Introduction
Mucormycosis is an opportunistic infection that is
caused by Mucorales fungi of the Zygomycetes class. The term
zygomycosis, the previous designation for infections caused by
fungi of the order Mucorales, is no longer appropriate due to a
recent taxonomic reclassification that abolished Zygomycetes as a
class (1). Mucorales fungi are
ubiquitous, saprophytic and not fastidious fungi located in soil or
decaying organic matter, with three genera that are known to be
human pathogens, namely, Rhizopus, Absidia and
Mucor. The optimal temperature for growth is 28 to 30°C
under aerobic conditions, with an incubation period of 2 to 5 days.
Incubation begins with inhalation of the spores or their direct
inoculation into abraded skin (2).
Six distinct clinical presentations of mucormycosis are now
recognized: Rhinocerebral, cutaneous, pulmonary, gastrointestinal
and central nervous system mucormycosis, and a miscellaneous form
involving the bones, breasts, mediastinum and kidneys. The first
case of pulmonary mucormycosis was described in 1876 by Furbringer
(3). The estimated incidence of the
disease is 1.7 cases per million people per year in the United
States (4). In a review of 116 cases
of mucormycosis, 22% were pulmonary mucormycosis (5). However, the incidence of pulmonary
mucormycosis has increased with the development of modern medicine.
Numerous predisposing clinical factors have been described,
including uncontrolled diabetes mellitus, diabetic ketoacidosis,
chemotherapy, hematological malignancies (leukemia and lymphoma),
immunosuppressive therapy, acquired or congenital neutropenia,
antibiotic therapy, metabolic acidosis due to chronic salicylate
poisoning, elastoplast bandages, renal failure, a prolonged
post-operative course, solid tumors, solid organ transplantation,
agammaglobulinemia and burns (6–8). It is
well known that iron metabolism has a key role in mucormycosis
pathogenesis. Therefore, patients in an iron overload state,
including those individuals undergoing deferoxamine chelation
therapy, are uniquely predisposed to mucormycosis (9). Only 6.25% patients do not have any
underlying risk factor (10,11). In patients with hematological
malignancies, mucormycosis most commonly affects the lungs (58–81%)
