Introduction
Hepatocellular carcinoma (HCC) is the fifth most
commonly observed malignant tumor globally, and the third leading
cause of cancer-associated mortality (1–3). Surgery,
chemotherapy, radiotherapy, immunotherapy and liver transplantation
are typically used to treat patients with HCC (4). However, the 5-year age-standardized
relative survival rates for HCC have not improved significantly
over the past 10 years (5). Knowledge
of the molecular mechanisms of HCC brings opportunities for
therapeutic interventions against this type of cancer using novel
approaches.
Suicide gene therapy, based on gene-directed enzyme
prodrug therapy, is a promising alternative treatment to
conventional chemotherapy. The suicide gene system makes use of an
enzyme, which converts a non-toxic prodrug to its cytotoxic form
(6). The combinations of the enzyme
cytosine deaminase (CD) with the prodrug 5-fluorocytosine (5-FC)
and of the enzyme thymidine kinase (TK) with the prodrug
ganciclovir (GCV) are two of the most common suicide gene therapy
systems (7,8). In each case, the prodrug (5-FC or GCV)
is non-toxic to normal human cells when administered on its own,
but its enzyme (CD or TK, respectively) metabolizes the prodrug
into a monophosphate form that subsequently acts as a
chemotherapeutic agent against cancer cells (9). However, the current low efficiency of
suicide gene systems limits their application (10). A number of studies have attempted to
enhance the therapeutic effect of suicide gene therapy by combining
it with other gene therapies (11,12).
Vascular growth has a significant role in tumor
development and metastasis (13).
Anti-angiogenic therapy has been demonstrated to be safe and
effective for the treatment of solid tumors (14). Vascular endothelial growth factor
(VEGF), a critical proangiogenic regulator, is overexpressed in
HCC, whereas it has low levels of expression in normal liver
tissues (15–17). Thus, VEGF is an ideal target for HCC
treatment (18).
In the present study, a combination suicide gene
system using both CD and TK was utilized, along with a VEGF
promoter (VEGFp), and this was delivered into HCC cells, followed
by assessment of the in vitro and in vivo effects.
The present study provides an experimental basis for additional
application of double suicide gene therapy strategies.
Materials and methods
Cell lines
Human HCC cell lines (BEL-7402 and HepG2) and human
embryonic kidney-293 (HEK-293) cells were obtained from the Animal
Experimental Center of Sun Yet-Sen University (Guangzhou, China).
Human umbilical vein vascular endothelial cells (HUVEC) were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Cells were cultivated in Dulbecco's modified Eagle's medium with
10% fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). All cells were maintained at 37°C in a
humidified atmosphere containing 5% CO2.
Construction of the recombinant
plasmids
A 1.3 kb gene fragment encoding the CD gene and a
1.1 kb fragment encoding the TK gene were amplified by polymerase
chain reaction (PCR) from Escherichia coli JM109 DNA and
pREP8-TK, respectively (kindly provided by Cell biology department
of Southern medical University, Guangzhou, China). These two DNA
fragments were then inserted into the plasmid pMD18-T (catalog no.,
D101A; Takara Bio, Inc., Otsu, Japan) to generate pMD18-CD and
pMD18-TK. Following confirmation by sequencing (Beijing Genomics
Institution, Beijing, China), the CD and TK fragments were digested
and inserted into pcDNA3 (provided by Department of Cell Biology,
Southern medical University) to build the plasmid pcDNA3-CDglyTK.
pcDNA3-CDglyTK was then cut by the restriction enzymes
HindIII and PvuII (New England BioLabs, Inc.,
Ipswich, MA, USA) to generate CDglyTK-pA. The human VEGFp region
was cut from pEGFP-1-SV-VEGFp (kindly provided by Professor Jiro
Kishimoto, Shiseido Research Center, Yokohama, Japan) and amplified
by PCR using the 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The cycling conditions
were as follows: 98°C for 5 min, followed by 28 cycles at 94°C for
40 sec, 50°C for 40 sec, 72°C for 1 min and 72°C for 10 min.
Subsequently, VEGFp was ligated to pAdtrack (Shanghai GenePharma
Co., Ltd., Shanghai, China) to construct pAdtrack-VEGFp. Finally,
CDglyTK-pA was added to generate pAdtrack-VEGFp-CDglyTK
(Ad-VEGFp-CDglyTK). PCR primers are listed in Table I.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
| Gene | Size, bp | Primers |
|---|
| CD | 1,300 | Forward
5′-GGGAAGCTTAGGCTAGCAATGTCGAATAACGCT-3′ |
|
|
| Reverse
5′-GGGGGATCCCTCCACGTTTGTAATCGATGGCTTC-3′ |
| TK | 1,100 | Forward
5′-GGGGGATCCGGCGGGGGCGGTGGAGGAGGGGGTATGGCTTCGTAC-3′ |
|
|
| Reverse
5′-GGGTCTAGATTAGTTAGCCTCCCCCATCTC-3′ |
| VEGFp | 572 | Forward
5′-TCACCGCCTCGGCTTGTCACATCT-3′ |
|
|
| Reverse
5′-ATGAACTTTCTGCTGTCTTGGGTG-3′ |
Amplification and identification of
recombinant adenovirus
The vector (Ad-VEGFp-CDglyTK) was transfected into
HEK-293 cells and purified using cesium chloride gradient
ultracentrifugation at 10,000 × g. Viral production and
amplification were monitored with the aid of green fluorescent
protein (GFP), and the titer of the purified recombinant
adenoviruses was as high as 2.0×1012 pfu/ml. The
recombinant adenovirus was verified by PCR.
Transfection efficiency
To measure the efficiency of transfection with
Ad-VEGFp-CDglyTK, HUVEC and the human HCC cell lines BEL-7402 and
HepG2 (the latter is deficient in VEGF) were used. Cells were
plated into six-well culture plates (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) at a density of 2×105 cells/well, and
transfected with Ad-VEGFp-CDglyTK using various multiplicities of
infection (MOI; 0, 10, 20, 50, 100, and 200 pfu/cell). Fluorescence
microscopy (GFM600 microscope, Leica Microsystems GmbH, Wetzlar,
Germany) was used to assess GFP expression following 24 and 48 h of
incubation at 37°C.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using
TRIzol® reagent according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.) and digested with
RNase-free DNase (Promega Corporation, Madison, WI, USA) to clear
residual genomic DNA. The reverse transcription reaction was
performed using the RevertAid™ First-Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) in a final volume of 20
µl containing 2 µg total RNA, 1 µg oligo (dT) 18primer, 10 mmol/l
deoxynucleotidetriphosphate mixture, 4 µl 5X reverse transcription
buffer, 20 units RiboLock™ Ribonuclease inhibitor,
diethylpyrocarbonate-treated water and 200 units RevertAid™ M-Mulv
reverse transcriptase. Following incubation at 42°C for 60 min, the
reverse transcription reaction was terminated by heating at 70°C
for 10 min. The reaction contained 2 µl cDNA template. The cDNA was
amplified by PCR using the 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The 25 µl reaction
mixture contained 2.5 units of Taq polymerase (Promega
Corporation), 1 µl of cDNA template (Promega Corporation), 1.5
mmol/l MgCl2 (Promega Corporation) and 0.5 µmol/l CDglyTK primers.
The CDglyTK primers were synthesized by Beijing Genomics
Institution (forward, 5′-GGGAAGCTTAGGCTAGCAATGTCGAATAACGCT-3′ and
reverse, 5′-GGGTCTAGATTAGTTAGCCTCCCCCATCTC-3′; generating a DNA
fragment of 2,400 bp). Expression was normalized to the
glyceraldehyde-3-phosphate dehydrogenase gene (forward,
5′-CTCAGACACCATGGGGAAGGTGA-3′ and reverse,
5′-ATGACTTGAGGCTGTTGTCATA-3′; generating a DNA fragment of 450 bp;
Beijing Genomics Institute, Beijing, China). The reaction
conditions were as follows: Preliminary denaturation at 94°C (5
min), followed by 30 cycles of denaturation at 94°C (40 sec),
annealing at 58°C (60 sec) and extension at 72°C (90 sec), with a
final extension step at 72°C for 10 min. The PCR products were run
on a 1.5% agarose gel, and examined on a CX2000 UV illuminator (UVP
Inc., Upland, CA, USA) and photographed using a Canon EOS 60D
camera (Canon, Inc., Tokyo, Japan). This experiment was performed
three times.
