Introduction
Myeloid sarcoma is a form of solid tumor, and is
composed of immature white blood cells known as myeloblasts
(1,2).
The disease is classified into four groups according to tumor cell
type, namely granulocytic sarcoma, primitive monocytic sarcoma,
myeloid sarcoma of hematopoietic cell and primary myeloid sarcoma
without the presentation of other hematological diseases (1,2). Myeloid
sarcoma may develop at any age, but occurs most commonly in
children and young individuals (3).
The incidence of myeloid sarcoma in females is slightly higher than
in males, with a ratio of 1.42:1 (3)
The proportion of patients with acute myelocytic leukemia (AML)
accompanied with myeloid sarcoma is 2–8%, and ~10% of type M2 AML
cases develop into myeloid sarcoma (1,4). Genetic
or chromosomal abnormalities are associated with the disease
(5), and factors including
malnutrition, cellular immune dysfunction, increased white blood
cells (3), and myeloblasts expressing
T-cell surface markers, cluster of differentiation (CD)13, or CD14
(6) serve as risk factors. The
mortality rate of patients is extremely high, and in a study of 23
non-leukemic myeloid sarcoma cases, it was observed that the
average progression free survival was 12.5 months, the average
survival rate was 32.9 months and the expected 3-year survival rate
was 41% (7).
No universally accepted treatment for the disease is
currently available. For myeloid sarcoma other than leukemia,
surgical removal of the tumor followed by local radiotherapy may be
performed. In certain cases, myeloid sarcoma is accompanied by
other hematological diseases, in which case systemic chemotherapy
or combined treatment of surgery, local radiotherapy and systemic
chemotherapy may be performed (8) In
the majority of cases, myeloid sarcomas are accompanied by other
hematological diseases, including AML, chronic myeloproliferative
diseases and myelodysplastic syndrome. However, Kohli et al
(9) reported a case in which myeloid
sarcoma occurred without developing acute myeloid leukaemia or
other hematological diseases, it only formed multiple metastatic
deposits. The patient received systemic chemotherapy in addition to
radiotherapy, due to the limited therapeutic effect of chemotherapy
alone (9).
Although myeloid sarcoma is able to develop in the
absence of other systemic diseases, it is a complicated disease
with high mortality and low survival rates in itself, and patients
presenting with features of the disease should receive combined
treatment as soon as possible.
Case report
A 65-year-old male patient was admitted to The
Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
China) on November 28, 2013, with a 6-month history of bilateral
purple-red papules and nodules on the upper limbs, which had spread