(12). A previous single-center
autopsy study over a 15-year period in patients with hematological
malignancy reported a significant 3-fold increase in the incidence
of autopsy-proven mucormycosis cases, from 0.9–3%, during the study
period (13). The present study
reports the case of a patient with a definite histological
diagnosis of pulmonary mucormycosis.
Case report
A previously healthy 15-year-old male was admitted
to The First Affiliated Hospital of Soochow University (Suzhou,
Jiangsu, China) in January 2012 with a 3-day history of anergy and
epistaxis. There was no history of hemoptysis, fever, chills, night
sweats, chest pain, diabetes mellitus or weight loss. The patient
reported no history of steroid use. Physical examination showed a
well-developed male with facial pallor and fresh petechia on the
lower limbs. The patient had a temperature of 36.7°C (normal range,
36–37°C), a pulse rate of 80 beats/min (normal range, 60–100
beats/min), a respiratory rate of 20 breaths/min (normal range,
12–20 breaths/min) and a blood pressure of 120/60 mmHg (normal
range, <130/85 mmHg). Further physical examination results were
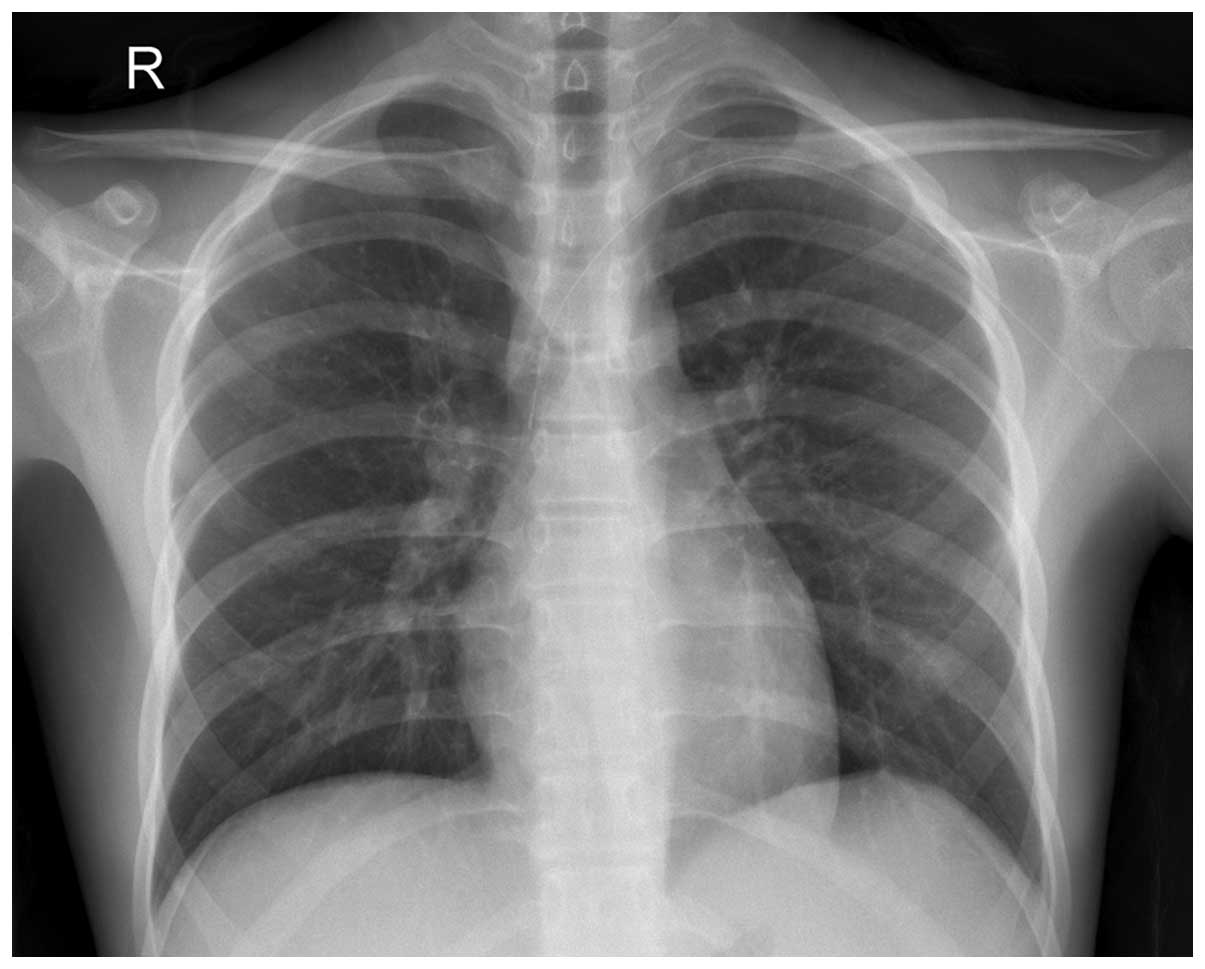
unremarkable. The chest radiograph showed no abnormalities
(Fig. 1). A full blood count revealed
a hemoglobin level of 76 g/l (normal range, 120–150 g/l), a
platelet count of 1.5×1010/l (normal range,
10.0–30.0×1010/l) and a white blood cell count of
1.24×1011/l (normal range, 4.00–10.00×109/l),
with 1.24×109/l neutrophils (normal range,
1.80–6.30×109/l), 2.98×1010/l lymphocytes
(normal range, 1.10–3.20×1010/l), and
9.3×1010/l protocells and juvenile cells (normal range,
0 cells). Bone marrow examination revealed 89.1% of the naive
population were T lymphocytic cells (normal range, 0%). The patient
was therefore diagnosed with T-cell acute lymphoblastic leukemia
(T-ALL). At 3 days post-evaluation, the patient received induction
chemotherapy with an IVP regimen (10 mg idamycin on days 1–4; 4 mg
vindesine once a week for four weeks; and 10 mg dexamethasone every
day). On the 15th hospital day the patient developed a fever, with
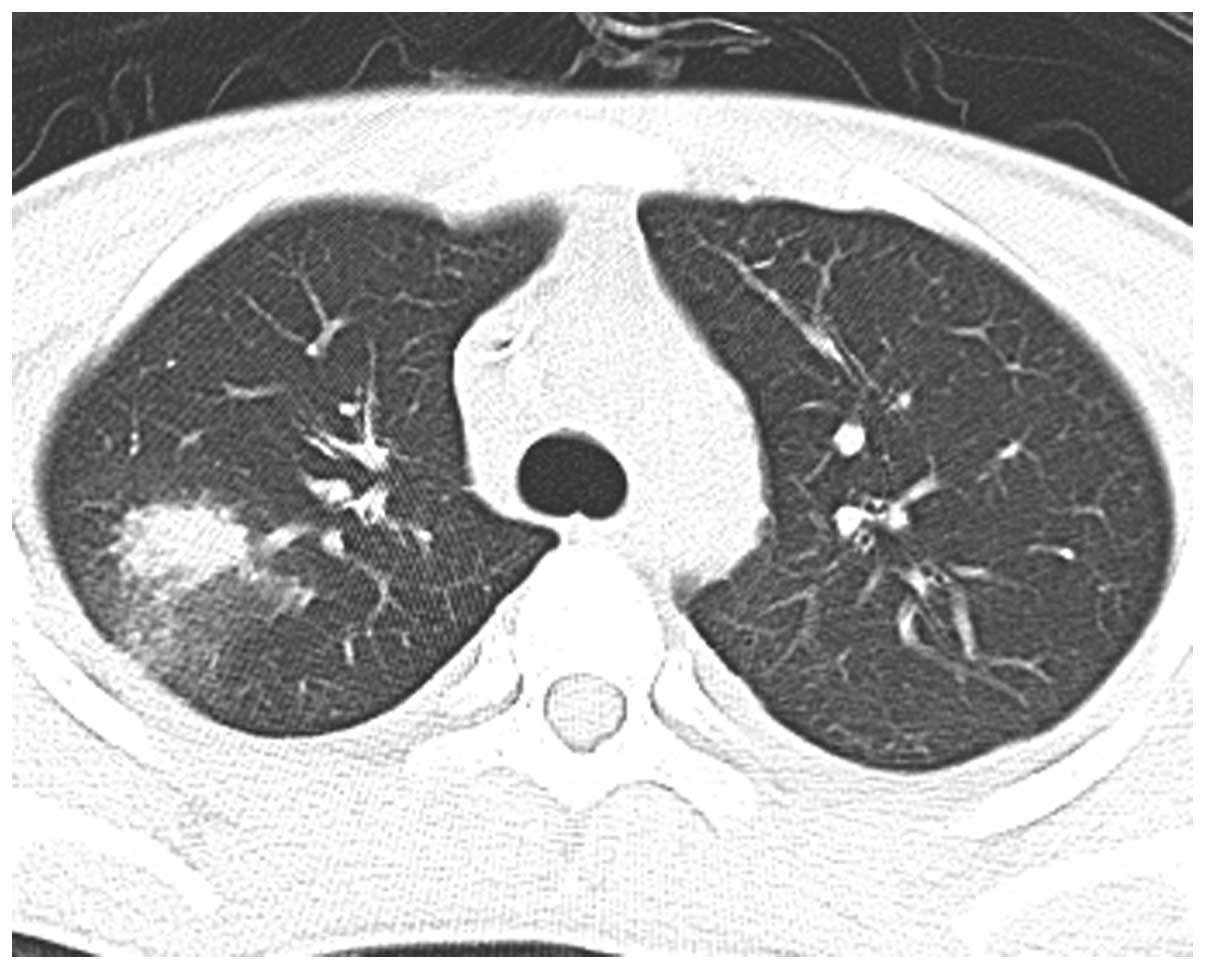
a temperature of 39°C. Computed tomography (CT) scans showed right
upper lobe (RUL) infiltration (Fig.