In vitro study
Cell proliferation assay
To investigate the biological effect induced by
suicide gene systems, cytotoxicity (the effect on cell viability)
was assessed using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Sigma-Aldrich, St. Louis, MO, USA] assay. HUVEC, BEL-7402, and
HepG2 cells were seeded into 96-well plates (Guangzhou RiboBio Co.,
Ltd.) at a density of 5×103 cells/well for 24 h.
Following incubation at 37°C for 24 h, the culture medium was
removed, and cells were infected with Ad-VEGFp-CDglyTK (100
pfu/cell) and incubated for 24 h at 37°C. Uninfected cells
incubated at 37°C for the same duration served as a control.
Following incubation, the medium was replaced with various
concentrations of GCV (0, 5, 10, 50, 100, or 200 µg/ml; Roche
Diagnostics GmbH, Mannheim, Germany) or 5-FC (0, 20, 40, 60, 80, or
100 µg/ml; Sigma-Aldrich), or a combination of the two. The cells
were subsequently cultured for 48 h and incubated with 10 µl MTT
(10 mg/ml; Sigma-Aldrich). The medium was removed and the remaining
purple-blue sediment was dissolved in 150 µl dimethyl sulfoxide
(Sigma-Aldrich) for 10 min. The relative optical density (OD) of
each well was determined at the test wavelength (490 nm) using a
Bio-Rad 2550 EIA Reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Viability of the cells was calculated using the following
equation: Cell survival rate (%) = (OD value of experimental group
/ OD value of control group) × 100%.
Bystander effect assay
Cells infected with recombinant virus were mixed
with uninfected cells in various ratios (5:95, 10:90, 20:80, 40:60,
60:40, and 80:20) and seeded into 96-well plates at
1×104 cells/well. Cells were treated with GCV (100 mg/l)
and 5-FC (80 mg/l) together for 48 h and analyzed by MTT assay, as
described above.
Flow cytometry analysis
To determine the effect of recombinant viruses on
apoptosis, Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA) were
used for flow cytometry (FCM) analysis to assess apoptosis. Cells
infected with recombinant virus and uninfected cells were treated
with GCV (100 mg/l), 5-FC (80 mg/l) or a combination of the two for
24 h. The harvested cells were washed twice with phosphate-buffered
saline (PBS), and adjusted to a concentration of 5×105
cells/ml by dilution with PBS. The cell suspension (200 µl) was
added to each labeled tube, followed by 5 µl of Annexin V-FITC and
10 µl PI. The tube was incubated for at least 10 min at room
temperature in the dark. Cells were assayed using a FACSCalibur™
Flow Cytometer (BD Biosciences). Apoptotic cells were defined as
the population that were Annexin V-FITC positive and
PI-negative.
In vivo study
Tumor cell xenograft
Animal experiments were approved by the
Institutional Animal Care and Use Committee of Southern Medical
University (Guangzhou, China). In total, 20 male nude mice (4–6
weeks of age, 18–20 g) were purchased from the Laboratory Animal
Center of Sun Yet-Sen University. The mice were kept in a
specific-pathogen-free room at 26–28°C in a 10 h light and 14 dark
cycle, and had free access to food and water. The mice were
randomly divided into four groups with 5 mice in each group. The
BEL-7402 cell suspension (either infected with Ad-VEGFp-CDglyTK or
uninfected) was injected subcutaneously (0.5×107 cells
in 200 µl serum-containing medium). Mice were observed daily to
ensure that the injection site was healthy. GCV (200 mg/kg/day) and
5-FC (100 mg/kg/day) were injected into the tumor daily for 10
days. At 1 week subsequent to injection, tissue samples and other
biological data (tumor weight, and long and short diameter) were
collected. The tumor volume was calculated using the following
formula: Volume=(axb2)/2 mm3 (a, longer
diameter of the tumor; b, shorter diameter of the tumor) (19). The rate of inhibition of tumor growth
was subsequently calculated as the tumor inhibition rate as
follows: (1-tumor weight of treatment group/tumor weight of control
group)x100%.
Microvessel density assay
To assess tumor angiogenesis, cluster of
differentiation 34 antibody was used to determine microvessel
density (MVD). Tumor tissue was collected and fixed in 10%
formaldehyde (Shanghai Chemical Reagent Co., Ltd., Shanghai, China)
for 24 h. The tumor tissue was embedded in paraffin and 4-µm
sections were cut using the RM2245 microtome (Leica Microsystems
GmbH). To block endogenous peroxidase activity, 3% H2O2 (Shanghai
Chemical Reagent Co., Ltd.) was added for 10 min. Following antigen
retrieval with sodium citrate (0.01 mmol/l, pH 6.0; Shanghai
Chemical Reagent Co., Ltd.) for 10 min, rabbit anti-human cluster
of differentiation 34 monoclonal antibody (catalog no., sc-19621;
1:150 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was incubated with samples overnight at 4°C, followed by incubation
with the secondary biotin-labeled goat anti-mouse immunoglobulin G
antibody (1:500 dilution; catalog no., A0286; Beyotime Institute of
Biotechnology, Haimen, China) at 37°C for 30 min. Following
staining with 3,3′-diaminobenzidine (OriGene Technologies, Inc.,
Beijing, China) and hematoxylin (Shanghai Chemical Reagent Co.),
MVD was assessed under a microscope (TS100; Nikon Corporation,
Tokyo, Japan), as described previously by Weidner et al
(20). Under ×100 magnification, five
areas were identified with the highest vascular density (‘hot
spots’), and the number of vessels in each of these regions was
counted under ×200 magnification. Microvessels were counted by two
independent observers, and the mean value was used for
analysis.