over the whole body of the patient one month prior to admission to
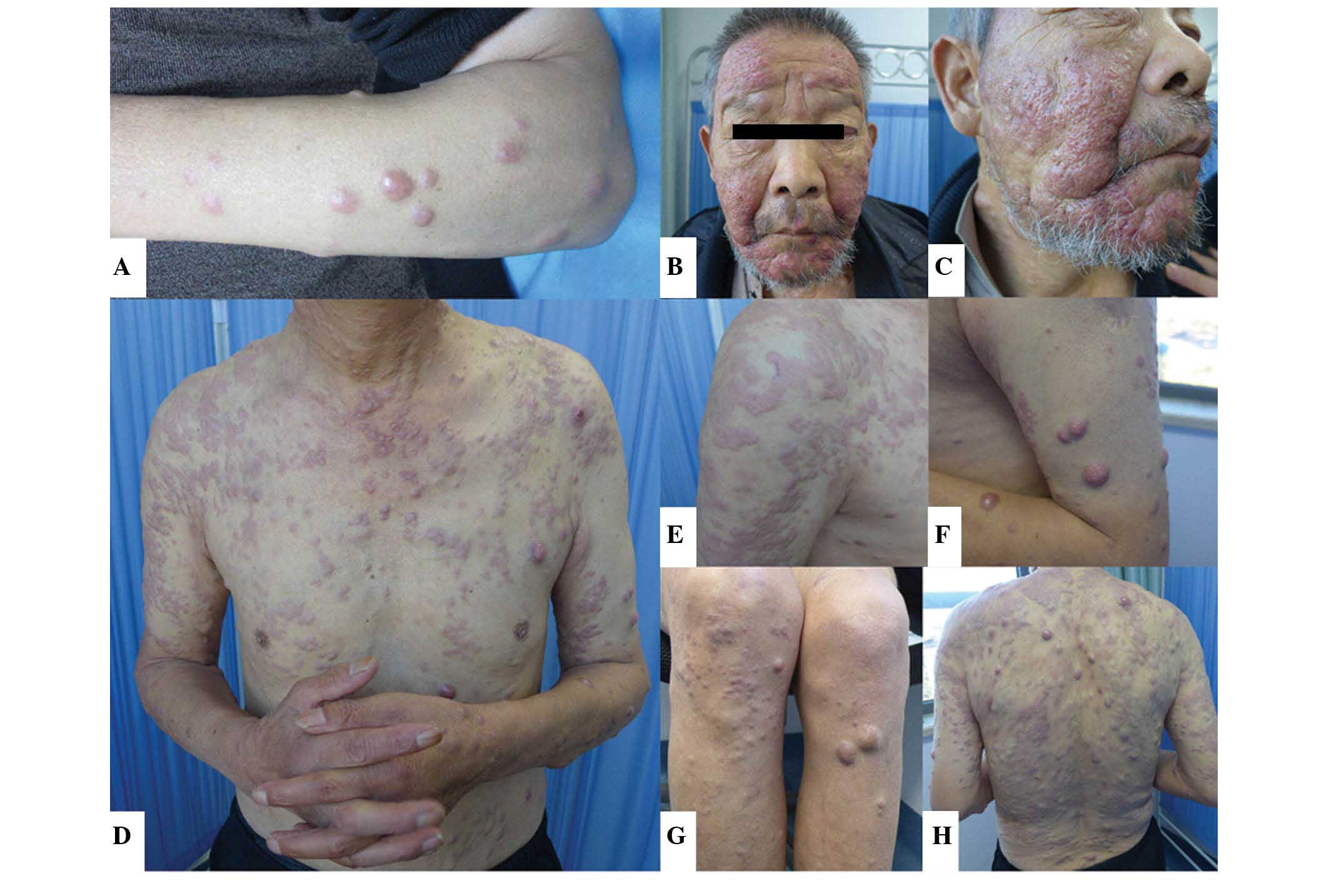
the hospital (Fig. 1A). The
purple-red papules and nodules measured between 3.0 mm and 1.0 cm
in size, and had appeared bilaterally on the upper limbs six months
prior to admission to the hospital, with no apparent identifiable
cause. A proportion of the papules and nodules were observed to be
merged together, forming large nodules with clear borders and a
hard texture. Generally, there was no mobility of the papules and
nodules. Since the patient experienced no other symptoms, with the
exception of slight itching, the skin lesions were not considered
to be of serious concern, even though the number of the papules and
nodules had increased and spread to the whole body with no apparent
cause. The patient was admitted to The Second Affiliated Hospital
of Xi'an Jiaotong University for a skin biopsy of the left elbow.
Following the biopsy, the skin samples were stained with
hematoxylin and eosin (catalog no., AR1180-100; Wuhan Boster
Biological Technology, Ltd., Wuhan, China). The patient refused to
undergo an immunohistochemical examination, due to the relatively
high cost associated with the test.
One of the skin lesions of the patient became
aggravated 10 days following admission to the hospital, without any
apparent cause. The patient was then admitted to a local hospital,
where was diagnosed with Sjogren's syndrome. The patient was
provided with oral drugs, the name and dose of which are not known.
However, the effects of the treatment were limited, and the lesion
progressed. The patient experienced pain in his throat and the
sensation of a foreign body three days after treatment. The patient
also had dysphagia and did not eat or drink water for three days.
In consequence, the patient returned to The Second Affiliated
Hospital of Xi'an Jiaotong University for additional diagnosis and
treatment. The patient's throat was assessed by the naked eye with
a mirror and a laryngoscope, and abnormal parenchyma were
identified. Therefore, the tumor was speculated to have not only
invaded the throat, but to have also invaded the esophagus and
other digestive organs. Although the patient was conscious
following the onset of the disease, his mental state was poor. In
addition, the patient had not defecated for three days, although
urinary function was normal according to a 24-hour urine volume
test. The weight of the patient had decreased by 4.5 kg, compared
with the weight recorded during his visit to the hospital one month
earlier. The patient had no fever, infection, anemia or joint pain,
but had a history of hypertension and cerebral infarction, and was
suffering from hemiplegia.