2). The white blood cell count was 0.3×109/l and the
neutrophil count was 0.03×109/l. Throat swab, blood,
urine and sputum cultures for bacteria and fungus were repeatedly
obtained, but did not reveal any pathogens. Sputum smears for
acid-fast bacilli were negative, and galactomannan testing (GM) for
diagnosing invasive aspergillosis was negative. The patient
clinically improved following intravenous meropenem (0.5 g; every 8
h), teicoplanin (3 mg/kg; every 12 h), amphotericin B (1 mg on day
1, then add 5 mg every day until 0.5 mg/kg/day reached) and
caspofungin (70 mg on day 1, then 50 mg/day) empirically for one
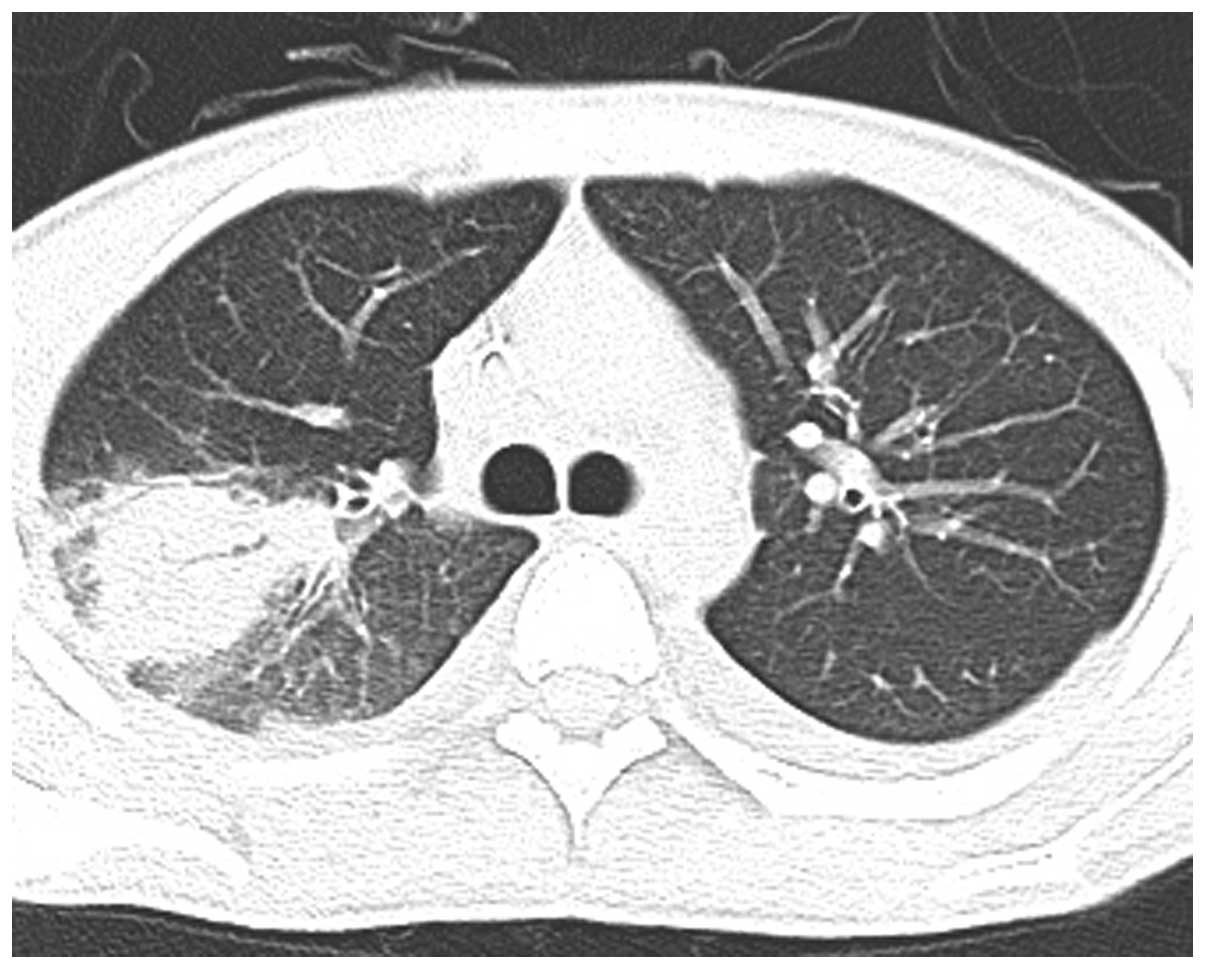
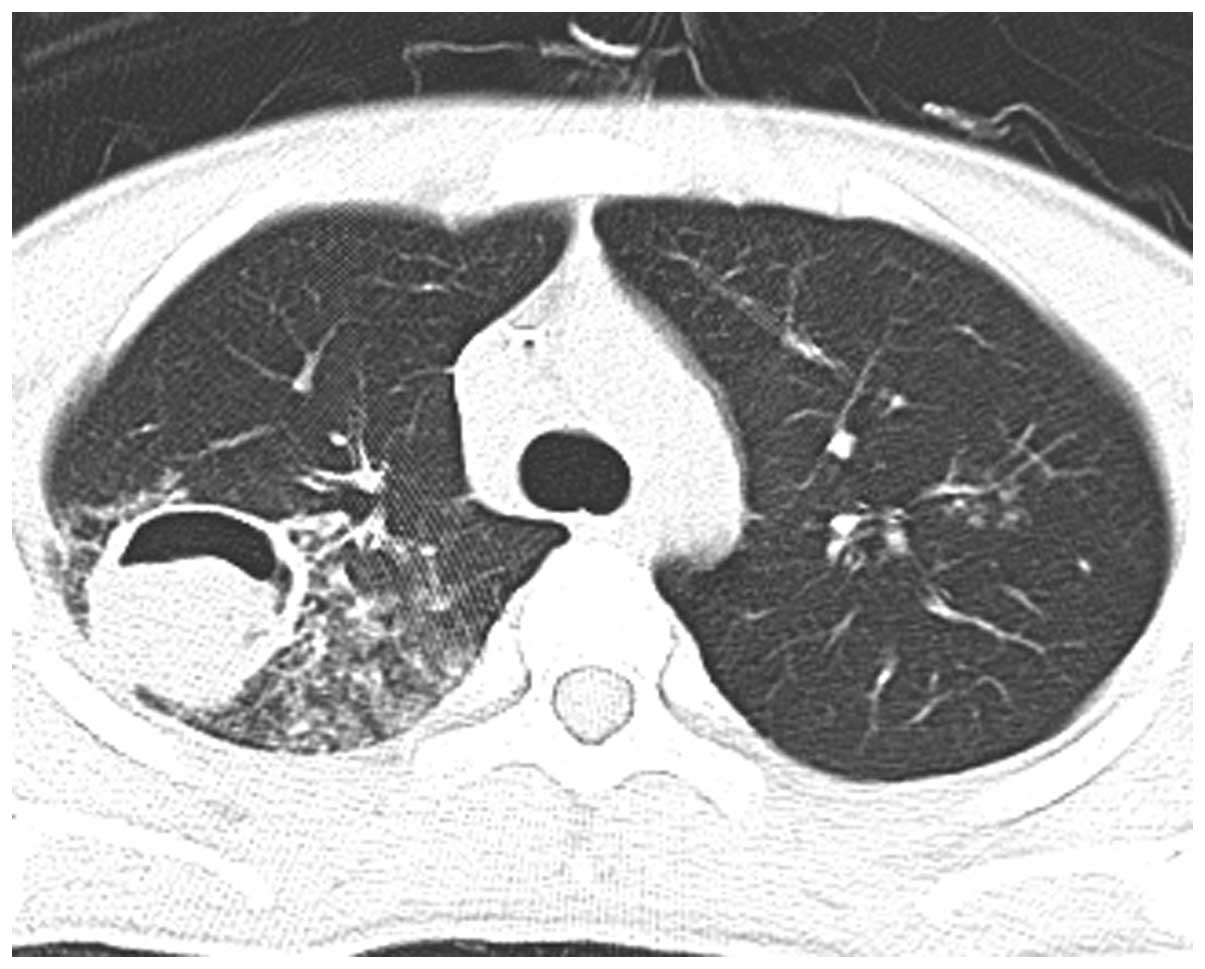
week, however, radiography showed progression of the infiltrating
lesion in the RUL (Figs. 3–5). Following chemotherapy with
L-asparaginase (10,000 units every other day, three times), the
patient had a white blood cell count of 8.27×109/l, a
hemoglobin level of 84 g/l and a platelet count of
195×109/l. Bone marrow morphology showed complete
remission had been attained. The patient underwent a lobectomy of
the RUL at 40 days post-admission, during which the infected area
and necrotic tissue were resected. During surgery, a mass measuring
5×5×5 cm was revealed arising from the posterior segment of the
RUL, which was tightly adherent to the chest wall. The pulmonary
hilar lymph nodes were slightly enlarged. Histological study of the
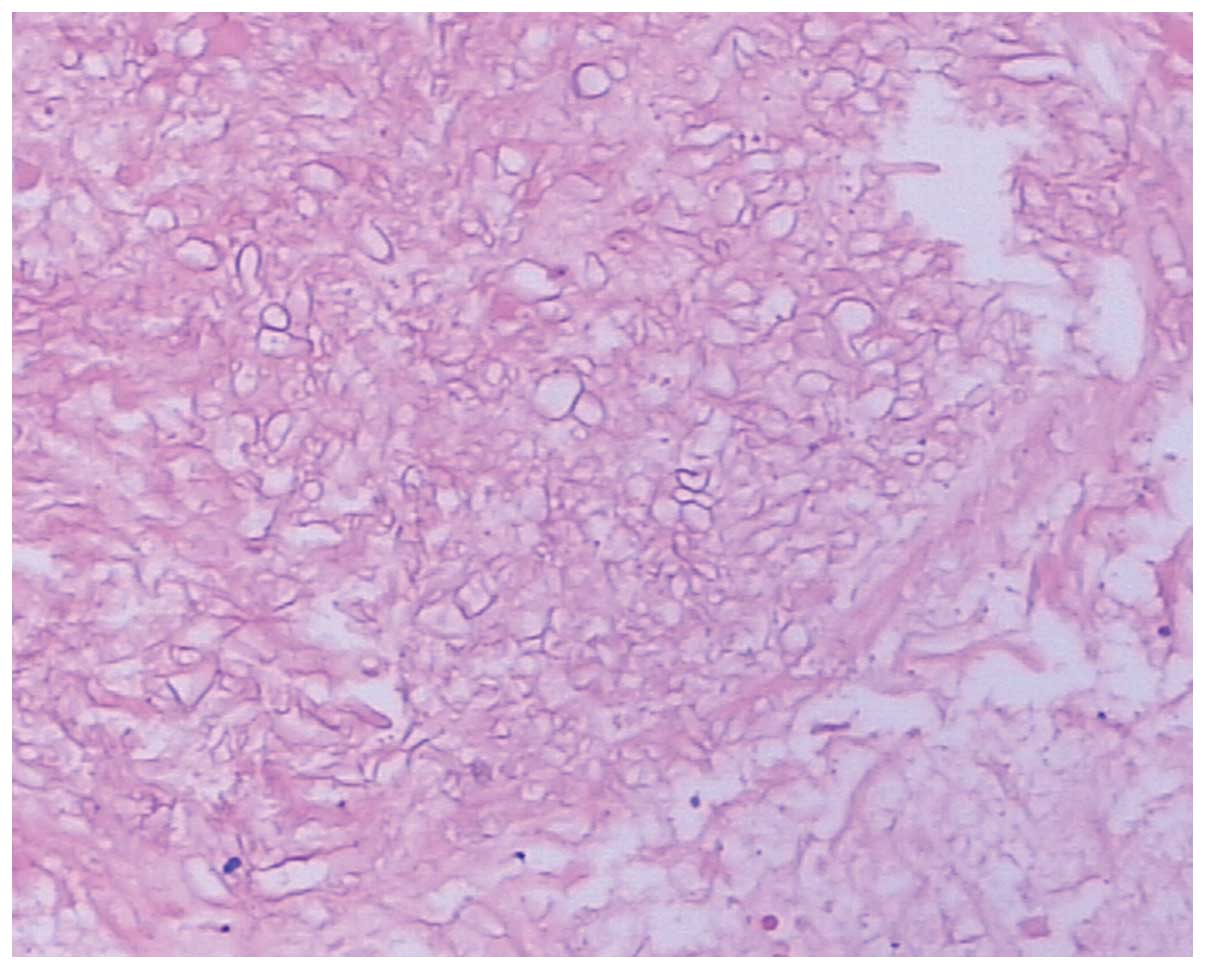
