIGO
clinical stage, tumor size, CA125 level and metastatic status. The
results showed that the expression of B7-H4 was significantly
correlated with histological type, clinical stage, tumor size and
tumor metastasis (all at P<0.05), but not with patients' age,
cell differentiation and CA125 level (all P>0.05, Table I). In addition, B7-H1 expression had
no relationship with patients' age, cell differentiation,
histological type, tumor size and CA125 level, but was relevant to
clinical stage and tumor metastasis (P<0.05, Table I).
 | Table I.Association of B7-H1 and B7-H4
expression in ovarian carcinoma tissue with clinical pathological
parameters for patients with ovarian cancer. |
Table I.
Association of B7-H1 and B7-H4
expression in ovarian carcinoma tissue with clinical pathological
parameters for patients with ovarian cancer.
|
|
| Cases with B7-H4
expression | Cases with B7-H1
expression |
|---|
|
|
|
|
|
|---|
| Variable | Total cases | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.56 | 0.46 |
|
| 0.55 | 0.46 |
|
<55 | 45 | 15 | 30 |
|
| 23 | 22 |
|
|
| ≥55 | 67 | 27 | 40 |
|
| 39 | 28 |
|
|
| Histological
typing |
|
|
| 7.98 | 0.05a |
|
| 3.62 | 0.31 |
|
Serous | 93 | 40 | 53 |
|
| 50 | 43 |
|
|
|
Mucinous | 12 | 1 | 11 |
|
| 6 | 6 |
|
|
|
Endometrioid | 3 | 1 | 2 |
|
| 2 | 1 |
|
|
| Clear
cell cancer | 4 | 0 | 4 |
|
| 4 | 0 |
|
|
| Cell grading |
|
|