Physical examination of the patient's temperature,
respiration, pulse and blood pressure demonstrated that the vital
signs of the patient were stable. However, the patient had
difficulty closing his eyes. Hemiplegic gait and swollen lymph
nodes behind the right ear were also observed, which were painful
when pressed. In addition, nodules of ~1.0 cm in size were observed
on the throat. Examination of the skin revealed the presence of red
papules and nodules that measured between 3.0 mm and 2.5 cm in
size, which had spread over the body. The distribution of the
papules and nodules were diffuse or focused, and the shape of the
papules and nodules were round or oval. The nodules protruded above
the skin surface, and their borders were defined, while their
texture was hard. The surface of certain papules and nodules had
burst. Several papules and nodules had merged together to form
irregular nodules. The nodules at the bilateral zygomatic regions
and cheeks were observed to be merged together, with pitting
surface edema. A red nodule of ~1.0 cm in size was observed at each
of the palpebral conjunctiva of the patient. Sporadic purple-red
petechia or bruising was also observed at the flexor side of the
bilateral lower limbs, with defined margins, and the color did not
disappear when pressure was applied (Fig.
1B–H).
Laboratory examinations revealed that the neutrophil
ratio of the patient was 72.24% (normal range, 51–75%), while the
monocyte ratio was 2.74% (normal range, 3–8%). On chest X-ray
(SOMATOM Definition AS 64, Siemens AG, Munich, Germany), increased
bronchovascular shadows on the lung were observed. Computed
tomography (LightSpeed VCT XT; GE Healthcare Life Sciences,
Chalfont, UK) also revealed increased bronchovascular shadows on
the lung, and bilateral cord-like lesions at the apex of the lung
(Fig. 2A and B).
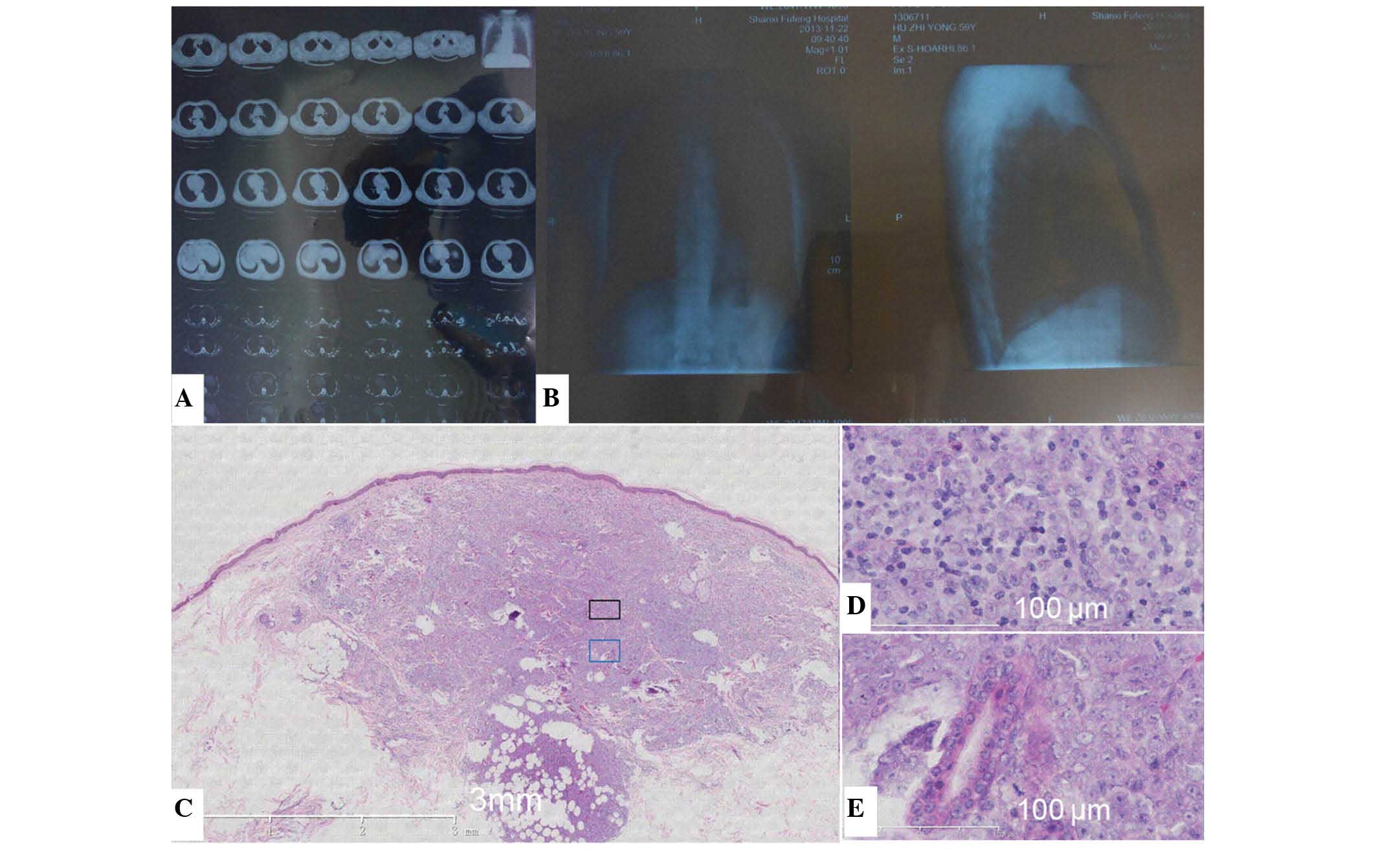 | Figure 2.CT, X-ray and HE examination of the
