Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide and non-small-cell lung cancer (NSCLC) accounts
for ~88% of the cases (1). In
Malaysia specifically, lung cancer is the third leading cause of
cancer-related mortality accounting for ~19.8% of cancer-related
deaths (2). In spite of several
advances in the treatment modalities, the burden of lung cancer in
Malaysia remains high, with an incidence rate of 13.8% in males and
3.8% in females (2). Adenocarcinoma,
a histological variant of NSCLC, is the most common type of cancer
observed in Malaysian patients irrespective of their smoking status
(1). Guidelines for the management of
NSCLC strongly recommend testing for epidermal growth factor
receptor (EGFR) mutation (3).
This specific mutation has a predilection for Asians, females,
non-smokers and patients with adenocarcinoma (4,5). The
presence of EGFR mutation was found to be a strong
predictive biomarker for the clinical efficacy of EGFR
tyrosine kinase inhibitors (TKI) such as gefitinib (6).
A number of studies have reported improved outcomes
with gefitinib monotherapy in terms of prolonged progression-free
survival (PFS) and improvements in time to treatment failure (TTF)
when used as a first-line treatment in East Asian patients with
advanced NSCLC positive for EGFR mutations (7–10).
Gefitinib (IRESSA®, AstraZeneca) is a once-daily oral medication
(usually given at a dose of 250 mg) indicated in advanced NSCLC,
for patients with EGFR mutations. IPASS (IRESSA® Pan-Asia
Study) was a randomized, large-scale, double-blinded study; the
study compared gefitinib versus carboplatin/paclitaxel as a first
line treatment in 1,217 patients in Asia with advanced NSCLC. The
IPASS study established gefitinib as a potential first-line therapy
for patients with EGFR mutation-positive tumors and showed
superior PFS for gefitinib over intravenous carboplatin/paclitaxel
chemotherapy in clinically selected Asians with advanced NSCLC
[Hazard ratio (HR) 0.74, 95% confidence interval (CI) 0.65 to 0.85,
P<0.0001]. IPASS also reported there was a significantly higher
response rate with an improved tolerability profile and superior
quality of life rates with gefitinib compared with
carboplatin/paclitaxel chemotherapy (6).
Although data exists on the use of gefitinib in
Asian population, there is very limited data on the use of this
drug in patients of Malaysian descent (11,12).
Therefore, the present study was conducted to investigate the
Malaysian experience with gefitinib from a single-center in
EGFR mutation-positive NSCLC patients.
Patients and methods
Study design
The present retrospective, single-center study was
conducted to evaluate the response and survival rate of Malaysian
patients who had been treated with gefitinib (IRESSA®, Astrazeneca,
London, UK) for EGFR-positive NSCLC. The primary end point was the
objective response rate (ORR). Secondary end points were PFS, and
safety. This study followed the ethical principles approved by the
institutional review board of the hospital. All patients' data was
kept anonymous. The list of patients who had received gefitinib
prior to December 2013 for their lung cancer was traced, these
patients' charts were obtained for review and the appropriate data
was extracted.
Patient population
All patients with Stage IV, EGFR mutation-positive
NSCLC who received gefitinib as first- line treatment between May
2008 and July 2013 (Subang Jaya Medical Center, Subang Jaya,
Malaysia) were identified and included in this analysis, after
approval from the local ethics committee. Medical charts of the
patients were reviewed. Patients were selected based on the
following inclusion criteria: Male or female aged ≥18 years;
patients diagnosed with NSCLC, which was confirmed histologically
or cytologically as adenocarcinoma or adenosquamous carcinoma;
patients with Stage IV disease that was not curable with surgery or
radiotherapy; and patients with an Eastern Cooperative Oncology
Group (ECOG) performance status of 0-3. Patients who had a prior
history of chemotherapy with other drugs such as erlotinib,
carboplatin/gemcitabine, zoledronic acid and
carboplatin/pemetrexed, were also included as long as they switched
to gefitinib. Only patients who were EGFR negative (and hence, not
given gefitinib) were excluded from this analysis. All the patients
in the study underwent comprehensive baseline assessments that
included clinical laboratory tests and imaging studies. Follow-up
assessments and monitoring of all the patients were also carried
out at weekly intervals. Toxicity evaluations followed the National
Cancer Institute Common Terminology Criteria for Adverse Events,
version 3.0 (v3.0). With the exception of 3 patients, all were
non-smokers.
