Introduction
Angiosarcomas are uncommon tumors of
endothelial-type cells that line vessel walls. These tumors account
for 2–3% of adult soft tissue sarcoma cases (1), which are characterized by rapid
proliferation and extensive infiltration. Soft tissue sarcomas may
occur in any area of the body, typically the head and neck region
or breast (2–4).
Hepatic angiosarcoma is an extremely rare condition,
with only a small number of cases having been reported previously.
It has been associated with exposure to colloidal solutions of
thorium dioxide, vinyl chloride, arsenic and radiation (5,6). Early
hepatic angiosarcoma typically does not cause pain, and patients
may exhibit no obvious symptoms (7).
As the disease progresses, signs and symptoms may include abdominal
fullness and abdominal pain (7).
Clinical staging of angiosarcoma is based on the
American Joint Committee on Cancer (AJCC) staging system for soft
tissue sarcoma (8). In addition to
the size of the tumor and spread to lymph nodes or distant sites,
the histological grade, based on cellular differentiation, mitotic
rate and degree of necrosis, may be recorded for such patients
(8). However, little has been
established regarding the optimal treatment and outcomes according
to disease stage. Various kinds of treatments, including surgery,
chemotherapy and radiation, have been used previously, with varying
outcomes (9–11). The overall 1-year, 3-year and 5-year
survival rates associated with surgical treatment were 100.0, 80.0
and 40.0% based on a retrospective analysis of 6 cases (10). Studies focusing on the efficacy of
systemic chemotherapy are limited; however, a study of 4 patients
indicated that chemotherapy may improve quality and duration of
survival (11). The present study
reviewed 28 cases of hepatic angiosarcoma from studies published
between January 2000 and December 2012, in addition to 6 cases
diagnosed at our center, Tri-Service General Hospital (Taipei,
Taiwan). Information was registered and analyzed for all 34
patients regarding initial stage, treatment and prognosis, with the
aim of evaluating the association between tumor stage (according to
the current staging system) and prognosis.
Patients and methods
Patients and study design
A review of the available literature on PubMed was
conducted using the following key words: ‘Liver angiosarcoma’,
‘hepatic angiosarcoma’ or ‘hepatic hemangiosarcoma’. Articles
published between January 2000 and December 2012 and with available
clinical data were included in the analysis. In addition, 6 cases
diagnosed at Tri-Service General Hospital in the most recent 10
years were included. All cases contained detailed patient
information, including medical history, tumor size, pathological
diagnosis, treatment records and prognosis. Clinical stage was
based on the AJCC staging system (8).
All cases were stage T1b or T2b, indicating deep tumors, as hepatic
angiosarcoma is located beneath the superficial fascia.
Histological grade will be determined as ‘grade cannot be assessed’
if tumor grade is not mentioned. Studies were excluded if tumor
stage could not be determined on the basis of the published
information. The presence of vascular invasion within the tumor and
the number of tumors was also noted. Due to the tumor location in
the liver, overall survival was assessed in relation to the TNM
staging system for hepatocellular carcinoma (12).
Statistical analysis
Survival analysis was conducted using the
Kaplan-Meier method and the log-rank test. Data were analyzed using
SPSS software version 21 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 65 cases of primary hepatic angiosarcoma
were identified in the literature, of which 37 were excluded due to
inadequate staging information, such as tumor size. In addition, 6
cases from Tri-Service General Hospital were included. Finally, 34
patients in 16 case reports and from our hospital were enrolled in
the analysis (10,13–27). The
mean age at diagnosis was 58.9 years (range, 29–83 years). The
samples included 23 males and 11 females, giving a male:female
ratio of ~2:1 (Table I). This
comprised 25 patients (74%) with localized disease (stage I), and 9
patients (26%) of an advanced stage (stage IV), with metastatic
disease on presentation. The locations of extrahepatic metastasis
included spleen, lung, bone, brain, stomach, peritoneum and
pericardium.
 | Table I.Summary of 34 cases of hepatic
angiosarcoma. |
Table I.