mass using hematoxylin and eosin staining revealed features
consistent with pulmonary mucormycosis. The mass was composed of a
large amount of right-angled branching, broad, non-septate hyphae.
Epithelioid cells and an intense chronic inflammatory reaction were
noted (Fig. 6). The patient
experienced 12 months of uneventful follow-up post-surgery;
however, the patient died from severe septic shock 13 months
following surgery.
Written informed consent was obtained from the
patient for the publication of the study.
Discussion
The present study reports the case of a young boy
with T-ALL that developed agranulocytosis following chemotherapy at
the same time as pneumonia. RUL infiltration progressed after
empirical antibiotic therapy, which included antifungal agents.
Surgery was the first choice of treatment for the patient and,
fortunately, pulmonary mucormycosis was diagnosed subsequent to the
lobectomy, so amphotericin B therapy was continuously provided. The
patient's pulmonary mucormycosis was successfully treated.
Pulmonary mucormycosis occurs due to the inhalation
of fungi spores into the bronchioles and alveoli, which typically
results in the rapid progression of pneumonia or endobronchial
disease. Rarer results include endobronchial lesions and
complications associated with airway occlusion. Hemoptysis commonly
occurs with vascular invasion, which can occasionally be fatal. The
symptoms of pulmonary mucormycosis are typically non-specific, even
at late stages of infection, and may include fever, dyspnea,