Efficacy and safety assessments
The response rate [complete response (CR), partial
response (PR), stable disease (SD), and progressive disease (PD)
were recorded] were evaluated as the main outcome for the patients
treated with gefitinib in the present study. Disease progression
was monitored by x-rays, computerized tomography (CT), positron
emission tomography (PET), magnetic resonance imaging (MRI) and
bone scans. The duration of response from gefitinib was recorded in
months. The adverse event (AE) profile of gefitinib was also
evaluated and graded as mild (Grade 1), moderate (Grade 2), and
severe (Grade 3). Patients were considered non-evaluable if they
did not have adequate reports available for response rate or
progression of disease. PFS was measured from Day 1 of the
treatment until clinical signs of disease progression. EGFR
mutation testing was performed by quantitative polymerase chain
reaction (qPCR) amplification and bidirectional sequencing. All
patients were confirmed as having EGFR-positive Grade IV
adenocarcinoma. Diagnosis was performed in the study mainly by
CT-guided lung biopsy. Other related procedures that were used to
aid diagnosis were percutaneous lung biopsy, bilateral neck nodes
biopsy, pleural biopsy, paraspinal mass biopsy, pleural cytology
and bronchoscopic biopsy. The actual clinical and CT findings were
captured, but the radiological findings had to be interpreted as
their primary interpretation could not be performed
retrospectively.
Statistical analysis
Response data were analyzed using descriptive
statistical methods. The results are reported as the mean and
standard deviation for continuous data and counts and percentages
for categorical data. The PFS curves were estimated using the
Kaplan-Meier method, and sub-group analyses of treatment responses
were carried out by baseline characteristics using 95% CI and
logistic regression. All comparative analyses are performed at
significance level 0.05. All analyses were carried out using the
SAS 9.3 software version (SAS Institute, Cary, NC, USA).
Results
Demographic and baseline
characteristics
During May 2008 to July 2013, the study identified
60 patients with NSCLC Stage IV, of these, 27 patients were
excluded from the study for reasons such as gefitinib not being
prescribed (n=3), EGFR mutation negative (n=10), or missing data
(n=14). A total of 33 patients with median age 58.0 (32.0-77.0)
years were included in the study, of these, 32 (97.0%) patients had
adenocarcinoma and 1 (3%) had adenosquamous carcinoma. All the
patients received gefitinib 250 mg/day orally until disease
progression occurred. The median treatment duration was 10.9 months
(±11.6). Treatment remains ongoing in 5 patients, and 2 patients
were lost to follow-up. The details of population characteristics
are presented in Table I.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| Characteristics | Overall (n=33) |
|---|
| Gender, n (%) |
|
|
Female | 29 (87.9%) |
| Male | 4 (12.1%) |
| Age (years) |
|
| Mean
(SD) | 57.1 (11.7) |
| Median
(Range) | 60.0 (32.0–77.0) |
| Age category (years),
n (%) |
|
| 60 years
and below | 18 (54.5%) |
| Above 60
years | 15 (45.5%) |
| ECOG performance
status, n (%) |
|
| 0 | 8 (24.2%) |
| 1 | 20 (60.6%) |
| 2 | 4 (12.1%) |
| 3 | 1 (3.0%) |
| Disease condition, n
(%) |
|
|
Adenocarcinoma | 32 (97.0%) |
|
Adenosquamous carcinoma | 1 (3.0%) |
| Disease stage, n
(%) |
|
| IV | 33 (100.0%) |
| Smoking history, n
(%) |
|
|
Current/former | 3 (9.1%) |
|
Non-smoker | 30 (90.9%) |
| Significant PMH/FH, n
(%) |
|
| No | 14 (42.4%) |
| Yes | 19 (57.6%) |
| EGFR mutation, n
(%) |
|
| Detected
[15bp del (nt2235–2249) | 2 (6.1%) |
|
CD 746–750] in
EXON 19 |
|
| Detected
[15bp del (nt2236–2250) | 1 (3.0%) |
|
CD 746–750] in
EXON 19 |
|
| Detected
[Deletion] in EXON 19 | 18 (54.5%) |
| Detected
[Deletion] in EXON 19, [Insertion] in EXON 20 and [L858R] in EXON
21 | 1 (3.0%) |
| Detected
[L858R] in EXON 21 | 3 (9.1%) |
| Detected
[T to G (NT 2573) | 2 (6.1%) |
|
L858R] in EXON
21 |
|
| Detected
[T790M] in EXON 20 and | 1 (3.0%) |
|
[L858R] in EXON
21 |
|
| Mutation
detected | 5 (15.2%) |
Response and survival
A total of 18 (54.5%) and 2 (6.1%) patients achieved
PR and CR to gefitinib therapy, respectively. An ORR of 60.6% (95%
CI, 42.1-77.1%) was achieved. A total of 5 (15.2%) patients each
achieved SD and PD. A total of 2 patients with CR were non-smoker
female adenocarcinoma patients with EGFR mutation in exon 19.