Summary of 34 cases of hepatic
angiosarcoma.
|
| TNM stage |
|
|---|
|
|
|
|
|---|
| First author,
year | Country | Age
(years)/gender | Tumor size (cm) | T | N | M | Stage | Pathological
diagnosis | Extrahepatic
metastasis | Treatment | Liver rupture | Follow-up
(months) | Alive | Ref. |
|---|
| Weng, 1995 | Taiwan | 42/M | 12 | 2b | 0 | 0 | Ib | Biopsy | None | TAE+surgery | No | 2.25 | No | (13) |
| Tsai, 1998 | Taiwan | 56/M | 7 | 2b | 0 | 0 | Ib | Biopsy | None | None | No | 9 | No | (14) |
| Timaran, 2000 | America | 47/F | 20 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 120 | Yes | (16) |
| Cheng, 2003 | Taiwan | 58/F | 5.5 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 14 | Yes | (23) |
| Liang, 2003 | Taiwan | 59/F | 3 | 1b | 0 | 0 | Ia | Surgery | None | Surgery+C/T | No | 24 | Yes | (24) |
| Ozden, 2003 | Turkey | 54/F | 5.5 | 2b | 0 | 0 | Ib | Biopsy/surgery | None | Surgery+C/T | No | 64 | Yes | (17) |
| Ho, 2004 | Taiwan | 68/F | 5.5 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | Yes | 42 | Yes | (25) |
| Almogy, 2004 | Israel | 68/M | 12 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 9 | No | (9) |
| Nakayama, 2004 | Japan | 29/F | 5 | 1b | 0 | 0 | Ia | Surgery | None | Surgery | No | 102 | Yes | (18) |
| Kim, 2005 | Lebanon | 54/M | x | x | x | 1 | IV | Biopsy | Stomach | None | No | 5 | No | (19) |
| Leowardi, 2006 | Germany | 76/M | 12.5 | 2b | 0 | 0 | Ib | Surgery | None | Surgery+TAE | Yes | 1 | No | (20) |
| Kim, 2009 | Korea | 44/M | x | x | x | 1 | IV | Biopsy | Spleen | None | Yes | 0.27 | No | (21) |
| Kim, 2009 | Korea | 69/M | x | x | x | 1 | IV | Biopsy | Lung | C/T | No | 1.63 | No | (21) |
| Kim, 2009 | Korea | 49/M | x | x | x | 1 | IV | Biopsy | Spleen | C/T | No | 2.87 | No | (21) |
| Kim, 2009 | Korea | 41/M | x | x | x | 1 | IV | Biopsy | Pericardium | C/T | No | 15.8 | No | (21) |
| Kim, 2009 | Korea | 45/M | x | x | x | 1 | IV | Biopsy | Bone | C/T | No | 7.4 | No | (21) |
| Huang, 2011 | Taiwan | 56/M | 3.4 | 1b | 0 | 0 | Ia | Biopsy | None | TAE | No | 24 | No | (15) |
| Huang, 2011 | Taiwan | 67/M | x | x | x | x | IV | Surgery | Lung | Surgery+C/T | No | 44 | No | (15) |
| Huang, 2011 | Taiwan | 69/F | x | x | x | 1 | IV | Surgery | Brain | Surgery+C/T | No | 37 | No | (15) |