coughing and chest pain. Rare cases can present as progressive
subcutaneous emphysema, Pancoast syndrome, Horner's syndrome, or
chronic mediastinitis and bronchial perforation (7,14–17).
The radiological manifestations of pulmonary
mucormycosis are mostly non-specific. An abnormal chest
roentgenogram result is present in >80% of patients (18). The reported findings include
consolidation, cavitation, the air-crescent sign, the halo sign,
the reversed halo sign, solitary or multiple pulmonary nodules or
masses, bronchopleural fistulae, pulmonary artery pseudoaneurysms,
lymphadenopathy and pleural effusion. Cavitation is observed in as
many as 40% of cases, but the air-crescent sign is uncommon. CT can
show findings that alter the management or diagnostic approach in
as many as 26% of patients (19–24). The
presence of the air-crescent sign often portends a poor prognosis
if surgical therapy is delayed. Similar to invasive pulmonary
aspergillosis, pulmonary mucormycosis is detected with the highest
sensitivity when using high-resolution chest CT to determine the
extent of the disease. This technique also usually finds evidence
of the infection earlier than standard chest radiographs (2,4,9). The right lung is more commonly involved
than the left, and there is a predilection for the involvement of
the upper lobes, although the reason for this remains unknown. The
present case reported a lesion in the RUL, as in the majority of
the cases in the literature (25).