present patient with myeloid sarcoma. (A) CT revealed increased
bronchovascular shadows on the lung and bilateral cord-like lesions
at the apex of the lungs. (B) Chest X-ray revealed increased
bronchovascular shadows on the whole lung. Left image,
anteroposterior film; right image, lateral film. (C) A skin biopsy
of the left elbow revealed by HE staining that the lesions were
focused on the papillary dermis and subcutaneous tissues. (D and E)
Diffuse infiltration of round, medium-sized (40–60 µm), primitive
cells was observed. Generally, the shape of the cells was
identical, and the cell nuclei were primarily round or oval,
although lobate or kidney-shaped nuclei were also observed in
several cells. The cells had delicate chromatin and small nucleoli,
and a large number of cells were undergoing mitosis. CT, computed
tomography; HE, hematoxylin and eosin. |
Histopathological examination revealed that the
epidermis was not involved, and the lesions were primarily focused
on the papillary dermis and subcutaneous tissues. There was diffuse
infiltration of round, medium-sized, primitive cells. The majority
of the infiltrating cells were morphologically identical, and
compared with the mature cells, the ratio of cytoplasm to nuclear
volume in infiltrating cells was decreased and the nuclei of
infiltrating cells was increased. The nuclei of the cells were
primarily round or oval, although lobate or kidney-shaped nuclei
were also observed in several cells. The cells had delicate
chromatin and small nucleoli, and numerous cells undergoing mitosis
were observed (Fig. 2C–E).
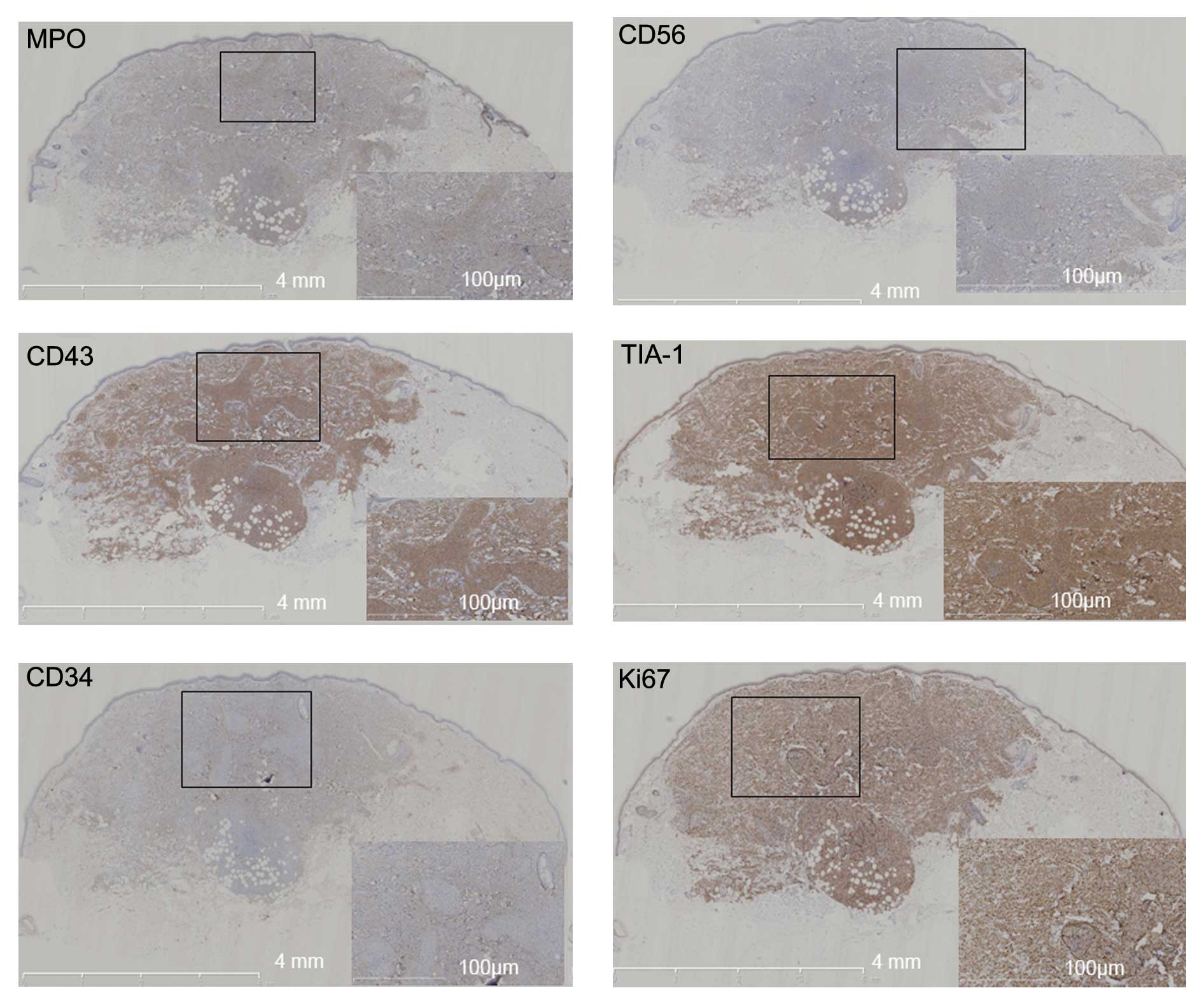
Immunohistochemical examination was performed using
the following antibodies: Monoclonal mouse anti-human CD56
(dilution, 1:800; catalog no., SC-106; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), monoclonal rabbit anti-human CD43