Statistical analysis
Each assay was performed at least three times, and
data are presented as the mean ± standard deviation. Analysis of
variance was used to determine the significance of differences in
multiple comparisons. All data were analyzed with SPSS (version
13.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
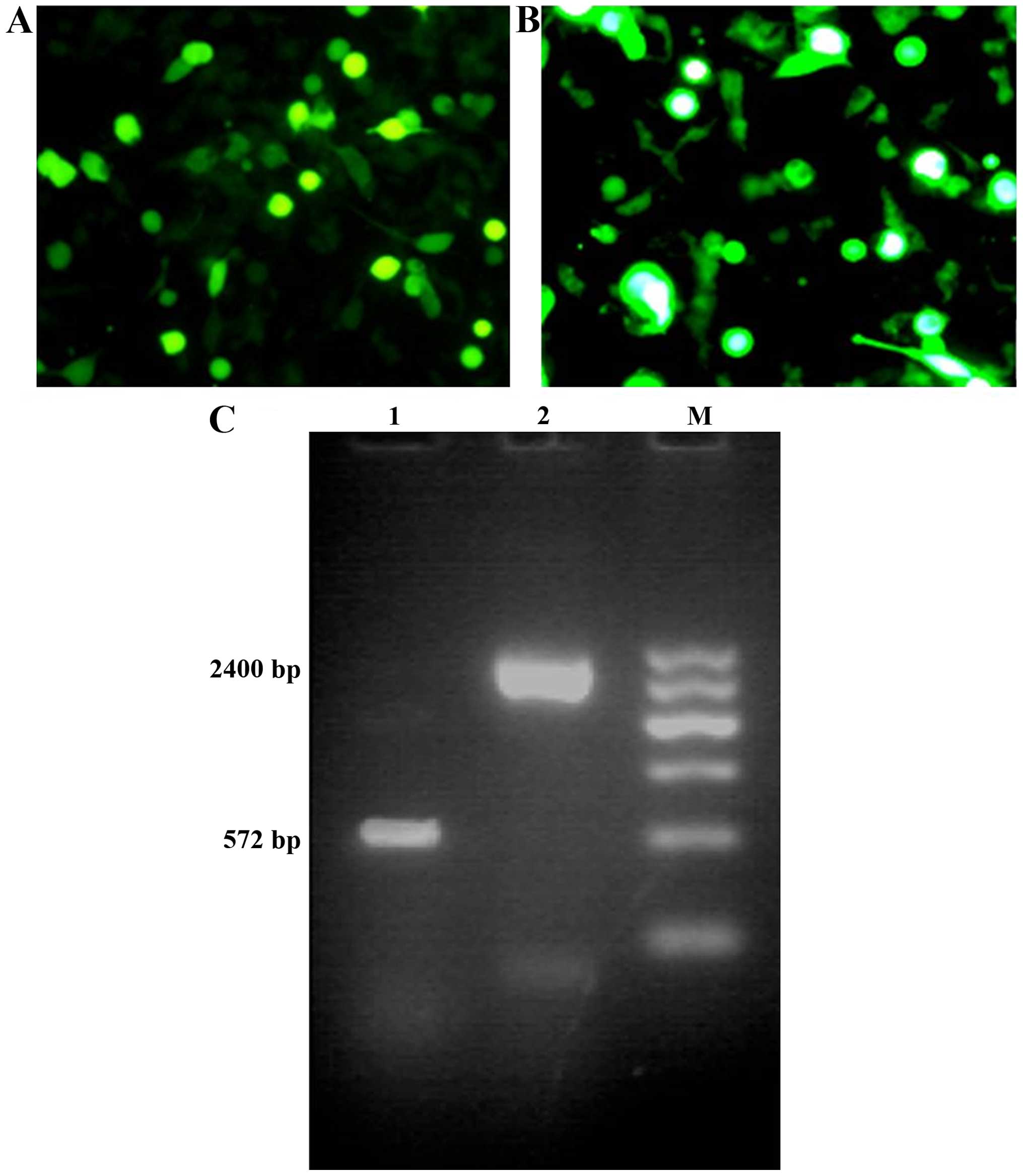
Recombinant viruses
At 24 h following transfection of recombinant virus
plasmids into HEK-293 cells, a stronger GFP fluorescence signal was
observed in the cells transfected with Ad-VEGFp-CDglyTK, while the
majority of the remaining HEK-293 cells expressed GFP protein at 48
h post-transfection. The recombinant adenoviruses were confirmed by
PCR and DNA electrophoresis, producing bands of 572 bp (same size
as VEGF) and 2,400 bp (same size as the CDglyTK gene fragment;
Fig. 1).
Transfection efficiency of
Ad-VEGFp-CDglyTK in various cell lines
To determine the infection efficiency of the
adenoviral vector, the BEL-7402, HUVEC and HepG2 cells were
infected with the Ad-VEGFp-CDglyTK at a MOI ranging from 10–200
pfu/cell. At a MOI of 10 pfu/cell, only a small number of cells
expressed GFP, whereas at a MOI of 100 pfu/cell, >95% of cells
were GFP-positive without demonstrating any marked adenoviral
toxicity (Fig. 2A). To investigate
expression of the CDglyTK gene in the infected cells, RNAs were
analyzed by RT-PCR. It was observed that the CDglyTK fusion gene
was expressed in the BEL-7402 cells and HUVEC, but not in HepG2
cells (Fig. 2B).
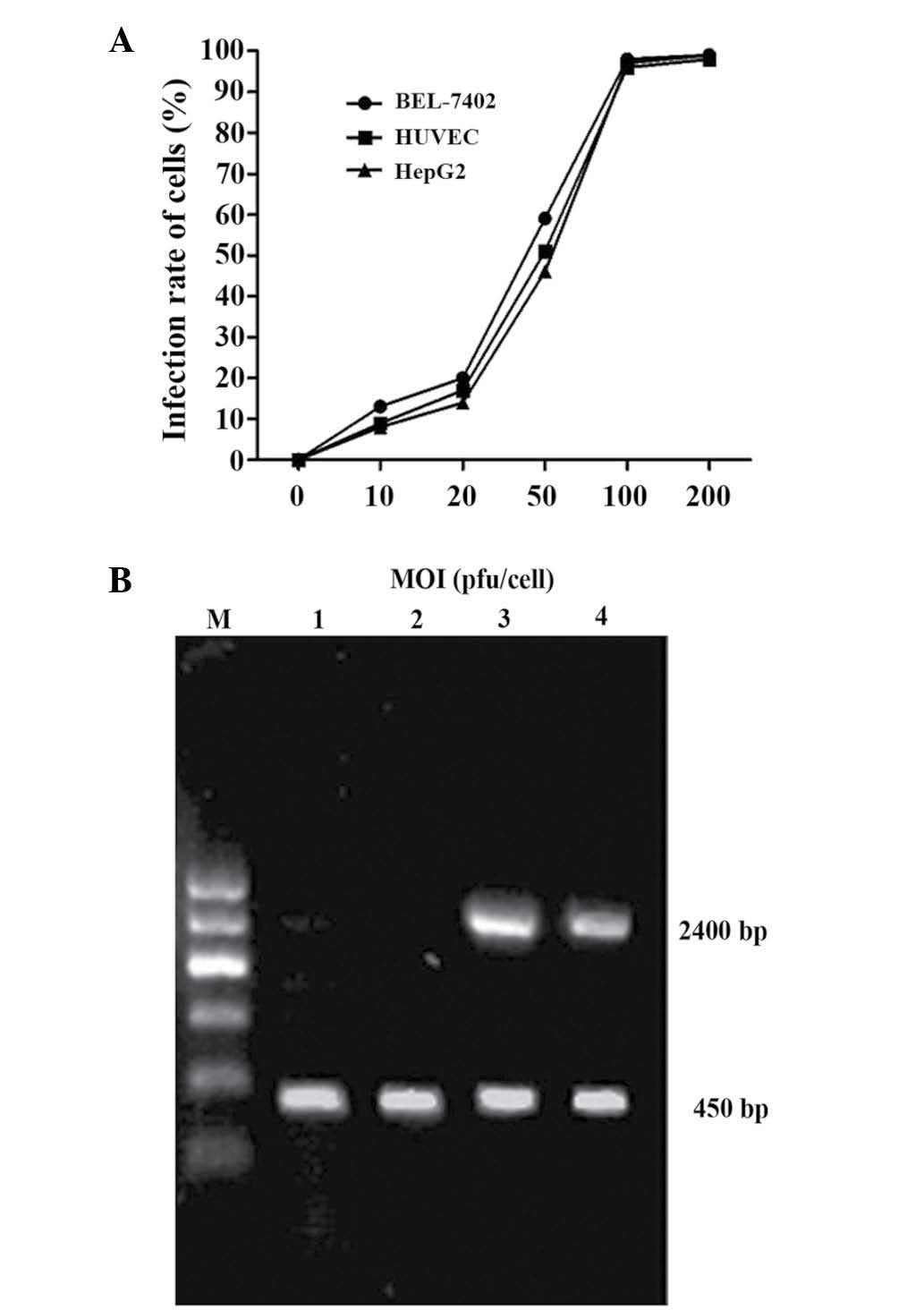 | Figure 2.Recombinant adenovirus transfer
efficiency and CDglyTK production in various cell lines. (A)
Transfer efficiencies of recombinant adenoviruses in various cell
lines. When the MOI was 100 pfu/cell, the transfer efficiency was
95% in all three cell lines. (B) Reverse transcription-polymerase
chain reaction analysis of the expression of CDglyTK lane (DNA
fragment of 2400 bp). Glyceraldehyde-3-phosphate dehydrogenase was
used as an internal control (DNA fragment of 450 bp). Lane M,
Marker; Lane 1, blank control group; Lane 2, no fragments of
CDglyTK gene detected in HepG2 cells; Lane 3, CDglyTK gene detected