Meanwhile, patients with exon 20 or 21 mutations (n=6, 66.7%)
tended to have improved ORR compared with exon 19 (n=22, 59.1%),
although may be due to unequal sample size. In 4 patients, the
response could not be assessed due to missing data (Table II).
 | Table II.Treatment response by baseline
characteristics. |
Table II.
Treatment response by baseline
characteristics.
|
| Type of EGFR
mutation | Histology | Smoking status | ECOG performance
status |
|---|
|
|
|
|
|
|
|---|
| Response | Overall | Exon 19 | L858R | Unknown | Adenocarcinoma | Adenosquamous
carcinoma | Never | Current/former | Overall | 0–1 | 2–3 |
|---|
| CR, n (%) | 2 (6.1%) | 2 (9.1%) | 0 (0.0%) | 0 (0.0%) | 2 (6.3%) | 0 (0.0%) | 2 (6.7%) | 0 (0.0%) | 2 (6.1%) | 2 (7.1%) | 0 (0.0%) |
| PR, n (%) | 18 (54.5%) | 11 (50.0%) | 4 (66.7%) | 3 (60.0%) | 17 (53.1%) | 1 (100.0%) | 15 (50.0%) | 3 (100.0%) | 18 (54.5%) | 16 (57.1%) | 2 (40.0%) |
| SD, n (%) | 5 (15.2%) | 4 (18.2%) | 0 (0.0%) | 1 (20.0%) | 5 (15.6%) | 0 (0.0%) | 5 (16.7%) | 0 (0.0%) | 5 (15.2%) | 5 (17.9%) | 0 (0.0%) |
| PD, n (%) | 5 (15.2%) | 2 (9.1%) | 2 (33.3%) | 1 (20.0%) | 5 (15.6%) | 0 (0.0%) | 5 (16.7%) | 0 (0.0%) | 5 (15.2%) | 4 (14.3%) | 1 (20.0%) |
| NE, n (%) | 3 (9.1%) | 3 (13.6%) | 0 (0.0%) | 0 (0.0%) | 3 (9.4%) | 0 (0.0%) | 3 (10.0%) | 0 (0.0%) | 3 (9.1%) | 1 (3.6%) | 2 (40.0%) |
| ORR, n/N (%) | 20/33 | 13/22 | 4/6 | 3/5 | 19/32 | 1/1 | 17/30 | 3/3 | 20/33 | 18/28 | 2/5 |
|
| (60.6%) | (59.1%) | (66.7%) | (60.0%) | (59.4%) | (100.0%) | (56.7%) | (100.0%) | (60.6%) | (64.3%) | (40.0%) |
| ORR, 95% CI | (42.1– | (36.4– | (22.3%- | (14.7%- | (40.6%- | (2.5%- | (37.4%- | (29.2%- | (42.1%- | (44.1– | (5.3– |
|
| 77.1%) | 79.3%) | 95.7%) | 94.7%) | 76.3%) | 100.0%) | 74.5%) | 100.0%) | 77.1%) | 81.4%) | 85.3%) |
It is noteworthy that there were 5 patients in the
study that develops brain metastasis, of which, 4 achieved PR and 1
achieved SD. On an interim assessment, it was observed that the
brain metastatic focus disappeared in 1 patient with PR. At the
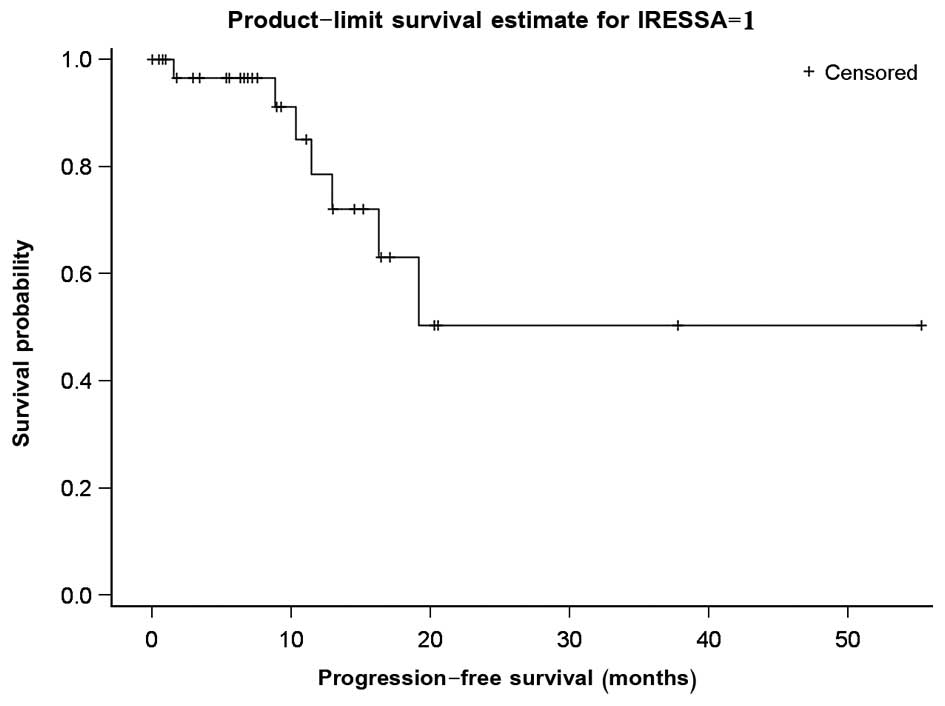
time of analysis, the median PFS was 8.9 months (Fig. 1). Univariate analysis revealed that an
Eastern Cooperative Oncology Group (ECOG) performance status of 0
or 1 was significantly associated with a longer PFS.
Drug safety and toxicity
Table III lists the
adverse events based on severity. The majority of the
treatment-related toxicity was mild in nature (National Cancer