(dilution, 1:1,000; catalog no., SAB5500067), monoclonal mouse
anti-human CD34 (dilution, 1:1,000; catalog no., SAB4700736),
monoclonal mouse anti-human Ki67 (dilution, 1:800; catalog no.,
P6834) (Sigma-Aldrich, St. Louis, MO, USA), polyclonal goat
anti-human T-cell intracytoplasmic antigen (TIA-1; dilution,
1:1,000; catalog no., SC-1751; Santa Cruz Biotechnology, Inc.),
monoclonal rabbit anti-human CD3 (dilution, 1:600; catalog no.,
SAB5500057), monoclonal rabbit anti-human CD4 (dilution, 1:600;
catalog no., SAB5500064), monoclonal rabbit anti-human CD7
(dilution, 1:600; catalog no., SAB5500071), monoclonal rabbit
anti-human CD20 (dilution, 1:1,000; catalog no., SAB5500049)
(Sigma-Aldrich), polyclonal rabbit anti-human CD123 (dilution,
1:800; catalog no., SC-681; Santa Cruz Biotechnology, Inc.),
monoclonal rabbit anti-human paired box 5 (PAX-5; dilution, 1:800;
catalog no., SAB5500160), polyclonal rabbit anti-human granzyme B
(dilution, 1:800; catalog no., HPA003418) and monoclonal rabbit
anti-human myeloperoxidase (MPO; dilution, 1:1,000; catalog no.,
HPA021147) (Sigma-Aldrich). The antibodies were diluted in 5%
bovine serum albumin, and the tissues were incubated overnight at
4°C. DBA color developing reagent was used to reveal the staining,
and the slides were subsequently analyzed through a microscope
(CX21; Olympus Corporation, Tokyo, Japan) aided by NanoZoomer
2.0-HT (Hamamatsu Photonics, Hamamatsu, Japan) and Image Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA). The results
demonstrated that all the cells expressed (MPO), CD56, CD43 and
TIA-1 (Fig. 3). In total, 70% of the
cells also expressed CD34, and 90% of the cells expressed Ki67.
However, the cells did not express CD3, CD4, CD7, CD123, CD20,
PAX-5, granzyme B or Epstein-Barr virus. In consequence, the
patient was diagnosed with myeloid sarcoma. The patient refused to
be hospitalized for treatment due to the relatively high cost.