| 4.12 | 0.25 |
|
| 0.95 | 0.81 |
|
Borderline | 5 | 1 | 4 |
|
| 2 | 3 |
|
|
| Poorly
differentiated | 78 | 34 | 44 |
|
| 6 | 3 |
|
|
|
Moderately differentiated | 20 | 5 | 15 |
|
| 11 | 9 |
|
|
| Highly
differentiated | 9 | 2 | 7 |
|
| 43 | 35 |
|
|
| FIGO staging |
|
|
| 29.64 | 0.00a |
|
| 15.44 | 0.00a |
| I | 26 | 0 | 26 |
|
| 7 | 19 |
|
|
| II | 7 | 2 | 5 |
|
| 5 | 2 |
|
|
| III | 72 | 33 | 39 |
|
| 43 | 29 |
|
|
| IV | 7 | 7 | 0 |
|
| 7 | 0 |
|
|
| Tumor size |
|
|
| 5.30 | 0.02a |
|
| 0.28 | 0.60 |
| <120
cm3 | 79 | 35 | 44 |
|
| 45 | 34 |
|
|
| ≥120
cm3 | 33 | 7 | 26 |
|
| 17 | 16 |
|
|
| CA125 |
|
|
| 2.05 | 0.15 |
|
| 0.03 | 0.87 |
|
Normal | 30 | 8 | 22 |
|
| 16 | 14 |
|
|
|
Uprising | 82 | 34 | 48 |
|
| 45 | 37 |
|
|
| Metastasis |
|
|
| 17.34 | 0.00a |
|
| 12.47 | 0.00a |
|
Yes | 85 | 41 | 44 |
|
| 55 | 30 |
|
|
| No | 27 | 1 | 26 |
|
| 7 | 20 |
|
|
Survival analysis and COX multi-factor
regression
In the current study, 112 patients with ovarian
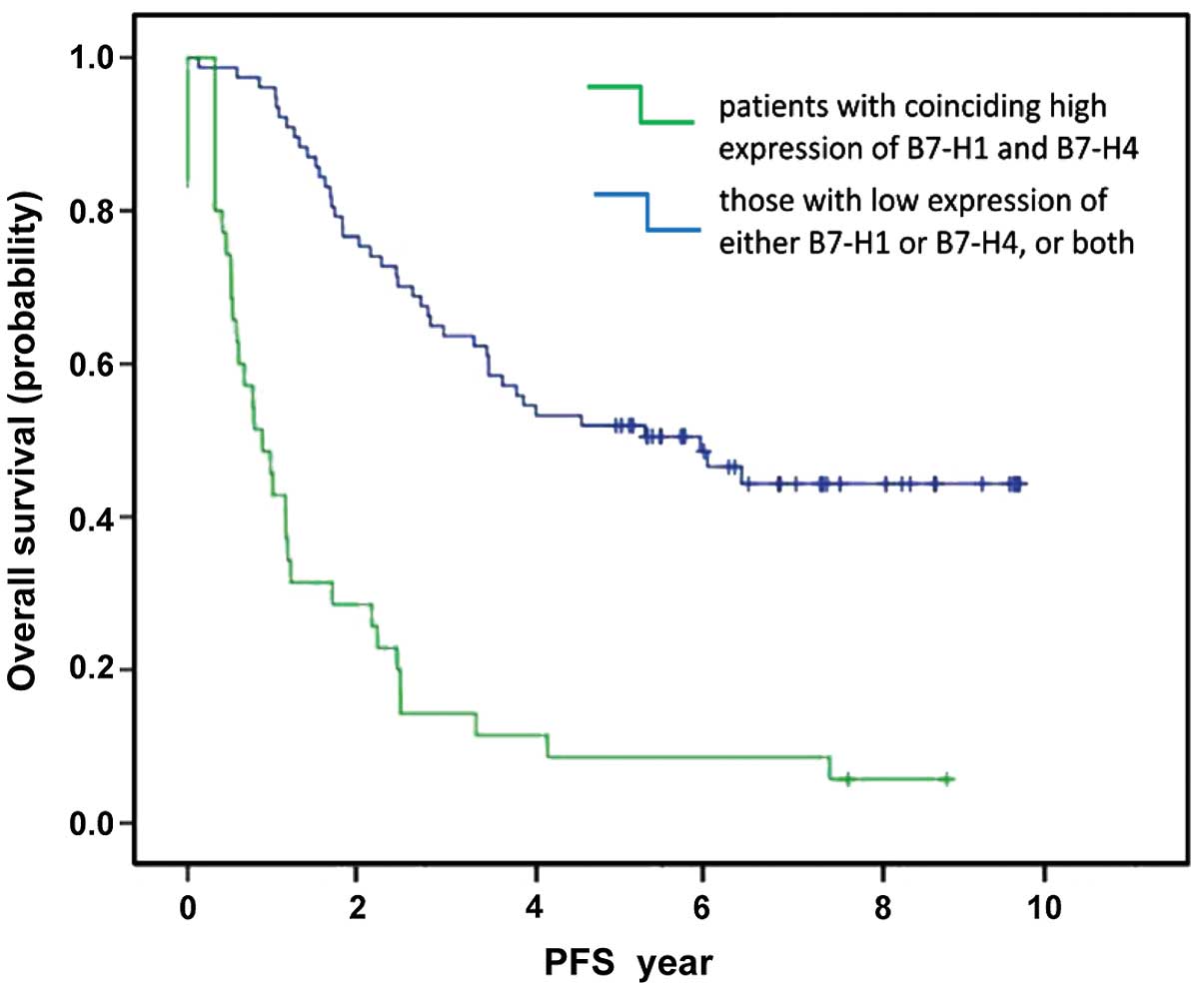
cancer underwent 5–10 years follow-up. Fig. 2 compares the profiles of PFS in
patients with a coinciding high expression of B7-H1 and B7-H4 to
those without this expression. We found that the PFS of patients
with a coinciding high expression of B7-H1 and B7-H4 was
significantly shorter when compared to those without this
expression (18.2±139.5 vs. 2,108.2±153.7 days, P<0.001).