Institute-Common Toxicity Criteria grade 1 or 2). Missing toxicity
information of patients was reported conservatively with grade 3
(severe) toxicity level. The most frequently reported AEs of grade
1 or 2 included dry skin (39.4%), skin rash (27.2%), and dermatitis
acneiform (15.2%). One patient developed progressive dyspnea due to
pneumonitis, which was severe in nature. Symptomatic treatment was
given following leading to patient improvement, and was eventually
switched over to erlotinib. Other toxicities were generally
tolerable, and no unexpected toxicities were observed.
 | Table III.Summary of patients with adverse
events by severity. |
Table III.
Summary of patients with adverse
events by severity.
| AEs | Mild | Moderate | Severe | Overall |
|---|
| Dermatitis
acneiforma | 5 (15.2%) | 1 (3.0%) | 0 (0.0 %) | 4 (12.1%) |
| Diarrhea | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Dry mouth | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Dry
skinb | 13 (39.4%) | 0 (0.0 %) | 0 (0.0 %) | 12 (36.4%) |
| In-growing
nail | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Koilonychias | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Mouth
ulceration | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Onychalgia | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Pneumonitis,
dyspnea | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) | 1 (3.0%) |
| Rashc | 6
(21.2%) | 0 (0.0 %) | 1 (3.0%) | 4 (12.1%) |
| Erythematous
rash | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Maculopapular
rash | 1 (3.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
| Xerosis | 2 (6.0%) | 0 (0.0 %) | 0 (0.0 %) | 1 (3.0%) |
Discussion
The results of this retrospective study conducted in
Malaysian patients with EGFR-positive NSCLC, demonstrated
that gefitinib therapy is effective in this population, as assessed
by ORR of 60.6% and median PFS of 8.9 months. Gefitinib therapy was
well tolerated, with patients reporting mild-to-moderate skin
toxicity, and AEs consistent with the characterized
tolerability/safety profile for gefitinib (8–10,13). These findings revealed that gefitinib
therapy was associated with improved outcomes in
EGFR-positive NSCLC in Malaysian patients. The findings of
this study were consistent with results from the IPASS study
(6), and previous IRESSA studies
conducted in the relapsed setting and underlying disease; the most
commonly reported AE with IRESSA in these studies being
mild-to-moderate rash and diarrhea (8,10,12).