Histopathologically, vascular invasion with tissue
necrosis and neutrophilic infiltration of the tissue is common to
all types of mucormycosis. Diagnosis is achieved by demonstrating
broad (diameter, 6–16 µm), non-septate (coenocytic), ribbon-like
hyphae, with right-angled branching in a tissue biopsy specimen
stained with routine hematoxylin and eosin. Special fungal stains
are usually not necessary for diagnosis. The less common and less
specific features of pulmonary mucormycosis include bronchial
invasion, pneumonia, lung abscesses and granulomatous pneumonitis
(20,21).
As pulmonary mucormycosis demonstrates rapid
clinical progression and is often fatal, patient survival is
dependent on an early diagnosis. Mucorales fungi are ubiquitous
saprophytic fungi that grow in decaying organic matter,
particularly fruit with a high sugar content, soil and manure.
Although the fungi are able to grow in anaerobic, aerobic and
microaerophilic conditions, clinical specimen cultures often prove
to be negative, making the diagnosis difficult. There has
previously been no serological test for mucormycosis. The symptoms,
signs and radiographic manifestations of pulmonary mucormycosis are
non-specific. Pulmonary mucormycosis is associated with bacterial
pneumonia in 30% of cases, which can delay the diagnosis of the
fungal infection (26). Diagnostic
options are largely limited to the clinical and radiographic
findings, together with staining and culture. A definitive
diagnosis depends on the identification of mucoraceus hyphae in
affected tissues (9,21); diagnostic techniques used to achieve
this identification include percutaneous needle biopsy, open lung
biopsy and pleural fluid culture. Fiberoptic bronchoscopy is a
useful diagnostic method, and an adequate bronchoalveolar lavage
specimen provides enough diagnostic material to form a cytological
diagnosis (27). The differential
diagnosis of the disease includes bacterial, viral and other fungal
pulmonary infections. Pulmonary mucormycosis has clinical
manifestations for which there is almost no differentiation from
those of other more common opportunistic molds such as
Aspergillus. The differentiation between mucormycosis and
aspergillosis is important as the treatments can differ, and since
the patient outcome may be improved by the appropriate early
treatment of mucormycosis (19). In
previous studies, polymerase chain reaction analysis from
deparaffinized sections performed from a selected paraffin block
showing both subtypes of hyphae allowed the identification of
aspergillosis and mucormycosis (28,29).
Successful treatment of pulmonary mucormycosis
relies on a timely diagnosis. Amphotericin B, along with surgical
resection of the involved areas of the lung and treatment of the
underlying disease, is the mainstay of treatment (25). Despite the risk of renal toxicity,
amphotericin B (1–1.5 mg/kg/day) remains the gold-standard
antifungal agent used against mucormycosis. Oral posaconazole is
also recommended, but these two types of drugs are often
ineffective without surgical intervention (9,10,30–32).
Voriconazole is ineffective against mucormycosis (33). Although the therapy duration is not
well defined, a total cumulative dose of 1.5 g of amphotericin is
usually sufficient in the selective group of patients who respond
only to amphotericin therapy (34).
Surgical therapy, such as wedge resection, lobectomy and
pneumonectomy, in combination with medical therapy, has been
associated with lower mortality rates in published series of
patients with Mucor infection, particularly in patients with
disease confined to one lung (35–38). In
order to prevent dissemination and erosion into the vessels, which
can result in potentially fatal massive hemoptysis, surgery should
be performed as soon as possible (39).
Unlike pulmonary aspergillosis, pulmonary
mucormycosis has a prognosis and outcome that have not
significantly improved over the last decade, mainly due to the
difficulty in forming an early diagnosis and the limited activity
of current antifungal agents against Mucorales (9,11).
Pulmonary mucormycosis is a rapidly fatal illness, with an overall
mortality rate of 76%, which increases to 95% with extrathoracic
dissemination (5,7,9,40–43). If
untreated, survival beyond 2 weeks is distinctly unusual (40). In total, <50% of patients are
diagnosed premortem (18). Delays in
the diagnosis result in a lethal clinical course due to fungal
sepsis, respiratory failure and hemoptysis (44,45). The
outcome is typically fatal when pulmonary mucormycosis develops in
a patient with hematological disease (41). Combined surgical/medical treatment may
provide a better survival outcome than medical therapy alone
(46).
References
|
1
|
Hibbett DS, Binder M, Bischoff JF,
Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM,
Lücking R, et al: A higher-level phylogenetic classification of the
Fungi. Mycol Res. 111:509–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spellberg B, Edwards J Jr and Ibrahim A:
Novel perspectives on mucormycosis: Pathophysiology, presentation
and management. Clin Microbiol Rev. 18:556–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fürbringer P: Observations on pulmonary
mucormycosis in humans. Virchows Arch Path Anat. 66:330–365.