in BEL-7402 cells; Lane 4, CDglyTK gene detected in HUVEC. MOI,
multiplicity of infection; CD, cytosine deaminase; TK, thymidine
kinase; HUVEC, human umbilical vein vascular endothelial cells. |
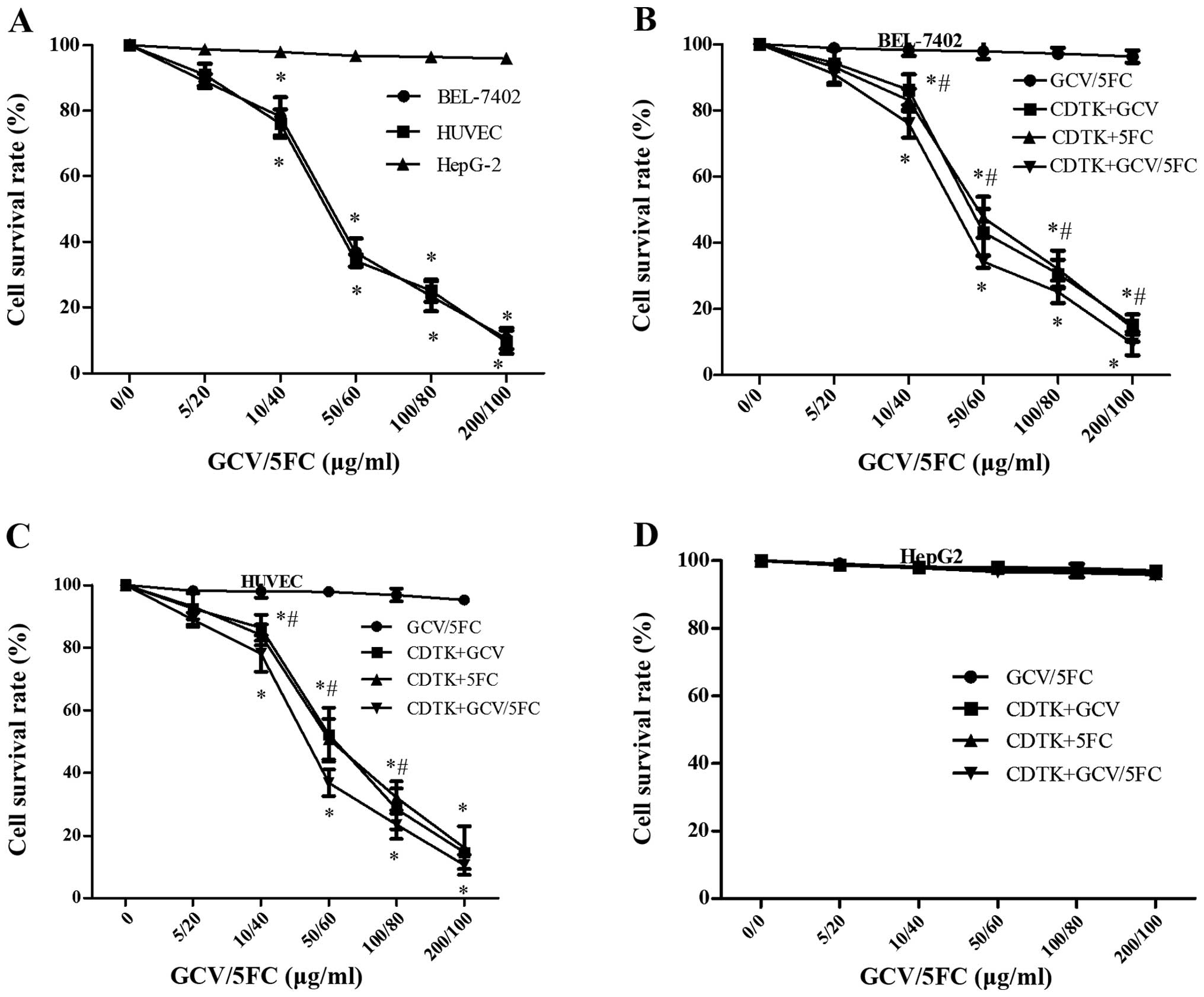
Cytotoxicity analysis of the
Ad-VEGFp-CDglyTK on transfected cells in vitro
To investigate the biological effect induced by
Ad-VEGFp-CDglyTK, cytotoxicity was assessed in HUVEC, BEL-7402, and
HepG2 cells infected with Ad-VEGFp-CDglyTK and treated with GCV,
5-FC or GCV+5-FC. As shown in Fig.
3A, HUVEC and BEL-7402 cells were highly sensitive to the
prodrugs, but HepG2 cells were not sensitive to the prodrugs
(P=0.0006). Cell survival rates significantly decreased in line
with increasing concentrations of the prodrugs in the GCV+5-FC
group and in the individual prodrug treatment groups (P=0.027). The
sensitivity of CDglyTK-transfected cells to GCV+5-FC was greater
compared with their sensitivity to GCV or 5-FC alone in the
BEL-7402 and HUVEC groups (P=0.013; Fig.
3B and C); however, there was no significant difference in the
sensitivity of HepG2 cells to either of the single drugs or to the
combination treatment (P=0.923; Fig.
3D).
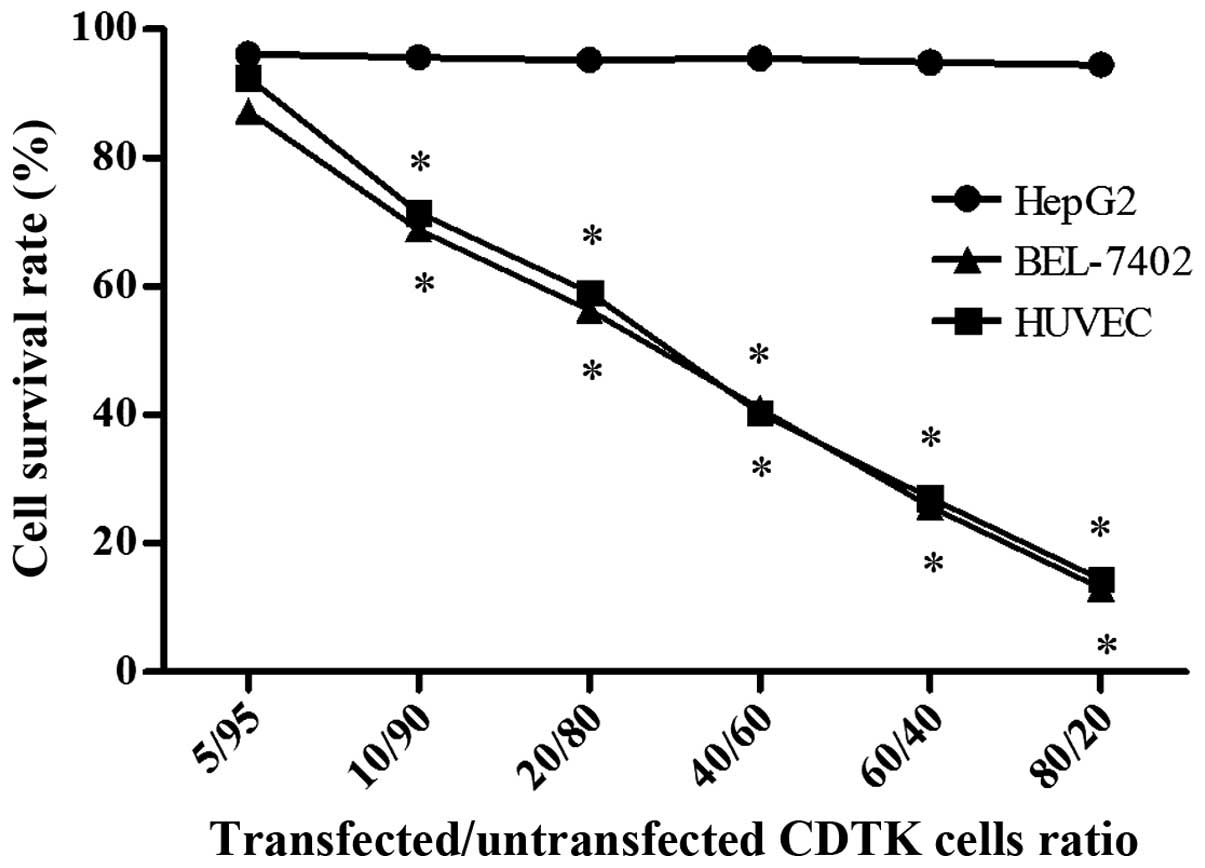
Bystander effect of
Ad-VEGFp-CDglyTK
The bystander effect of the CDglyTK gene was
assessed by mixing transfected and untransfected cells in various
ratios. In the BEL-7402 and HUVEC groups, the survival rate
decreased significantly as the proportion of
Ad-VEGFp-CDglyTK-transfected cells increased (P=0.041; Fig. 4); however, the HepG2 cells did not
exhibit this phenomenon (P=0.718). In addition, cell survival rates
were all markedly lower in the transfected cells compared with the
untransfected cells for HUVEC and BEL-7402 cells, but not for HepG2
cells. The cell survival rate was ~81% at a ratio of 95%
untransfected to 5% transfected BEL-7402 cells, but when the
proportion of untransfected cells was reduced to 90%, the cell
viability fell to 69%. These results indicate that GCV and 5-FC are
able to kill transfected cells, but are additionally able to kill
untransfected cells via a bystander effect.