The percentage of patients in IRESSA Dose Evaluation
in Advanced Lung Cancer study 1 and 2 (IDEAL 1 and IDEAL 2), and
IRESSA Survival Evaluation in Lung Cancer (ISEL) study with
adenocarcinoma were only 61.5, 68.6, and 47.9%, respectively
(13–15), whereas in the present study, 97% of
the patients had adenocarcinoma, with >50% of the patients being
treatment naive. Adenocarcinoma histology, East-Asian ethnicity,
female gender, and no smoking history are all clinical predictive
factors of response in patients treated with gefitinib (5,6); of note,
≥50% of the patients recruited in the present study had these
baseline characteristics. A correlation between specific
EGFR mutations and tumor response to gefitinib was
demonstrated by Lynch et al (16). Furthermore, an ORR of 71.2% was
observed with first-line gefitinib treatment in patients with
EGFR mutation-positive adenocarcinoma in the IRESSA Pan-Asia
Study (IPASS) (6). In the present
study, a similar but slightly lower ORR of 60.6% was observed, even
though all the patients were EGFR mutation-positive, maybe
because of the small sample size, patients lost to follow-up or
patients not included in the analysis because of incomplete data.
The median survival was shown to be improved to over 2 years with
gefitinib in mutation-positive NSCLC patients in the IRESSA
Combined Analysis of the Mutation Positives study (17). In a small sub-group of 44 EGFR
mutation-positive patients, an improved PFS and higher RR were seen
with gefitinib than docetaxel (18).
Liam et al reported that 26 patients (36.6%) achieved PR
whereas no patient had CR. Moreover, 26 (36.6%) and 19 (26.8%)
patients had SD and PD, respectively, with gefitinib treatment
(11). A small retrospective analysis
also revealed a 48% PR and 13% SD in a Malaysian population treated
with gefitinib (12). It can be
inferred from these studies that gefitinib offers good efficacy in
Asian EGFR mutation-positive patients; hence, EGFR
mutation status must be determined before starting treatment with
gefitinib especially in a first-line setting. The proportion of
non-smokers who responded to gefitinib was more than double
compared with those who had smoked, due to the small sample size. A
higher RR has been observed in non-smokers in previous studies
(11,12). A good ECOG performance status of 0-1
independently predicted the response to gefitinib monotherapy in
our patients. Poor performance status has been identified as a
predictor of poor response to gefitinib (13,19).
In a trial with 41 Japanese patients with brain
metastases from EGFR-mutant lung adenocarcinoma, an ORR of
87.8% and a median PFS of 14.5 months were observed with gefitinib
monotherapy without radiation (20).
In our study, 4 patients with brain metastases achieved PR with
gefitinib, and 1 patient achieved SD. The AEs of gefitinib therapy
in the patients in the present study were generally mild and
consisted mainly of skin reactions as has been reported by others
(13). The incidence of diarrhea was
relatively uncommon as observed with other studies (5). Only 1 patient in the present study
reported severe pneumonitis leading to dyspnea, which was
symptomatically treated and gefitinib treatment was stopped.
The present study has several limitations. Due to
its retrospective nature, access to medical data was unimpeded.
However, the recordings of observation were not standardized, and
often there were missing data. The timing of scans to assess
response was also not uniform amongst the clinicians. The sample
size was small and the study was conducted in a single tertiary
center in Malaysia, making it difficult to extrapolate these
results.
In conclusion, this retrospective analysis
demonstrated that Malaysian patients with locally advanced and
metastatic EGFR mutation-positive lung adenocarcinoma
responded favorably to gefitinib therapy in terms of ORR, median
PFS, and tolerability, the results of which were consistent with
IPASS study conducted in Asian population. Considering the efficacy
and safety profile of gefitinib, it is a favorable option for the
first-line treatment of Malaysian patients with EGFR
mutation-positive NSCLC. However, future long-term studies in
larger population of Malaysian patients are required to support
whether the prolonged PFS conferred by gefitinib will translate
into prolonged overall survival.
Conflict of interest
The present study was funded by AstraZeneca. IRESSA
(gefitinib) is a trademark of the AstraZeneca group of companies.