Therefore, no treatment was administered to the patient. The
patient died three days later due to a throat blockage, which
prevented the patient from eating or drinking water resulting in
hypoxia and malnourishment. The course of the disease was eight
months from the initial presentation of symptoms. Written informed
consent was obtained from the patient for the publication of the
present study and use of accompanying images.
Discussion
Myeloid sarcoma is composed of primitive and
immature cells, which originate, mature and differentiate in the
bone marrow (1). Myeloid sarcoma is
classified into four subtypes according to the various cell types
involved, as follows: i) Granulocytic sarcoma; ii) primitive
monocytic sarcoma; iii) myeloid sarcoma of hematopoietic cells; and
iv) primary myeloid sarcoma without the presentation of other
hematological diseases (1,2).
Previous studies have suggested that genetic or
chromosome abnormalities are associated with myeloid sarcoma
(10). Several gene rearrangements
and the presence of three copies of the chromosome 8 are relatively
common chromosome abnormalities observed in myeloid sarcoma
(1). Malnutrition, cellular immune
dysfunction and increased levels of white blood cells are also risk
factors for myeloid sarcoma (3).
Gastrointestinal tract, ovary, testes, skeletal muscle, nervous
tissue, normal brain cells and natural killer (NK) cells express
CD56 (11). In addition, cells,
tissues and tumors generated from the ectoderm, as well as tumors
generated from the mesoderm and NK/T-cell lymphoma also express
CD56 (12). Previous
immunohistochemical examinations have revealed that the majority of
patients with myeloid sarcoma do not express CD56 (12). However, this was not the case for the
present patient. Previous studies have reported that certain
chromosomal abnormalities such as t(8;21), are often associated
with CD56 expression, which increases the incidence of myeloid
sarcoma or relapse of myeloid sarcoma following treatment (11). Myeloid sarcoma that expresses CD56 may
be associated with a poor prognosis, which was confirmed by the
outcome observed in the present patient. In addition, 90% of the
cells in the myeloid sarcoma of the present patient expressed Ki67,
which is also known to lead to a poor prognosis (7).
The clinical symptoms of myeloid sarcoma are
determined by the tumor features, which are primarily characterized
by dysfunction or functional disorders of the tissues or organs
that are affected (13). The
immunophenotypic features of myeloid sarcoma include expression of
CD68, MPO, CD13, CD33, CD117 and CD43; and no expression of CD3,
CD20 or CD7 (12,14–16). In
certain patients, the expression of CD34 and terminal
deoxynucleotidyl transferase (TdT) has been reported (17). However, the majority of patients with
myeloid sarcoma do not express CD56, with the exception of rare
examples, including the present patient (12).
The incidence of myeloid sarcoma is extremely low
(1,18); the exact data is not clear as the
disease may be easily misdiagnosed as malignant lymphoma,
metastatic cancer and other diseases (8,19).
However, the incidence of myeloid sarcoma in AML is 2–9% (8). In certain cases, myeloid sarcoma is
accompanied by hematological malignant tumors, and the symptoms may
be similar to those of autoimmune diseases (20). Therefore, a differential diagnosis
between myeloid sarcoma and lymphoblastic lymphoma, blastic
plasmacytoid dendritic cell neoplasm and small-blue-round-cell
tumor must be confirmed (10).
Lymphoblastic lymphoma may present with symptoms of
skin invasion and MPO expression (21). Myeloid cell differentiation also
distinguishes lymphoblastic lymphoma from myeloid sarcoma (12). Immunophenotypic features are extremely
important for a differential diagnosis between lymphoblastic
lymphoma and myeloid sarcoma (22).
Thus, although myeloid sarcoma may express TdT, CD34, PAX-5, CD7
and CD10, these markers are not generally observed in myeloid
sarcoma, but are frequently expressed in lymphoblastic lymphoma
(22,23).
Blastic plasmacytoid dendritic cell neoplasm is
primarily observed in elderly male patients (24). The pathological features of this
disease are similar to myeloid sarcoma (24). The morphological characteristics of
the tumor cells are similar to myeloblasts or lymphocytes, but no
blood vessels are identified in the tumor cells, which, by
contrast, is frequently observed in myeloid sarcoma (24). In addition, inflammation or necrosis
surrounding the tumor is also rare in blastic plasmacytoid
dendritic cell neoplasm, but not in myeloid sarcoma (24). Tumor cells in blastic plasmacytoid
dendritic cell neoplasm generally express CD4, but do not express
MPO or TIA-1 (24).