| Huang, 2011 | Taiwan | 63/F | x | x | x | 1 | IV | Surgery | Peritoneum | Surgery+C/T | No | 3 | No | (15) |
| Murawaki, 2011 | Japan | 72/M | 3 | 1b | 0 | 0 | Ia | Biopsy | None | TACE+interleukin
2 | No | 34 | No | (26) |
| Okano, 2012 | Japan | 69/M | 6.5 | 2b | 0 | 0 | Ib | Surgery | None | TAE+Surgery | Yes | 17 | No | (22) |
| Duan, 2012 | China | 46/M | 9 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 23 | No | (10) |
| Duan, 2012 | China | 78/M | 11 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 1 | No | (10) |
| Duan, 2012 | China | 46/M | 8 | 2b | 0 | 0 | Ib | Surgery | None | Surgery+C/T | No | 41 | No | (10) |
| Duan, 2012 | China | 45/M | 7 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 84 | Yes | (10) |
| Duan, 2012 | China | 65/M | 13 | 2b | 0 | 0 | Ib | Surgery | None | Surgery | No | 40 | No | (10) |
| Duan, 2012 | China | 52/F | 3 | 1b | 0 | 0 | Ia | Surgery | None | Surgery | No | 69 | No | (10) |
| TSGH | Taiwan | 76/F | 8.8 | 2b | 0 | 0 | Ib | Biopsy | None | None | Yes | 2 | No |
|
| TSGH | Taiwan | 83/M | 5 | 1b | 0 | 0 | Ia | Biopsy | None | None | No | 11 | No |
|
| TSGH | Taiwan | 68/F | 5.1 | 2b | 0 | 0 | Ib | Biopsy | None | C/T | No | 3 | No |
|
| TSGH | Taiwan | 79/M | 3.7 | 1b | 0 | 0 | Ia | Biopsy | None | Cyberknife | No | 17 | No |
|
| TSGH | Taiwan | 59/M | 4 | 1b | 0 | 0 | Ia | Biopsy | None | Surgery | No | 21 | No |
|
| TSGH | Taiwan | 50/M | 2.4 | 1b | 0 | 0 | Ia | Biopsy | None | C/T+cyberknife
radiosurgery | No | 43 | Yes |
|
Tumor size data was available for 25 patients. The
mean size was 7.24 cm, with a range of 2.4–20.0 cm. Histological
grade was not mentioned for all cases. Treatment varied according
to stage: The majority of patients with stage I disease (18 of 25
patients; 72%) were initially treated with surgery; 1 case in our
hospital received cyberknife treatment alone, whilst 3 cases
received only supportive care (pain management, and nutritional and
psychosocial support), including 2 cases in our hospital, due to
poor performance status or advanced decompensated liver disease.
Chemotherapy alone or combined surgery and chemotherapy was
reported in 8 of 9 patients (89%) with advanced stage disease
(stage IV).
Survival outcome
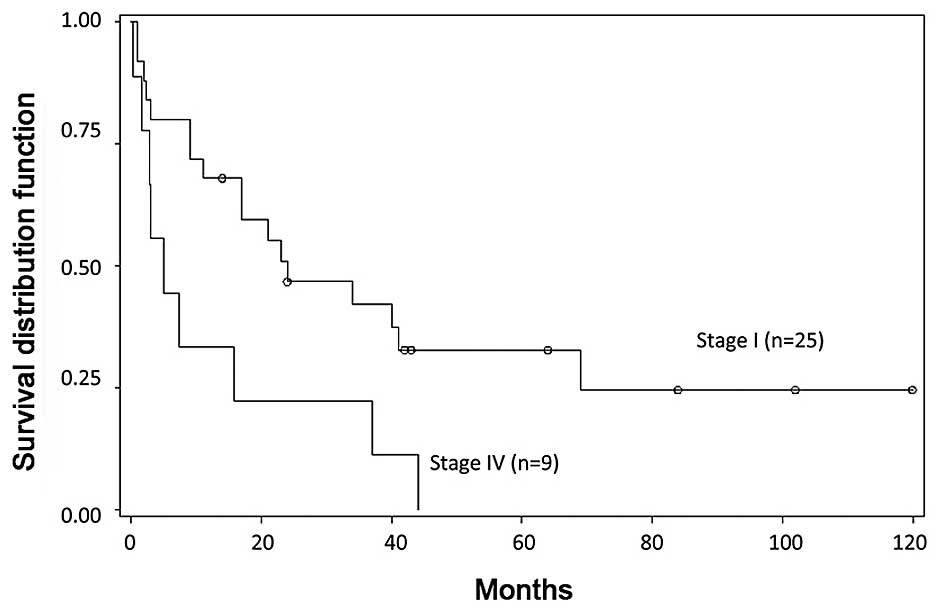
Survival information was available in all cases.