Dr Matin Mellor Abdullah received honorarium from Astra Zeneca for
the previous engagement.
References
|
1
|
Liam CK, Pang YK, Leow CH, et al: Changes
in the distribution of lung cancer cell types and patient
demography in a developing multiracial Asian country: Experience of
a university teaching hospital. Lung Cancer. 53:23–30. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Registry (2007). Malaysia
Cancer Statistics - Data and Figure, National Cancer Registry,
Malaysia. http://www.care.upm.edu.my/dokumen/13603_NCR2007.pdfAccessed.
September 08–2014
|
|
3
|
National Comprehensive Cancer Network:
2011 NCCN Clinical Practice Guidelines in Oncology: Non-small Cell
Lung Cancer, Version 1. National Comprehensive Cancer Network
(2011) NCCN Clinical Practice Guidelines in Oncology.
2011.http://www.nccn.org/patients/guidelines/nscl/index.htmlAccessed.
September 08–2014
|
|
4
|
Liam CK, Leow HR, How SH, Pang YK, Chua
KT, Lim BK, Lai NL, Kuan YC, Pailoor J and Rajadurai P: Epidermal
growth factor receptor mutations in non-small cell lung cancers in
a multiethnic malaysian patient population. Asian Pac J Cancer
Prev. 15:321–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho C, Murray N, Laskin J, Melosky B,
Anderson H and Bebb G: Asian ethnicity and adenocarcinoma histology
continues to predict response to gefitinib in patients treated for
advanced non-small cell carcinoma of the lung in North America.
Lung Cancer. 49:225–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang CH, Yu CJ, Shih JY, Chang YC, Hu FC,
Tsai MC, Chen KY, Lin ZZ, Huang CJ, Shun CT, et al: Specific EGFR
mutations predict treatment outcome of stage IIIB/IV patients with
chemotherapy-naive non-small-cell lung cancer receiving first-line
gefitinib monotherapy. J Clin Oncol. 26:2745–2753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang A, Parikh P, Thongprasert S, Tan EH,
Perng RP, Ganzon D, Yang CH, Tsao CJ, Watkins C, Botwood N and
Thatcher N: Gefitinib (IRESSA) in patients of Asian origin with
refractory advanced non-small cell lung cancer: Subset analysis
from the ISEL study. J Thorac Oncol. 1:847–855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liam CK, Ruthranesan M, Lee CH, Pang YK,
Chua KT and Lim BK: Outcomes of Malaysian patients with advanced
lung adenocarcinoma and unknown epidermal growth factor receptor
mutation status treated with gefitinib. Asia Pac J Clin Oncol.
8:267–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liam CK, Pang YK and Leow CH: Epidermal
growth factor receptor targeted therapy with gefitinib in locally
advanced and metastatic primary lung adenocarcinoma. Respirology.
11:287–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin
Oncol. 21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thatcher N, Chang A, Parikh P, Pereira
Rodrigues J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell lung cancer: Results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morita S, Okamoto I, Kobayashi K, Yamazaki
K, Asahina H, Inoue A, Hagiwara K, Sunaga N, Yanagitani N, Hida T,
et al: Combined survival analysis of prospective clinical trials of
gefitinib for non-small cell lung cancer with EGFR mutations. Clin
Cancer Res. 15:4493–4498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim ES, Hirsh V, Mok T, Socinski MA,
Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, et al:
Gefitinib versus docetaxel in previously treated non-small-cell
lung cancer (INTEREST): A randomised phase III trial. Lancet.
372:1809–1818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller VA, Kris MG, Shah N, Patel J,
Azzoli C, Gomez J, Krug LM, Pao W, Rizvi N, Pizzo B, et al:
Bronchioloalveolar pathologic subtype and smoking history predict
sensitivity to gefitinib in advanced non-small-cell lung cancer. J
Clin Oncol. 22:1103–1109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iuchi T, Shingyoji M, Sakaida T, Hatano K,
Nagano O, Itakura M, Kageyama H, Yokoi S, Hasegawa Y, Kawasaki K
and Iizasa T: Phase II trial of gefitinib alone without radiation
therapy for Japanese patients with brain metastases from
EGFR-mutant lung adenocarcinoma. Lung Cancer. 82:282–287. 2013.
View Article : Google Scholar : PubMed/NCBI
|