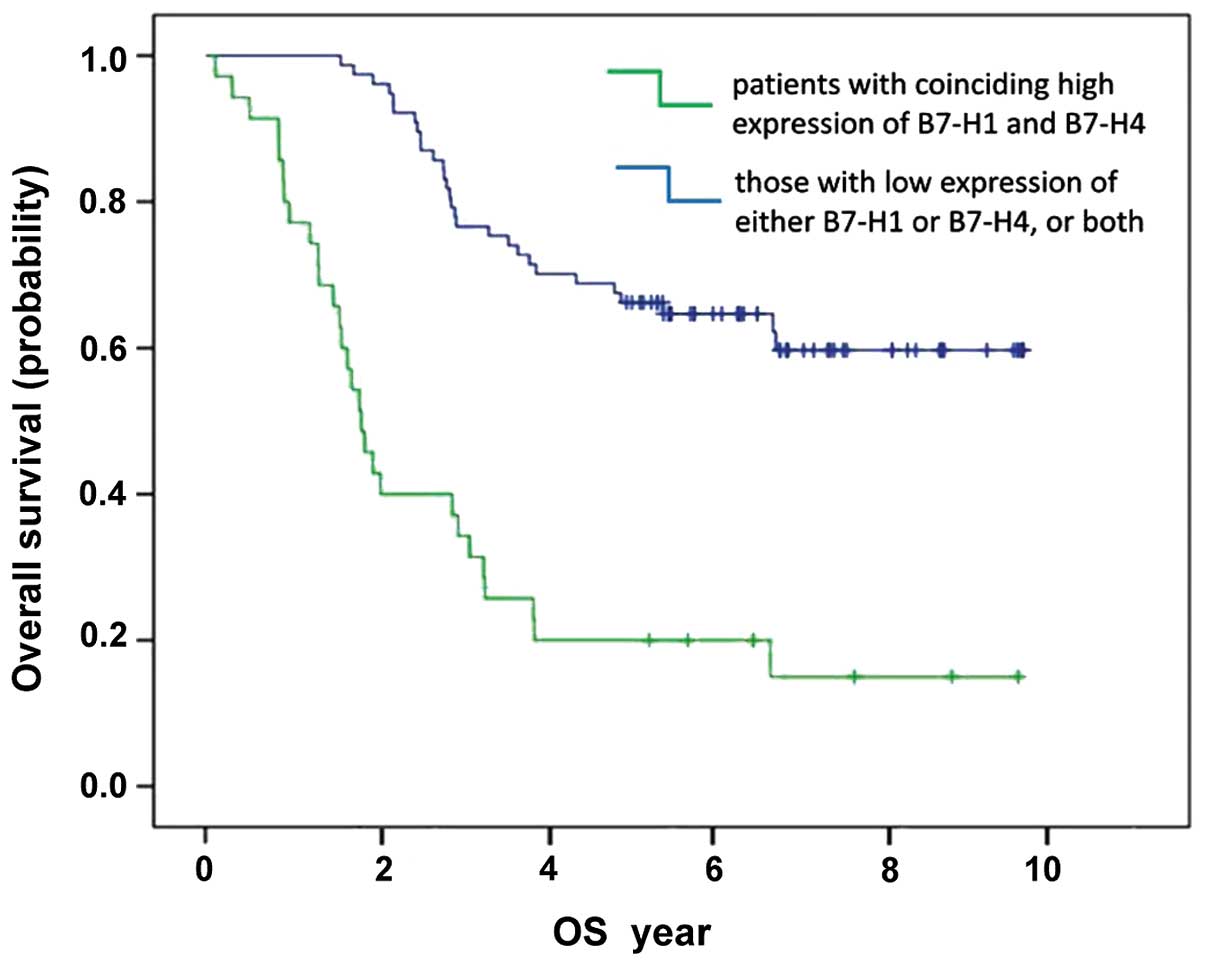
Similarly, OS of patients with a coinciding high expression of
B7-H1 and B7-H4 was 1,169.0±188.2 days, in contrast to
2,612.2±133.3 days for other patients (Fig. 3). The differences were statistically
significant (χ2=45.60 and 37.99, respectively. P<0.001).
The results from the COX multi-factor regression
analysis releaved that for the expression of B7-H1, the regression
coefficient (B) was 0.74, and the relative risk (RR) was 2.10, with
P=0.03, while for the expression of B7-H4, B was 0.81, and RR was
2.25, with P=0.01 (data not shown). These findings indicated that
the expression of B7-H1 and B7-H4 was an independent prognostic
factor that influenced the prognosis of patients with ovarian
cancer.