Flow cytometric analysis
To additionally analyze the effect of
Ad-VEGFp-CDglyTK on tumor cells, cell apoptosis was assessed by
FCM. As demonstrated in Fig. 5,
apoptosis rates were significantly increased (P<0.0001) in the
Ad-VEGFp-CDglyTK-transfected BEL-7402 cells and HUVEC compared with
the HepG2 cells. The apoptosis rate was additionally higher in the
recombinant virus-transfected group compared with the untransfected
groups for the BEL-7402 cells and HUVEC (P=0.012), but not for the
HepG2 cells (P=0.872). The combination of GCV+5-FC exerted a
stronger effect than either drug alone (P=0.023; Fig. 5D).
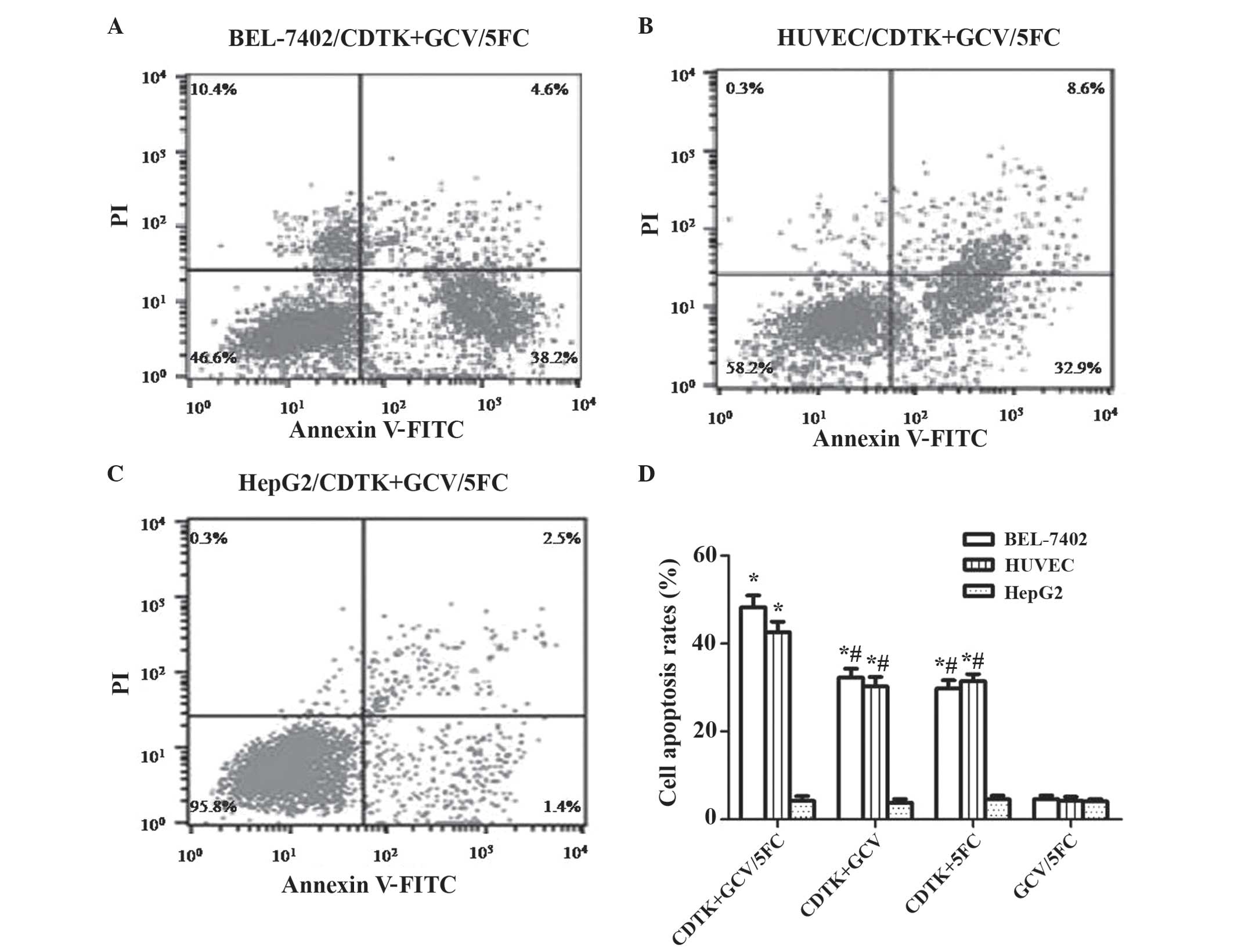 | Figure 5.Flow cytometric analysis of the
effect of GCV combined with 5-FC on cell apoptosis. Cell lines
transfected with Ad-VEGFp-CDglyTK; (A) BEL-7402; (B) HUVEC and (C)
HepG2 cells. (D) Cell apoptosis rate induced by the prodrugs GCV
and/or 5-FC in the cell lines BEL-7402, HUVEC and HepG2. *P<0.05
compared with the untransfected group; #P<0.05 GCV or
5-FC alone compared with GCV+5-FC. GCV, gancivlovir; 5-FC,
5-fluorocytosine; VEGFp, vascular endothelial growth factor
promotor; CD, cytosine deaminase; TK, thymidine kinase; HUVEC,
human umbilical vein vascular endothelial data; PI, propidium
iodide; FITC, fluorescein isothiocyanate; Ad, adenovirus. |
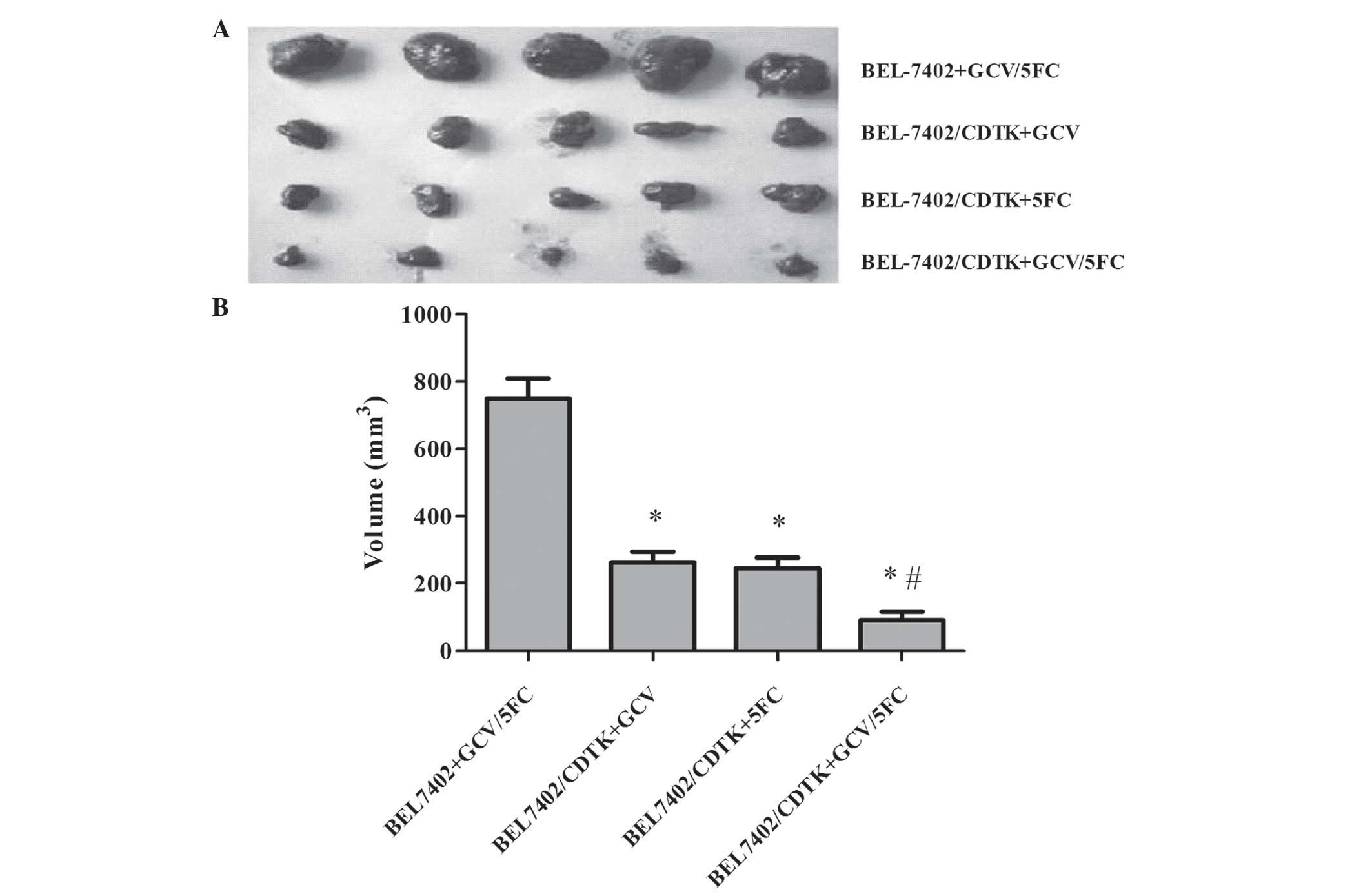
Anti-tumor effect of Ad-VEGFp-CDglyTK
in vivo
Based on the results of cytotoxicity and FCM