With a mean follow-up of 27.5 months (range, 0.27–102 months), 18%
(6/34) of the patients had survived. The 1-, 3- and 5-year survival
rates were 68.0±9.3, 42.1±10.2 and 32.7±9.8% in patients with stage
I disease at diagnosis (mean follow-up, 32.7 months); for patients
with stage IV disease, the 1- and 3-year survival rates were
33.3±15.7 and 22.2±13.9% (mean follow-up, 13.0 months). Survival
significantly differed between patients with stage I and with stage
IV disease at presentation, based on Kaplan-Meier survival curves
(P=0.0157); however, no apparent difference in survival was
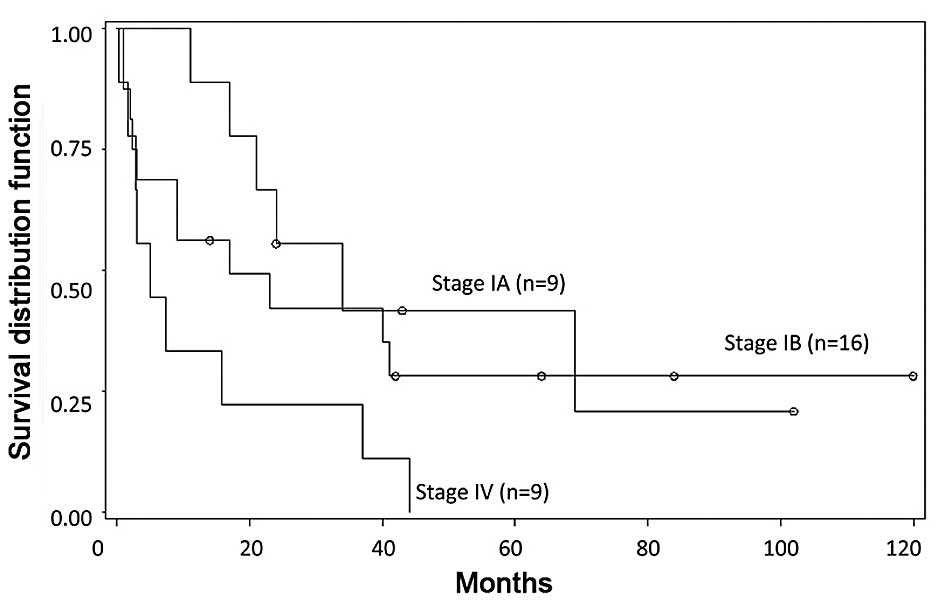
observed between those with stage IA and those with stage IB
(P=0.4806). Thus, survival time worsened with advanced tumor stage
at diagnosis compared with early stage at diagnosis (Figs. 1 and 2).