Small-blue-round-cell tumors, including
neuroblastoma, rhabdomyosarcoma and Ewing's sarcoma, are primarily
observed in children, and generally do not express CD43 or MPO,
which aids the differential diagnosis from myeloid sarcoma
(18,19).
There is no general treatment method for myeloid
sarcoma currently available. For myeloid sarcomas other than
leukemia, surgical treatment for the removal of the tumor, followed
by local radiotherapy may be performed (8). For certain patients, where myeloid
sarcomas are accompanied by other hematological diseases, systemic
chemotherapy or combined treatment with surgical removal, local
radiotherapy and systemic chemotherapy may be performed, depending
on the particular situation of each individual patient (8). Although previous studies have reported
the spontaneous remission of myeloid sarcomas (25), the majority of these were accompanied
with other hematological diseases (18). Therefore, treatment is required to
improve the survival rate of patients with myeloid sarcoma.
In the present study, the initial symptom of the
elderly male patient was the presence of purple-red papules and
nodules. The symptoms were not deemed to be too serious until the
skin lesions spread to the entire body. A month following the
initial admission to the hospital, the patient experienced throat
pain with the sense of a foreign object and dysphagia, and was
unable to ingest water and food for three days. Subsequently, the
patient underwent additional diagnosis, and following examination,
the presence of a tumor that had invaded the throat, esophagus and
other digestive organs was observed. The progression of the myeloid
sarcoma in the present patient was eight months between the initial
development of the skin lesion and the date when the patient
succumbed to the disease.
In conclusion, myeloid sarcoma may either occur
alone or be accompanied by a hematological disease (19). In either case, it is a comprehensive
disease, with rapid development and a poor prognosis. A synergistic
method, which may be adapted according to the requirements of the
patient and includes local or systemic chemotherapy and surgical
treatment for the removal of the tumor, should be selected for
treatment as soon as features of disease onset are identified
(9). Future research on this disease
is required in order to develop a more effective treatment.
Acknowledgements
The abstract of the present case report was
presented at the Third Eastern Asia Dermatology Congress September
24–26, 2014 in Jeju, Korea, and was published by the Japanese
Dermatological Association as abstract no. P-152 in Poster
Abstracts, Wang X, Li WS, Zheng Y and Zheng JF: A case report of
CD56+myeloid sarcoma: Natural history and literature review. J
Dermatol 41 (Suppl 1): 35, 2014.
References
|
1
|
Di Veroli A, Micarelli A, Cefalo M,
Ceresoli E, Nasso D, Cicconi L, Mauramati S, Ottaviani F, Venditti
A and Amadori S: Recurrence of a t(8;21)-positive acute myeloid
leukemia in the form of a granulocytic sarcoma involving cranial
bones: A diagnostic and therapeutic challenge. Case Rep Hematol.
2013:2453952013.PubMed/NCBI
|
|
2
|
Aboutalebi A, Korman JB, Sohani AR,
Hasserjian RP, Louissaint A Jr, Le L, Kraft S, Duncan LM and
Nazarian RM: Aleukemic cutaneous myeloid sarcoma. J Cutan Pathol.
40:996–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vishnu P, Chuda RR, Hwang DG and Aboulafia
DM: Isolated granulocytic sarcoma of the nasopharynx: A case report
and review of the literature. Int Med Case Rep J. 7:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YF, Li Q, Xu WG, Xiao JY, Pang QS,
Yang Q and Zhang YZ: Rare myeloid sarcoma/acute myeloid leukemia
with adrenal mass after allogeneic mobilization peripheral blood
stem cell transplantation. Cancer Biol Med. 10:232–235.
2013.PubMed/NCBI
|
|
5
|
Nafil H, Tazi I and Mahmal L: Myeloid
sarcoma developing in prexisting hydroxyurea-induced leg ulcer in a
polycythemia vera patient. Case Rep Med. 2013:4975932013.PubMed/NCBI
|
|
6
|
Byrd JC and Weiss RB: Recurrent
granulocytic sarcoma. An unusual variation of acute myelogenous
leukemia associated with 8;21 chromosomal translocation and blast
expression of the neural cell adhesion molecule. Cancer.
73:2107–2112. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He J, Zhu L, Ye X, Li L, Zhu J, Zhang J,
Xie W, Shi J, Zheng W, Wei G, et al: Clinical characteristics and
prognosis of nonleukemic myeloid sarcoma. Am J Med Sci.