analysis, BEL-7402 cells, with or without the Ad-VEGFp-CDglyTK
system, were injected subcutaneously into nude mice. As
demonstrated in Fig. 6, the tumor
cells transfected with recombinant virus formed smaller tumors
compared with the untransfected cells, and the volumes of the
tumors were significantly smaller for the transfected group
compared with the untransfected group (P=0.0003). The inhibition
rate was increased for the Ad-VEGFp-CDglyTK cells treated with
GCV+5-FC group compared with the untransfected group (P=0.003).
Therefore, combination of the recombinant virus with the prodrugs
(GCV, 5-FC or GCV+5-FC) was able to suppress the growth of HCC
cells significantly in vivo. The GCV+5-FC combination
exerted a stronger tumor-suppressor effect than either drug alone
(P<0.001). It is notable that this difference was more marked in
the in vivo experiment compared with the in vitro
studies.
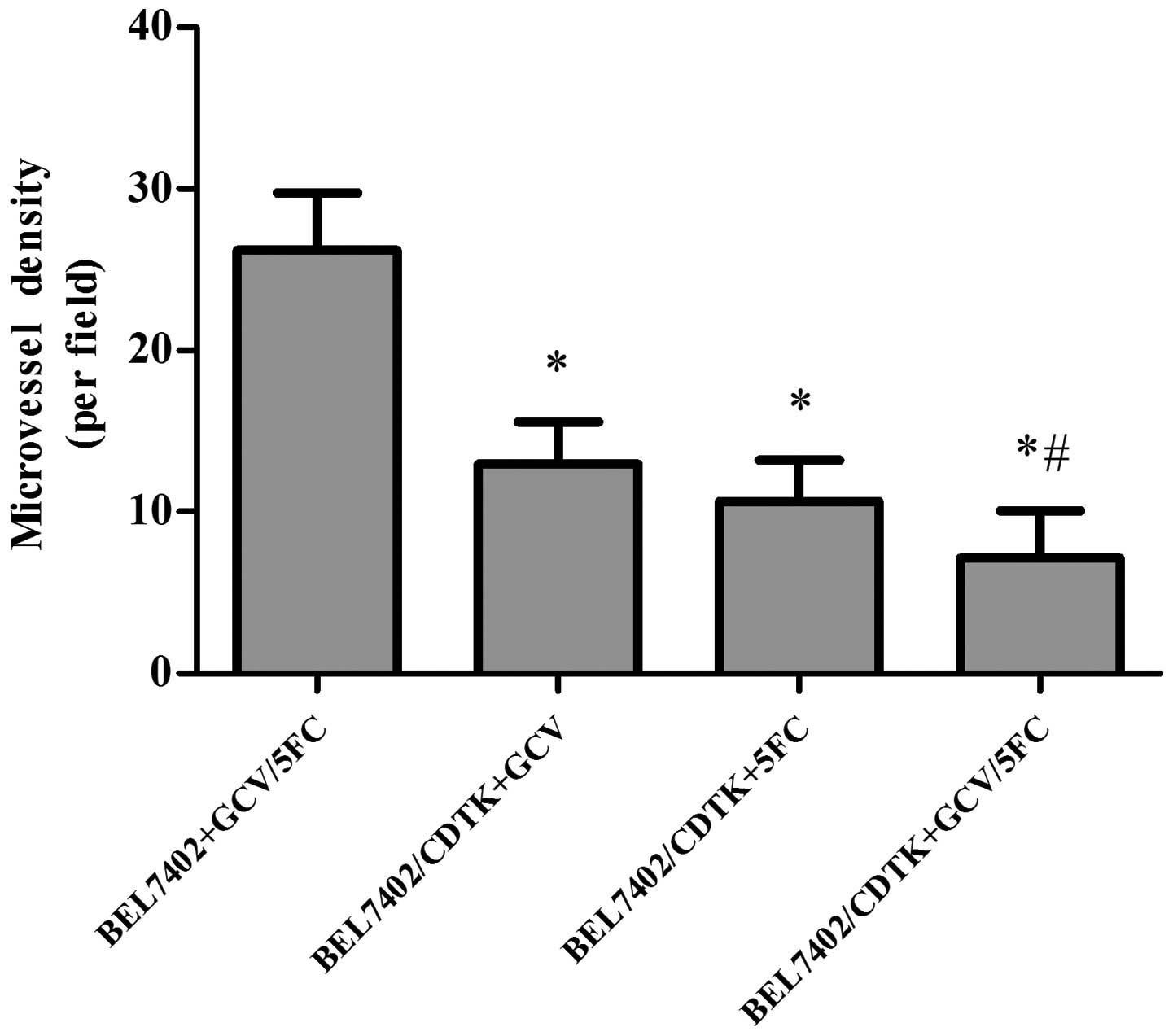
Effect of Ad-VEGFp-CDglyTK on MVD of
HCC in nude mice
The MVD of the tumor tissue was assessed by cluster
of differentiation 34 immunohistochemistry. The MVD of the tumor
tissue was decreased more significantly (P=0.0008) in the group
transfected with Ad-VEGFp-CDglyTK and treated with prodrugs (GCV,
5-FC, or GCV+5-FC) compared with the control group (Fig. 7). The MVD was slightly but
significantly (P=0.012) lower in the GCV+5-FC group compared with
the groups treated with either drug alone.