Staging system for hepatocellular
carcinoma and survival outcome
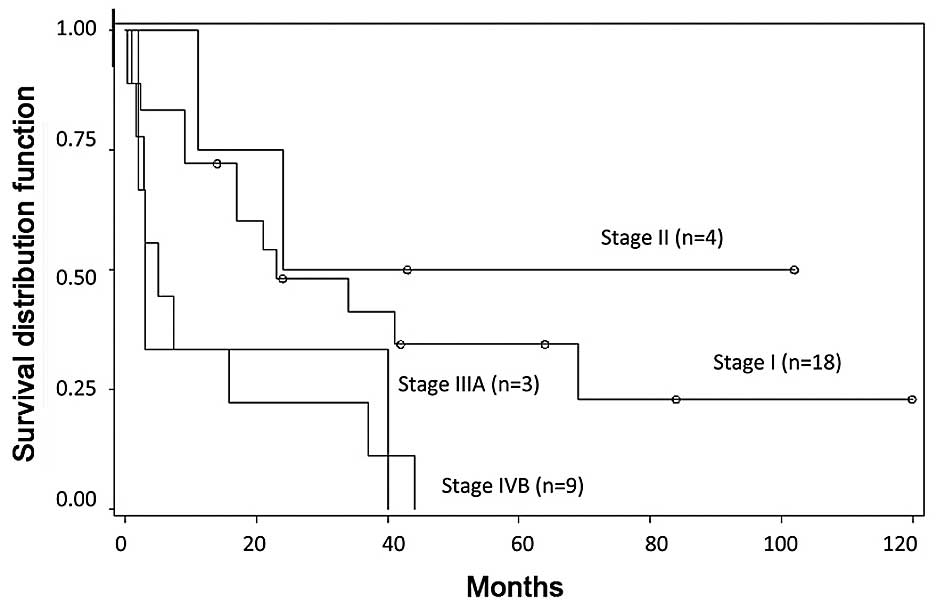
According to the AJCC TNM staging system for
hepatocellular carcinoma, 18 patients (53%) had stage I disease, 4
patients (12%) had stage II disease, 3 patients (9%) had stage IIIA
disease, and 9 patients (26%) had stage IVB disease (Fig. 3). The overall survival of stages I and
IVB was significantly different (P=0.0182); however, there was no
statistically significant difference between any other two stages
(I vs. II, P=0.4743; I vs. IIIA, P=0.1487; II vs. IIIA, P=0.1531;
II vs. IVB, P=0.0629; IIIA vs. IVB, P=0.9972). Thus, this did not
appear to be superior to the staging system for sarcoma.
Treatment outcome
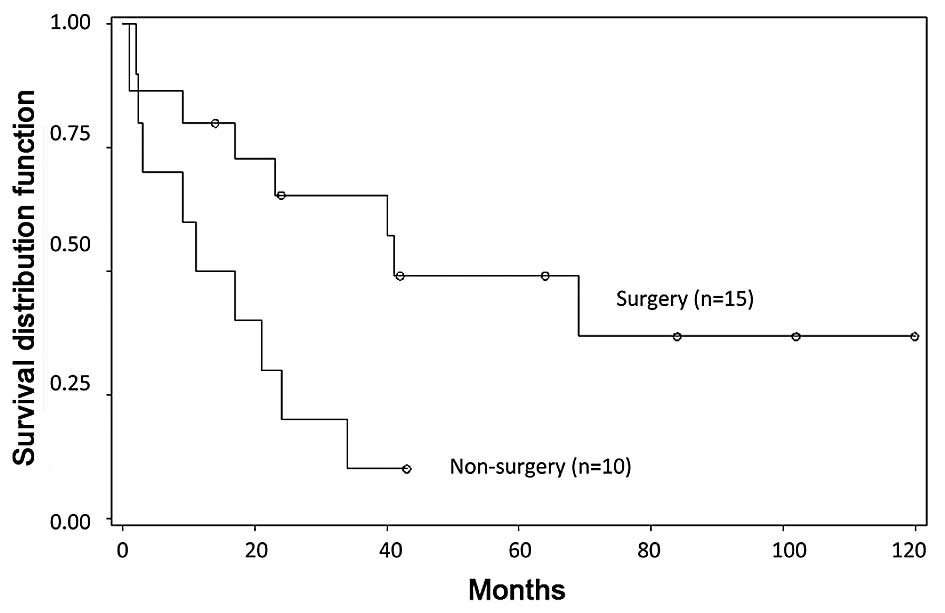
Survival rates were better for those who underwent
site-directed surgery as part of first-line treatment than for
those who did not (P=0.0209; Fig. 4).