347:434–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brähler S, Thielen I, Schwabe H, Engels M,
Kreuzer KA, Wolf J and Ansén S: Rapid remineralization of multiple
disseminated bone lesions after high-dose cytarabine in a patient
with isolated myeloid sarcoma. Eur J Haematol. 92:537–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohli S, Lee M and Marshall S: A case
report on the progression of myeloid sarcoma to form multiple
metastatic deposits without developing acute myeloid leukaemia.
Case Rep Hematol. 2015:1621542015.PubMed/NCBI
|
|
10
|
Pileri SA, Ascani S, Cox MC, Campidelli C,
Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero
D, et al: Myeloid sarcoma: clinico-pathologic, phenotypic and
cytogenetic analysis of 92 adult patients. Leukemia. 21:340–350.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Audouin J, Comperat E, Le Tourneau A,
Camilleri-Broët S, Adida C, Molina T and Diebold J: Myeloid
sarcoma: Clinical and morphologic criteria useful for diagnosis.
Int J Surg Pathol. 11:271–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho T, Sedarat F, Rao N and Pullarkat ST:
Diagnostic confusion resulting from CD56 expression by cutaneous
myeloid sarcoma. Rare Tumors. 1:e512009.PubMed/NCBI
|
|
13
|
Yilmaz AF, Saydam G, Sahin F and Baran Y:
Granulocytic sarcoma: A systematic review. Am J Blood Res.
3:265–270. 2013.PubMed/NCBI
|
|
14
|
Amador-Ortiz C, Hurley MY, Ghahramani GK,
Frisch S, Klco JM, Lind AC, Nguyen TT, Hassan A, Kreisel FH and
Frater JL: Use of classic and novel immunohistochemical markers in
the diagnosis of cutaneous myeloid sarcoma. J Cutan Pathol.
38:945–953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vachhani P and Bose P: Isolated gastric
myeloid sarcoma: A case report and review of the literature. Case
Rep Hematol. 2014:5418072014.PubMed/NCBI
|
|
16
|
Bain EE, Rothman I and Lin L: De novo
myeloid sarcoma in a 4-month-old infant: A case report and review
of the literature. J Cutan Pathol. 40:321–325. 2013.PubMed/NCBI
|
|
17
|
Zhou J, Bell D and Medeiros LJ: Myeloid
sarcoma of the head and neck region. Arch Pathol Lab Med.
137:1560–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elyamany G, Khan M, El Hag I, El-Zimaity
M, Albalawi M and Al Abdulaaly A: Generalized lymphadenopathy as
the first presentation of granulocytic sarcoma: A diagnostic
challenge. Case Rep Med. 2013:4832912013.PubMed/NCBI
|
|
19
|
Raphael J, Valent A, Hanna C, Auger N,
Casiraghi O, Ribrag V, De Botton S and Saada V: Myeloid sarcoma of
the nasopharynx mimicking an aggressive lymphoma. Head Neck Pathol.
8:234–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Craig JW and Lin RJ: Paraneoplastic
autoimmunity associated with testicular myeloid sarcoma and chronic
myelomonocytic leukemia. Case Rep Hematol.
2013:6565432013.PubMed/NCBI
|
|
21
|
Chimenti S, Fink-Puches R, Peris K,
Pescarmona E, Pütz B, Kerl H and Cerroni L: Cutaneous involvement
in lymphoblastic lymphoma. J Cutan Pathol. 26:379–385. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brunning RD, Borowitz M, Matutes E, et al:
Precursor B-cell and T-cell neoplasms. In: WHO Classification of
Tumours. Pathology and Genetics of Tumours of Haematopoietic and
Lymphoid Tissues. Jaffe ES, Harris NL, Stein H and Vardiman JW:
3:IARC Press. (Lyon). 109–117. 2001.
|
|
23
|
Maitra A, McKenna RW, Weinberg AG,
Schneider NR and Kroft SH: Precursor B-cell lymphoblastic lymphoma.
A study of nine cases lacking blood and bone marrow involvement and
review of the literature. Am J Clin Pathol. 115:868–875. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y and Wang E: Blastic plasmacytoid
dendritic cell neoplasm: A clinicopathologic review. Arch Pathol
Lab Med. 138:564–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng Q, Yuan Y, Li P and Chen T:
Spontaneous remission in patients with acute myeloid leukemia with
t(8;21) or cutaneous myeloid sarcoma: Two case reports and a review
of the literature. Intern Med. 52:1227–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|