Discussion
Chemotherapy is an established and successful
therapy for retarding the growth of a variety of tumor types
(4). However, the majority of
chemotherapy drugs are not cancer-specific and may cause off-target
organ toxicity (21). Suicide gene
therapy, using enzymes that are not toxic to healthy tissues but
that produce highly toxic metabolites from a much less toxic
prodrug, is a safe and efficient therapeutic option for cancer
(22). As reported in several
studies, TK/GCV or CD/5-FC are the longest established of these
suicide gene therapy systems (23–25).
Single suicide gene systems (using one prodrug and one enzyme) are
able to inhibit tumor development, but a number of studies have
demonstrated that TK/GCV combined with CD/5-FC has a higher
efficacy for the treatment of solid tumors compared with single
suicide gene systems (26,27). However, the suicide gene systems
investigated in the present study (TK/GCV and CD/5-FC) have
demonstrated little efficacy in clinical practice, due to their low
targeting and poor gene-transfer efficiencies in tumors (28). Therefore, a gene that is able to
improve the targeting and gene-transfer efficiencies of these
systems is urgently required.
Angiogenesis, the formation of new blood vessels
from existing vasculature, is an important process in numerous
malignancies, including HCC (29).
VEGF is a critical proangiogenic factor that has a significant role
in the invasion and metastasis of HCC (30). A number of studies have verified that
VEGF expression levels are increased in vascular endothelial cells
of HCC compared with those of normal tissues (31,32). A
meta-analysis additionally revealed that the serum VEGF level is
associated with prognosis for patients with HCC (33). Inhibiting angiogenesis by using
anti-VEGF has been proposed as a potential anticancer strategy
(34). Therefore, the VEGF promoter
has been utilized to express target genes in HCC due to its
tumor-specific expression (32). In
the current study, the results demonstrated that VEGFp was able to
direct the CDglyTK gene in VEGF-high expressing cells
(BEL-7402 and HUVEC), but the transgenic CDglyTK double
suicide genes were not expressed in HepG2 cells owing a deficiency
in VEGF. Therefore, this suggests that when the VEGFp-driven
suicide gene is systemically administered, the systemic toxicity to
normal cells may be significantly reduced compared with that of
traditional chemotherapy.
In the present cytotoxicity experiment, BEL-7402
cells and HUVEC with high levels of CDglyTK expression were highly
sensitive to the prodrugs used (GCV, 5-FC and a combination of the
two). These prodrugs significantly decreased the survival rate of
BEL-7402 cells and HUVEC, and the cytotoxic effect increased in
line with increasing drug dose. However, such inhibition was not
observed in HepG2 cells, which have low CDglyTK expression.
Previous studies indicated an increased killing efficiency of a
combination suicide gene system compared with any single suicide
gene system, due to the synergetic cytotoxicity of the combined
gene system (6,35). Furthermore, Su et al (8) indicated that the effect of double
suicide genes was much stronger than that of individual suicide
genes in breast cancer cells. Although the present study used
different genes and cancer cells, the experiments of the current
study also revealed that although each single prodrug (GCV or 5-FC)
was able to kill tumor cells in the transgenic CDglyTK BEL-7402
cells and HUVEC, the combination of these two prodrugs exerted a
more powerful killing effect (P<0.05). Thus, combination
treatment may reduce the drug dose required and additionally
decrease the toxic side effects of suicide gene systems on other
organs.
The bystander effect is the primary driving force of
the suicide gene therapy strategy (36,37). The
results of the present study demonstrated that GCV+5-FC was not
only able to kill the transfected cells but was additionally able
to kill neighboring untransfected cells. Therefore, the bystander
effect greatly amplifies the efficacy of suicide gene therapy for
cancer.
Apoptosis is an important biological phenomenon in
tumor treatment. In the present cell apoptosis experiment, 5-FC
and/or GCV treatment significantly decreased cell viability in
HUVEC and BEL-7402 cells, but not in HepG2 cells. Treatment with
5-FC combined with GCV induced a more marked decrease in cell
viability compared with either prodrug alone.
In order to observe the antitumor effect in
vivo, HCC nude mouse models were established using human
BEL-7402 cells. The results of the present study revealed that
compared with the untransfected groups, tumors consisting of
BEL-7402 cells expressing the CDglyTK gene were
significantly suppressed by GCV and/or 5-FC treatment in
vivo.
Angiogenesis is closely correlated with tumor
growth, invasion and metastasis (38). MVD protein, which is released by tumor
and stroma cells, has a significant role in angiogenesis (39). A number of proteins, including cluster
of differentiation 31, cluster of differentiation 34, Factor VIII
and cluster of differentiation 105, are markers of angiogenesis
(40). As cluster of differentiation
34 is more sensitive and specific compared with other markers used
for staining endothelial cells induced by tumor neovascularization
(41), the present study assessed MVD
by the presence of cluster of differentiation 34. The results of
the present study revealed that the MVD of tumors was decreased by
treatment with prodrugs (GCV, 5-FC or GCV+5FC) in the
Ad-VEGFp-CDglyTK-transfected group, but not in the untransfected
group. This result confirmed that the double suicide genes
regulated by VEGFp are able to suppress tumor growth and
angiogenesis in vivo.
In conclusion, the results of the present study
indicate that the VEGFp-mediated double suicide gene system is able
to effectively inhibit human HCC cells and vascular endothelial
cells in vitro and in vivo. Expression of the
CDglyTK gene under the control of the VEGFp may represent a
promising gene therapy approach for the treatment of HCC, aiming to
improve long-term patient survival rates.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Guangdong Province (grant no.,
S2013010015998) and the National High Technology Research and
Development Program (‘863’ Program) of China (grant no.
2001AA217171).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang CH, Wey KC, Mo LR, Chang KK, Lin RC
and Kuo JJ: Current trends and recent advances in diagnosis,
therapy, and prevention of hepatocellular carcinoma. Asian Pac J
Cancer Prev. 16:3595–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhayani NH, Jiang Y, Hamed O, Kimchi ET,
Staveley-O'Carroll KF and Gusani NJ: Advances in the pharmacologic
treatment of hepatocellular carcinoma. Curr Clin Pharmacol.
10:299–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boucher PD, Im MM, Freytag SO and Shewach
DS: A novel mechanism of synergistic cytotoxicity with
5-fluorocytosine and ganciclovir in double suicide gene therapy.
Cancer Res. 66:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fogar P, Greco E, Basso D, Navaglia F,
Plebani M and Pedrazzoli S: Killer genes in pancreatic cancer
therapy. Cell Mol Biol (Noisy-le-grand). 51:61–76. 2005.PubMed/NCBI
|
|
8
|
Su GQ, Su G and Huang ZH:
Adenovirus-mediated tissue-targeted expression of the CDglyTk gene
for the treatment of breast cancer. Mol Med Rep. 6:321–329.
2012.PubMed/NCBI
|
|
9
|
Xu F, Li S, Li XL, Guo Y, Zou BY, Xu R,
Liao H, Zhao HY, Zhang Y, Guan ZZ and Zhang L: Phase I and
biodistribution study of recombinant adenovirus vector-mediated
herpes simplex virus thymidine kinase gene and ganciclovir
administration in patients with head and neck cancer and other
malignant tumors. Cancer Gene Ther. 16:723–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rainov NG: A phase III clinical evaluation
of herpes simplex virus type 1 thymidine kinase and ganciclovir
gene therapy as an adjuvant to surgical resection and radiation in
adults with previously untreated glioblastoma multiforme. Hum Gene
Ther. 11:2389–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong H, Tao L, Qi K, Wang Y, Li Q, Du J
and Huang Z: Thymidine kinase/ganciclovir and cytosine
deaminase/5-fluorocytosine suicide gene therapy-induced cell
apoptosis in breast cancer cells. Oncol Rep. 30:1209–1214.