1876.(In German). View Article : Google Scholar
|
|
4
|
Garg R, Marak RS, Verma SK, Singh J,
Sanjay and Prasad R: Pulmonary mucormycosis mimicking as pulmonary
tuberculosis: A case report. Lung India. 25:129–131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aboutanos MB, Joshi M and Scalea TM:
Isolated pulmonary mucormycosis in a patient with multiple
injuries: A case presentation and review of the literature. J
Trauma. 54:1016–1059. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bigby TD, Serota ML, Tierney LM Jr and
Matthay MA: Clinical spectrum of pulmonary mucormycosis. Chest.
89:435–439. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muqeetadnan M, Rahman A, Amer S, Nusrat S,
Hassan S and Hashmi S: Pulmonary mucormycosis: An emerging
infection. Case Rep Pulmonol. 2012:1208092012.PubMed/NCBI
|
|
8
|
Mohammadi A, Mehdizadeh A, Ghasemi-Rad M,
Habibpour H and Esmaeli A: Pulmonary mucormycosis in patients with
diabetic ketoacidosis: A case report and review of literature.
Tuberk Toraks. 60:66–69. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamilos G, Samonis G and Kontoyiannis DP:
Pulmonary mucormycosis. Semin Respir Crit Care Med. 32:693–702.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Scheven R, Lebiedz P, Spieker T,
Uekoetter A, Berdel WE and Kessler T: Fulminant invasive pulmonary
mucormycosis with Rhizopus oryzae in a patient with severe aplastic
anaemia and common variable immunodeficiency. Mycoses. 55:e32–e35.
2012.PubMed/NCBI
|
|
11
|
Roden MM, Zaoutis TE, Buchanan WL, Knudsen
TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH,
et al: Epidemiology and outcome of zygomycosis: A review of 929
reported cases. Clin Infect Dis. 41:634–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pagano L, Offidani M, Fianchi L, Nosari A,
Candoni A, Piccardi M, Corvatta L, D'Antonio D, Girmenia C, Martino
P, et al: Mucormycosis in hematologic patients. Haematologica.
89:207–214. 2004.PubMed/NCBI
|
|
13
|
Chamilos G, Luna M, Lewis RE, Bodey GP,
Chemaly R, Tarrand JJ, Safdar A, Raad II and Kontoyiannis DP:
Nvasive fungal infections in patients with hematologic malignancies
in a tertiary care cancer center: An autopsy study over a 15-year
period (1989–2003). Haematologica. 91:986–989. 2006.PubMed/NCBI
|
|
14
|
Bansal M, Martin SR, Rudnicki SA, Hiatt KM
and Mireles-Cabodevila E: A rapidly progressing Pancoast syndrome
due to pulmonary mucormycosis: A case report. J Med Case Rep.
5:3882011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koshy CG, Shah S and Mammen T:
Subcutaneous emphysema of the chest: Could it be pulmonary
mucormycosis? Thorax. 65:2802010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kotoulas C, Psathakis K, Tsintiris K,
Sampaziotis D, Karnesis L and Laoutidis G: Pulmonary mucormycosis
presenting as Horner's syndrome. Asian Cardiovasc Thorac Ann.
14:86–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HC, Jan MS, Lin YC, Lin WL, Wu TC,
Huang CN, Chen CM and Lu MC: A rare pulmonary zygomycosis
manifested as chronic mediastinitis and bronchial perforation. Eur
Respir J. 38:734–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donado-Uña JR, Díaz-Hellín V,
López-Encuentra A and Echave-Sustaeta JM: Persistent cavitations in
pulmonary mucormycosis after apparently successful amphotericin B.
Eur J Cardiothorac Surg. 21:940–942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung JH, Godwin JD, Chien JW and Pipavath
SJ: Case 160: Pulmonary mucormycosis. Radiology. 256:667–670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McAdams HP, de Christenson Rosado M,
Strollo DC and Patz EF Jr: Pulmonary mucormycosis: Radiologic
findings in 32 cases. AJR Am J Roentgenol. 168:1541–1548. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walsh TJ, Gamaletsou MN, McGinnis MR,
Hayden RT and Kontoyiannis DP: Early clinical and laboratory
diagnosis of invasive pulmonary, extrapulmonary and disseminated
mucormycosis (zygomycosis). Clin Infect Dis. 54(Suppl 1): S55–S60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ono A, Okada F, Ando Y, Maeda T, Saburi Y,
Kondo Y and Mori H: Multiple pulmonary arteriolar emboli in a
patient with disseminated mucormycosis and myelodysplastic
syndrome. Clin Radiol. 66:998–1000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Godoy MC and Marom EM: Reversed halo sign
in pulmonary zygomycosis. Thorax. 66:5442011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Busca A, Limerutti G, Locatelli F, Barbui
A, De Rosa FG and Falda M: The reversed halo sign as the initial
radiographic sign of pulmonary zygomycosis. Infection. 40:77–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butala A, Shah B, Cho YT and Schmidt MF:
Isolated pulmonary mucormycosis in an apparently normal host: A
case report. J Natl Med Assoc. 87:572–574. 1995.PubMed/NCBI
|
|
26
|
Pavie J, Lafaurie M, Lacroix C, Zagdanski
Marie A, Debrosse D, Socié G, Derouin F, Gluckman E and Molina
Michel J: Successful treatment of pulmonary mucormycosis in an
allogenic bone-marrow transplant recipient with combined medical
and surgical therapy. Scand J Infect Dis. 36:767–769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
al-Abbadi MA, Russo K and Wilkinson EJ:
Pulmonary mucormycosis diagnosed by bronchoalveolar lavage: A case
report and review of the literature. Pediatr Pulmonol. 23:222–225.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hofman V, Dhouibi A, Butori C, Padovani B,
Gari-Toussaint M, Garcia-Hermoso D, Baumann M, Vénissac N, Cathomas
G and Hofman P: Usefulness of molecular biology performed with
formaldehyde-fixed paraffin embedded tissue for the diagnosis of
combined pulmonary invasive mucormycosis and aspergillosis in an
immunocompromised patient. Diagn Pathol. 5:12010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi M, Togitani K, Machida H, Uemura
Y, Ohtsuki Y and Taguchi H: Molecular polymerase chain reaction
diagnosis of pulmonary mucormycosis caused by Cunninghamella
bertholletiae. Respirology. 9:397–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uchida Y, Tsukino M, Shigemori W, Hayashi
E, Watanabe I, Nakayama T, Yamada E and Moro K: Diagnosis of
pulmonary mucormycosis aiding the diagnosis of small cell lung
cancer. J Med Microbiol. 61:1610–1613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brugière O, Dauriat G, Mal H,
Marrash-Chalha R, Fournier M, Groussard O, Besnard M, Lesèche G and
Dupont B: Pulmonary mucormycosis (zygomycosis) in a lung transplant
recipient: Recovery after posaconazole therapy. Transplantation.