The 1- and 3-year survival rates of the non-surgery patient group
in stage I were 50.0±15.8 and 10.0±9.5%, whilst the 1-, 3- and
5-year survival rates for patients in stage I who underwent surgery
were 80.0±10.3, 65.5±12.6, 49.1±13.8%.
Discussion
Primary vascular neoplasms of the liver are rare and
have a poor prognosis (27). Tumor
size, depth, presence or absence of regional lymph nodes, distant
metastases and histological grade are commonly used to define the
stage of soft tissue sarcomas (8). A
size of 5 cm was the cutoff to determine tumor stage (IA or IB and
IIA or IIB), based on the AJCC TNM staging system for soft tissue
sarcoma (8). Superficial tumors are
located exclusively above the superficial fascia without invasion
of the fascia, whilst deep tumors are located in exclusively
beneath the superficial fascia, not accounting for disease site
(8). Thus, all hepatic angiosarcomas
are considered deep tumors (8).
Histological grading, which is based on cellular differentiation,
mitotic rate and degree of necrosis, is an important component of
staging, and has been demonstrated to be associated with degree of
malignancy and the probability of distant metastasis (28). However, pathological grade information
was absent in the cases included in the present study. T2 (>5
cm) tumors without spread to nearby lymph nodes (N0) or to distant
sites (M0) with well-differentiated (G1) or grade information not
available (GX) are classified as stage IB. However, if the tumor is
poorly differentiated (G3) it is classified as stage III.
Therefore, if definite tumor grade is taken into the consideration
during cancer staging, individual treatment strategies can be
developed and prognosis can be better predicted, possibly improving
overall survival.
Typical computed tomography or magnetic resonance
images of hepatic angiosarcoma reveal aggressive multifocal tumors
containing small heterogeneous hypervascular foci, which had early
arterial enhancement followed by progressive enhancement of the
lesion compared with early and delayed phase imaging (29). In addition, the presence of positive
nodes was not emphasized in cases reviewed in the present study.
This may be because sarcomas rarely spread to regional lymph nodes.
However, nodal metastasis has been reported in 13.5% of
angiosarcomas (30). Early
identification of lymph node involvement may result in appropriate
disease management and positively affect outcomes. Thus, stage II
and III of hepatic angiosarcoma are rare, and the majority of cases
included in the current study were stage I or IV. It was difficult
to predict the outcome due to the rarity of stage II and III
cases.
There are several treatment options for hepatic
angiosarcoma patients; surgery, chemotherapy and radiation have all
been used previously, with varying outcomes (9–11).
Complete surgical resection may provide an improved prognosis if
the lesion is resectable (10).
Responses to chemotherapy and radiation in advanced disease appear
to be limited (9,22); however, successful treatment with
liver transplantation and chemotherapy has been reported (31). Furthermore, for patients with
unresectable tumors or extrahepatic disease, chemotherapy may be
considered (10). In the current
analysis, the distribution of tumor stage at diagnosis was stage I
or IV; this may be due to the absence of tumor grade in the
included cases or the method of collecting samples; cases were
excluded if there was no information regarding tumor size and
staging was unavailable, but were included if the tumor had distant
metastasis, when they were determined to be stage IV. This
selection bias must be taken into account. However, the TNM stage
at presentation without grade information in patients with hepatic
angiosarcoma still appeared to be and important factor affecting
prognosis. As the results of the Kaplan-Meier analysis
demonstrated, patients in the early stages of disease at diagnosis
had better overall survival times.
In addition, we attempted to use the AJCC TNM
staging system for hepatocellular carcinoma to stage hepatic
angiosarcoma. The number of tumors and the presence and extent of
vascular invasion within the tumor were considered. However,
compared with the soft tissue sarcoma staging system, there was no
statistical association between stage and survival except for
stages I and IVB.
Many unsolved issues remain regarding this type of
cancer. In the previously published cases, standard prognostic
parameters have not been consistently taken into account. In
addition, different therapeutic strategies, treatment regimens and
their outcomes have not been systemically evaluated.