Discussion
B7-H1 is the third member in the B7 family. Previous
studies have indicated that B7-H1 plays an important role in
inducing tumor-specific T-cell apoptosis and tumor immune escape
(8). B7-H4, a new member in the B7
family, negatively regulated the T-cell immunologic response by
inhibiting cell proliferation, and obstructing the generation of
cytokines as well as the progression of cell cycle (5). Recent findings have shown that a high
expression of B7-H1 and B7-H4 in many malignant tumors was
assocaited with occurrence, development and prognosis of tumors
(8–10). Overexpression of B7-H1 and B7-H4 in
tumor tissues may become a new tumor marker or target for
immunotherapy. Previous findings have shown evidence of B7-H4
expression in ovarian carcinoma tissues, but reports on the
expression of B7-H1 are lacking (11).
Wu et al (12)
have conducted a study on the expression, clinical pathology and
prognostic correlation of B7-H1. Their results showed that B7-H1
had no expression in normal stomach, mild expression in gastric
adenoma, but a strong expression in 42.2% of gastric cancer
tissues. Additionally, its expression degree was correlated with
tumor size, depth of invasion, lymphatic metastasis, and patient
survival. Thus, B7-H1 may serve as an independent factor in
evaluating the prognosis of patients with gastric carcinoma.
Hamanishi et al (13) reported
that the expression level of B7-H1 in ovarian carcinoma tissues was
negatively correlated with the number of CD8+T in tumor
tissues and that B7-H1 was an independent factor in evaluating
patient prognosis. The results from our study demonstrated that
positive staining of B7-H1 was located in the cytoplasm and/or
cytomembrane of tumor cells. A low expression of B7-H1 was observed
in the 10 cases of benign ovarian cyst whereas 55.4% of the ovarian
carcinoma (or 62 of 112 cases) had a high expression (P<0.05).
Our data showed a slightly higher incidence than that reported by
Yu et al (11).
In another study, the expression of B7-H4 in ovarian
cancers of different pathological types was compared to that in
normal ovarian tissues (14). It was
found that B7-H4 mRNA had a high expression in 87.5% of ovarian
serous papillary adenocarcinoma tissues, which was ≥2-fold higher
than the average expression level in normal ovarian tissues.
However, the expression level of B7-H4 mRNA in mucus and border
ovarian tissues was equivalent to that in normal ovarian tissues.
Based on these findings, we hypothesized that the expression of
B7-H4 mRNA in ovarian cancers was tissue-specific and was located
in the cell membrane. The results of the present study confirmed
that the positive staining of B7-H4 was located in the cytoplasm
and/or cytomembrane of tumor cells. B7-H4 showed a low expression
in the 10 cases of benign ovarian cyst, in contrast to the
positive, high expression rate (37.5% or 42/112) in the 112 cases
of ovarian carcinoma tissues. Additionally, its expression in
ovarian cancers had tissue specificity (χ2=7.98,
P=0.046). Its expression rate reached 43% (40/93) in serous
cystadenocarcinoma, but in mucinous cystadenocarcinoma, it was
reduced (8.33% or 1/12). Furthermore, B7-H4 showed a high
expression in 1 of the 3 endometrioid carcinoma cases, but had a
low expression in the 4 subjects with clear cell carcinomas. By
contrast, Tringler et al reported that B7-H4 had 100%
expression in the primary ovarian serous carcinomas (32 cases),
endometrioid carcinomas (12 cases), clear cell carcinomas (15
cases) and all of the metastatic serous carcinomas (23 cases) and
metastatic endometrioid carcinomas (7 cases), while only 1 out of
11 cases with mucinous carcinomas had a positive B7-H4 expression
(15). The discrepancy between their
results and the results of the present study remain to be
elucitaded with regard to the specificity of B7-H4 expression on a
larger scale.
Our results have shown that the expression levels of
B7-H1 and B7-H4 were associated with FIGO stage and the occurrence
of metastasis (P<0.05), but they were not significantly
associated with cell differentiation, tumor site, occurrence of
combined CA125 and patient age (P>0.05). In other words, the
more advanced the FIGO stage, the higher the expression level.
Compared with the low expression, a higher expression of B7-H1 and
B7-H4 indicated a higher recurrence rate and mortality. Results
from the COX regression analysis demonstrated that the expression
levels of B7-H1 and B7-H4 were independent prognostic factors of
patients with EOC. Thus, the immunoinhibitory B7-H1 and B7-H4
molecules are potential targets in EOC management.