80:1361–1362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sharma SK, Agarwal N, Mukherjee A, Seth T,
Mishra P, Xess I, Mahapatra M and Sharma S: Coexisting pulmonary
tuberculosis and mucormycosis in a patient with aplastic anemia
post allogenic stem cell transplantation. Mediterr J Hematol Infect
Dis. 3:e20110362011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fanci R, Pecile P, Di Lollo S, Dini C and
Bosi A: Pulmonary mucormycosis with cervical lymph node involvement
in a patient with acute myeloid leukaemia: A case report. Mycoses.
51:354–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharma A, Gupta V, Singh RS, Kakkar N,
Singh S and Bambery P: Angioinvasive pulmonary mucormycosis
presenting as multiple bilateral pulmonary nodules in a patient
without obvious predisposing factors. Singapore Med J.
49:e269–e271. 2008.PubMed/NCBI
|
|
35
|
Schneidawind D, Nann D, Vogel W, Faul C,
Fend F, Horger M, Kanz L and Bethge W: Allogeneic hematopoietic
cell transplantation in patients with acute myeloid leukemia and
pulmonary mucormycosis. Transpl Infect Dis. 14:E166–E172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fitzpatrick MC and Carter BW: Pulmonary
mucormycosis complicating cutaneous blastic plasmacytoid dendritic
cell neoplasm. Proc (Bayl Univ Med Cent). 25:287–288.
2012.PubMed/NCBI
|
|
37
|
Serio B, Rosamilio R, Giudice V, Zeppa P,
Esposito S, Fontana R, Annunziata S and Selleri C: Successful
management of pulmonary mucormycosis with liposomal amphotericin B
and surgery treatment: A case report. Infez Med. 20(Suppl 2):
S43–S47. 2012.
|
|
38
|
Björkholm M, Runarsson G, Celsing F, Kalin
M, Petrini B and Engervall P: Liposomal amphotericin B and surgery
in the successful treatment of invasive pulmonary mucormycosis in a
patient with acute T-lymphoblastic leukemia. Scand J Infect Dis.
33:316–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JY, Yong TY, Jurisevic CA, Russ GR,
Grove DI, Coates PT and Disney AP: Successful treatment of
pulmonary mucormycosis in a renal transplant recipient with limited
pulmonary reserve by combined medical and surgical therapy. Heart
Lung Circ. 18:226–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agarwal R, Kumar V and Gupta D: Pulmonary
mucormycosis: Two of a kind. Eur J Intern Med. 17:63–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia ZK, Wang WL and Yang RY: Slowly
progressive cutaneous, rhinofacial and pulmonary mucormycosis
caused by mucor irregularis in an immunocompetent woman. Clin
Infect Dis. 56:993–995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chacko B, David VG, Tamilarasi V, Deepti
AN and John GT: Pulmonary mucormycosis in a nondiabetic renal
allograft recipient successfully managed by medical therapy alone.
Transplantation. 83:1656–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Petrikkos G, Skiada A, Lortholary O,
Roilides E, Walsh TJ and Kontoyiannis DP: Epidemiology and clinical
manifestations of mucormycosis. Clin Infect Dis. 54(Suppl 1):
S23–S34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chamilos G, Marom EM, Lewis RE, Lionakis
MS and Kontoyiannis DP: Predictors of pulmonary zygomycosis versus
invasive pulmonary aspergillosis in patients with cancer. Clin
Infect Dis. 41:60–66. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yagi S, Miyashita N, Fukuda M, Obase Y,
Yoshida K, Miyauchi A, Kawasaki K, Soda H and Oka M: Pulmonary
mucormycosis (Cunninghamella bertholletiae) with cavitation
diagnosed using ultra-thin fibre-optic bronchoscopy. Respirology.
13:312–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hua-Ping Z, Jian L, Jing-Bin H, Jie G,
Guo-Xin M, Yan-Hong J and Li-Xin X: Surgical resection and
liposomal amphotericin B to treat cavitary pulmonary zygomycosis in
a patient with diabetes. Respir Care. 56:1837–1931. 2011.
View Article : Google Scholar : PubMed/NCBI
|