In conclusion, hepatic angiosarcoma is a rare
disease for which the therapeutic guidelines have not been defined
due to the small number of cases. According to the current
meta-analysis, patients with early-stage disease appear to have
good prognosis following primary surgery or cyberknife therapy.
Definitive diagnosis and clinical stage are important for initial
assessment. Chemotherapy, surgery, radiation therapy or combined
therapy were all considered. New studies encompassing larger
patient populations are required to analyze and define standard
prognostic parameters and to standardize a treatment approach for
this rare neoplasm. The use of targeted therapies has markedly
improved outcomes for certain types of cancer (32), and this may be important in the
treatment of hepatic angiosarcoma in the future.
References
|
1
|
Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou
C, Smith KD, Phung TL, Pollock RE, Benjamin R, Hunt KK, et al:
Angiosarcoma: Clinical and molecular insights. Ann Surg.
251:1098–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wanebo HJ, Koness RJ, MacFarlane JK,
Eilber FR, Byers RM, Elias EG and Spiro RH: Society of Head and
Neck Surgeons Committee on Research: Head and neck sarcoma: Report
of the Head and Neck Sarcoma Registry. Head Neck. 14:1–7. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanchard DK, Reynolds CA, Grant CS and
Donohue JH: Primary nonphylloides breast sarcomas. Am J Surg.
186:359–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pandey M, Mathew A, Abraham EK and Rajan
B: Primary sarcoma of the breast. J Surg Oncol. 87:121–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buetow PC, Buck JL, Ros PR and Goodman ZD:
Malignant vascular tumors of the liver: Radiologic-pathologic
correlation. Radiographics. 14:153–166. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito Y, Kojiro M, Nakashima T and Mori T:
Pathomorphologic characteristics of 102 cases of thorotrast-related
hepatocellular carcinoma, cholangiocarcinoma and hepatic
angiosarcoma. Cancer. 62:1153–1162. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molina E and Hernandez A: Clinical
manifestations of primary hepatic angiosarcoma. Dig Dis Sci.
48:677–682. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC (American Joint Committee on Cancer):
Cancer Staging Manual (7th). Springer. New York: 2912010.
|
|
9
|
Almogy G, Lieberman S, Gips M, Pappo O,
Edden Y, Jurim O, Slasky Simon B, Uzieli B and Eid A: Clinical
outcomes of surgical resections for primary liver sarcoma in
adults: Results from a single centre. Eur J Surg Oncol. 30:421–427.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan XF and Li Q: Primary hepatic
angiosarcoma: A retrospective analysis of 6 cases. J Dig Dis.
13:381–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dannaher CL, Tamburro CH and Yam LT:
Chemotherapy of vinyl chloride-associated hepatic angiosarcoma.
Cancer. 47:466–469. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC (American Joint Committee on Cancer):
Cancer Staging Manual (7th). Springer. New York: 1752010.
|
|
13
|
Weng YJ, Chao Y, Wang SS, Chiang JH, Tsay
SH, Chen CC, Chau GY, Lui WY and Lee SD: Hepatic angiosarcoma:
Report of a case. Gastroenterol J Taiwan. 12:208–215. 1995.
|
|
14
|
Tsai CC, Cheng KS, Lai HC, Yu CJ and Hsu
CH: Ruptured angiosarcoma of the liver treated by emergency
TAE-case report. China Med Univ Reposit Taichung. 2007.