Closing the channel of B7-H1/PD-1 may be used as a
major joint antitumor treatment (16–18). By
using the hybridoma technique, Zhang et al (16) prepared the monoclonal antibody MAb 5G3
which combined with the B7-H4 molecules and promoted the apoptosis
of A549 lung carcinoma cells. Similarly, by using a specific siRNA
targeting B7-H4, the expression of B7-H4 mRNA and its protein was
knocked out, thereby inhibiting the growth of cancer by increasing
tumor cell apoptosis and inhibiting Erk1/2 signal channels
(19). The abovementioned
investigations indicated the potential of this therapeutic target
in treating cancer, particularly for EOC patients who had high
incidence but poor prognosis.
In conclusion, the results of the present study have
identified an extremely low or negative expression of B7-H1 and
B7-H4 in benign ovarian neoplasm tissues, but a significantly high
expression in EOC tissues. In addition, the co-expression of B7-H1
and B7-H4 was evident in >30% of the EOC cases. Patients with a
high coinciding B7-H1 and B7-H4 expression had lower survival, and
were prone to relapse. These findings demonstrate that B7-H1 and
B7-H4 expression in EOC tissues was significantly associated with
poor prognosis and a high relapse rate of EOC, suggesting that
B7-H1 and B7-H4 constitute negative prognostic markers for EOC and
a potential immunotherapeutic target for patients with EOC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varughese E, Kondalsamy-Chennakesavan S
and Obermair A: The value of serum CA125 for the development of
virtual follow-up strategies for patients with epithelial ovarian
cancer: A retrospective study. J Ovarian Res. 5:112012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Givens V, Mitchell GE, Harraway-Smith C,
Reddy A and Maness DL: Diagnosis and management of adnexal masses.
Am Fam Physician. 80:815–820. 2009.PubMed/NCBI
|
|
4
|
Schummer M, Drescher C, Forrest R, Gough
S, Thorpe J, Hellström I, Hellström KE and Urban N: Evaluation of
ovarian cancer remission markers HE4, MMP7 and Mesothelin by
comparison to the established marker CA125. Gynecol Oncol.
125:65–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He C, Qiao H, Jiang H and Sun X: The
inhibitory role of b7-h4 in antitumor immunity: association with
cancer progression and survival. Clin Dev Immunol. 2011:6958342011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M,
Duan W, Zhou X, Liang R and Tao M: B7-H1 and B7-H3 are independent
predictors of poor prognosis in patients with non-small cell lung
cancer. Oncotarget. 6:3452–3461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX,
Zhang F and Gai XD: B7-H1 and B7-H4 expression in colorectal
carcinoma: Correlation with tumor FOXP3(+) regulatory T-cell
infiltration. Acta Histochem. 116:1163–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang
W, Zhang Y and Geng W: B7-H4 overexpression impairs the immune
response of T cells in human cervical carcinomas. Hum Immunol.
75:1203–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song X, Liu J, Lu Y, Jin H and Huang D:
Overexpression of B7-H1 correlates with malignant cell
proliferation in pancreatic cancer. Oncol Rep. 31:1191–1198.
2014.PubMed/NCBI
|
|
11
|
Yu XW, Li CH, Zhang SL, et al: Study on
the expression of PD-L1 in patients with ovarian cancer. Chin J Lab
Diagn. 14:1854–1856. 2010.
|
|
12
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta Histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tringler B, Liu W, Corral L, Torkko KC,
Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J and Shroyer
KR: B7-H4 overexpression in ovarian tumors. Gynecol Oncol.
100:44–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Fang P and Gu ZJ: Preparation and
characterization of monoclonal antibody against human B7-H4
molecule. Monoclon Antib Immunodiagn Immunother. 33:270–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sznol M: Blockade of the B7-H1/PD-1
pathway as a basis for combination anticancer therapy. Cancer J.
20:290–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Y, Hong B, Shen L, Wu Z, Yao H and
Zhang L: B7-H4 enhances oncogenicity and inhibits apoptosis in
pancreatic cancer cells. Cell Tissue Res. 353:139–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|