2013.PubMed/NCBI
|
|
12
|
Qu L, Wang Y, Gong L, Zhu J, Gong R and Si
J: Suicide gene therapy for hepatocellular carcinoma cells by
survivin promoter-driven expression of the herpes simplex virus
thymidine kinase gene. Oncol Rep. 29:1435–1440. 2013.PubMed/NCBI
|
|
13
|
Sia D, Alsinet C, Newell P and Villanueva
A: VEGF signaling in cancer treatment. Curr Pharm Des.
20:2834–2842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertolini F, Shaked Y, Mancuso P and
Kerbel RS: The multifaceted circulating endothelial cell in cancer:
Towards marker and target identification. Nat Rev Cancer.
6:835–845. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suh YG, Lee EJ, Cha H, Yang SH and Seong
J: Prognostic values of vascular endothelial growth factor and
matrix metalloproteinase-2 in hepatocellular carcinoma after
radiotherapy. Dig Dis. 32:725–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bupathi M, Kaseb A and Janku F:
Angiopoietin 2 as a therapeutic target in hepatocellular carcinoma
treatment: Current perspectives. Onco Targets Ther. 7:1927–1932.
2014.PubMed/NCBI
|
|
17
|
Detwiller KY, Fernando NT, Segal NH, Ryeom
SW, D'Amore PA and Yoon SS: Analysis of hypoxia-related gene
expression in sarcomas and effect of hypoxia on RNA interference of
vascular endothelial cell growth factor A. Cancer Res.
65:5881–5889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siemann DW and Shi W: Efficacy of combined
antiangiogenic and vascular disrupting agents in treatment of solid
tumors. Int J Radiat Oncol Biol Phys. 60:1233–1240. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huber BE, Austin EA, Good SS, Knick VC,
Tibbels S and Richards CA: In vivo antitumor activity of
5-fluorocytosine on human colorectal carcinoma cells genetically
modified to express cytosine deaminase. Cancer Res. 53:4619–4626.
1993.PubMed/NCBI
|
|
20
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scripture CD and Figg WD: Drug
interactions in cancer therapy. Nat Rev Cancer. 6:546–558. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zarogoulidis P, Darwiche K, Sakkas A,
Yarmus L, Huang H, Li Q, Freitag L, Zarogoulidis K and Malecki M:
Suicide gene therapy for cancer - current strategies. J Genet Syndr
Gene Ther. 4:2013.PubMed/NCBI
|
|
23
|
Rogulski KR, Wing MS, Paielli DL, Gilbert
JD, Kim JH and Freytag SO: Double suicide gene therapy augments the
antitumor activity of a replication-competent lytic adenovirus
through enhanced cytotoxicity and radiosensitization. Hum Gene
Ther. 11:67–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer U, Steffens S, Frank S, Rainov NG,
Schulze-Osthoff K and Kramm CM: Mechanisms of thymidine
kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide
gene therapy-induced cell death in glioma cells. Oncogene.
24:1231–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu Y, Peng GL, Liu QC, Li FL, Zou XS and
He JX: Selective killing of lung cancer cells using
carcinoembryonic antigen promoter and double suicide genes,
thymidine kinase and cytosine deaminase (pCEA-TK/CD). Cancer Lett.
316:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fillat C, Carrió M, Cascante A and Sangro
B: Suicide gene therapy mediated by the Herpes Simplex virus
thymidine kinase gene/Ganciclovir system: Fifteen years of
application. Curr Gene Ther. 3:13–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang NH, Hwang KA, Yi BR, Lee HJ, Jeung
EB, Kim SU and Choi KC: Human amniotic fluid-derived stem cells
expressing cytosine deaminase and thymidine kinase inhibits the
growth of breast cancer cells in cellular and xenograft mouse
models. Cancer Gene Ther. 19:412–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konopka K, Spain C, Yen A, Overlid N,
Gebremedhin S and Düzgüneş N: Correlation between the levels of
survivin and survivin promoter-driven gene expression in cancer and
non-cancer cells. Cell Mol Biol Lett. 14:70–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan HY, Wang N, Tsao SW, Zhang Z and Feng
Y: Suppression of vascular endothelial growth factor via
inactivation of eukaryotic elongation factor 2 by alkaloids in
Coptidis rhizome in hepatocellular carcinoma. Integr Cancer Ther.
13:425–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li XM, Tang ZY, Zhou G, Lui YK and Ye SL:
Significance of vascular endothelial growth factor mRNA expression
in invasion and metastasis of hepatocellular carcinoma. J Exp Clin
Cancer Res. 17:13–17. 1998.PubMed/NCBI
|
|
31
|
Ranieri G, Ammendola M, Marech I, Laterza
A, Abbate I, Oakley C, Vacca A, Sacco R and Gadaleta CD: Vascular
endothelial growth factor and tryptase changes after
chemoembolization in hepatocarcinoma patients. World J
Gastroenterol. 21:6018–6025. 2015.PubMed/NCBI
|
|
32
|
Suzuki K, Hayashi N, Miyamoto Y, Yamamoto
M, Ohkawa K, Ito Y, Sasaki Y, Yamaguchi Y, Nakase H, Noda K, et al:
Expression of vascular permeability factor/vascular endothelial
growth factor in human hepatocellular carcinoma. Cancer Res.
56:3004–3009. 1996.PubMed/NCBI
|
|
33
|
Zhan P, Qian Q and Yu LK: Serum VEGF level
is associated with the outcome of patients with hepatocellular
carcinoma: A meta-analysis. Hepatobiliary Surg Nutr. 2:209–215.
2013.PubMed/NCBI
|
|
34
|
Cipriani G and Mazzanti R: Treatment with
inhibitors of angiogenesis in advanced hepatocellular carcinoma: A
new tool in our hands or simply a hope? Dig Liver Dis. 37:230–231.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma J, Li M, Mei L, Zhou Q, Liu L, Yu X and
Che G: Double suicide genes driven by kinase domain insert
containing receptor promoter selectively kill human lung cancer
cells. Genet Vaccines Ther. 9:62011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang JH, Wan MX, Yuan JY and Pan BR: Do
there exist synergistic antitumor effects by coexpression of herpes
simplex virus thymidine kinase with cytokine genes on human gastric
cancer cell line SCG7901? World J Gastroenterol. 10:147–151.
2004.PubMed/NCBI
|
|
37
|
Qiang L, Yanping L, Zonghai H, Fei C, Zhou
L and Jinlong Y: Study of the mechanism of bystander effect of
KDR-CDglyTK system mediated by adenovirus for the treatment of
gastric cancer. Cell Biochem Biophys. 67:1021–1027. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang W, Zhao CG, Sun HY, Zheng WE and
Chen H: Expression characteristics of KAI1 and vascular endothelial
growth factor and their diagnostic value for hepatocellular
carcinoma. Gut Liver. 8:536–542. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhai LL, Wu Y, Huang DW and Tang ZG:
Increased matrix metalloproteinase-2 expression and reduced tissue
factor pathway inhibitor-2 expression correlate with angiogenesis
and early postoperative recurrence of pancreatic carcinoma. Am J
Transl Res. 7:2412–2422. 2015.PubMed/NCBI
|
|
40
|
Zou Y, Guo CG and Zhang MM: Inhibition of
human hepatocellular carcinoma tumor angiogenesis by siRNA
silencing of VEGF via hepatic artery perfusion. Eur Rev Med
Pharmacol Sci. 19:4751–4761. 2015.PubMed/NCBI
|
|
41
|
Huang J, Ma X, Chen X, Liu X, Zhang B,
Minmin L, Nie W, Zhang L and Liu L: Microvessel density as a
prognostic factor in bladder cancer: A systematic review of
literature and meta-analysis. Cancer Biomark. 14:505–514.
2014.PubMed/NCBI
|