|
|
15
|
Huang NC, Wann SR, Chang HT, Lin SL, Wang
JS and Guo HR: Arsenic, vinyl chloride, viral hepatitis and hepatic
angiosarcoma: A hospital-based study and review of literature in
Taiwan. BMC Gastroenterol. 11:1422011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Timaran CH, Grandas OH and Bell JL:
Hepatic angiosarcoma: Long-term survival after complete surgical
removal. Am Surg. 66:1153–1157. 2000.PubMed/NCBI
|
|
17
|
Ozden I, Bilge O, Erkan M, Cevikbaş U and
Acarli K: Five years and 4 months of recurrence-free survival in
hepatic angiosarcoma. J Hepatobiliary Pancreat Surg. 10:250–252.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakayama H, Masuda H, Fukuzawa M, Takayama
T and Hemmi A: Metastasis of hepatic angiosarcoma to the gastric
vein. J Gastroenterol. 39:193–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim TO, Kim GH, Heo J, Kang DH, Song GA
and Cho M: Metastasis of hepatic angiosarcoma to the stomach. J
Gastroenterol. 40:1003–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leowardi C, Hormann Y, Hinz U, Wente MN,
Hallscheidt P, Flechtenmacher C, Buchler MW, Friess H and
Schwarzbach MH: Ruptured angiosarcoma of the liver treated by
emergency catheter-directed embolization. World J Gastroenterol.
12:804–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HR, Rha SY, Cheon SH, Roh JK, Park YN
and Yoo NC: Clinical features and treatment outcomes of advanced
stage primary hepatic angiosarcoma. Ann Oncol. 20:780–787. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okano A, Sonoyama H, Masano Y, Taniguchi
T, Ohana M, Kusumi F and Nabeshima M: The natural history of a
hepatic angiosarcoma that was difficult to differentiate from
cavernous hemangioma. Intern Med. 51:2899–2904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng SP and Jeng KS: Hepatic
angiosarcoma: Report of a case. Formos J Surg. 36:179–183.
2003.
|
|
24
|
Liang WB, Wang TE, Lin SC, Shin SC, Kao
CR, Chou SY and Chang KM: Primary angiosarcoma of the liver - a
case report. J Intern Med Taiwan. 14:290–294. 2003.
|
|
25
|
Ho SY, Tsai CC, Tsai YC and Guo HR:
Hepatic angiosarcoma presenting as hepatic rupture in a patient
with long-term ingestion of arsenic. J Formos Med Assoc.
103:374–379. 2004.PubMed/NCBI
|
|
26
|
Murawaki Y, Kono M, Miura M, Sugihara T,
Tanimura T, Oshima N, Tanaka S, Yoshimura T, Yamada M and Yoshida
M: A case of hepatic angiosarcoma detected as a small lesion. Nihon
Shokakibyo Gakkai Zasshi. 108:1252–1262. 2011.(In Japanese).
PubMed/NCBI
|
|
27
|
Groeschl RT, Miura JT, Oshima K, Gamblin
TC and Turaga KK: Does histology predict outcome for malignant
vascular tumors of the liver? J Surg Oncol. 109:483–486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fletcher CDM, Bridge JA, Hogendoorn PCW
and Mertens F: World Health Organization Classification of Tumours
of Soft Tissue and Bone (4th). IARC Press. Lyon, France: 2013.
|
|
29
|
Thapar S, Rastogi A, Ahuja A and Sarin S:
Angiosarcoma of the liver: Imaging of a rare salient entity. J
Radiol Case Rep. 8:24–32. 2014.PubMed/NCBI
|
|
30
|
Fong Y, Coit DG, Woodruff JM and Brennan
MF: Lymph node metastasis from soft tissue sarcoma in adults.
Analysis of data from a prospective database of 1772 sarcoma
patients. Ann Surg. 217:72–77. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maluf D, Cotterell A, Clark B, Stravitz T,
Kauffman HM and Fisher RA: Hepatic angiosarcoma and liver
transplantation: Case report and literature review. Transplant
Proc. 37:2195–2199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herrero AB, Martín-Castellanos C, Marco E,
Gago F and Moreno S: Cross-talk between nucleotide excision and
homologous recombination DNA repair pathways in the mechanism of
action of antitumor trabectedin. Cancer Res. 66:8155–8162. 2006.
View Article : Google Scholar : PubMed/NCBI
|