Introduction
Human renal cell carcinoma (RCC) is the most lethal
urinary malignancy and consists of a number of different
pathological types, including clear cell, chromophobe and papillary
RCC, which all arise from the epithelium of the renal tubules
(1,2).
The most prevalent RCC subtype is clear cell, accounting for 85% of
all RCC cases (3). Generally, RCC is
resistant to conventional radiotherapy and chemotherapy (4,5). Prognosis
for patients with metastatic RCC is poor, with a median survival
time of 8 months and a 5-year survival rate of 10% (6). Although certain genes have been
investigated as potential prognostic predictors and therapeutic
targets for RCC, the molecular mechanisms underlying the
development and progression of RCC remain largely unknown.
Targeting protein for Xenopus kinesin-like protein 2
(TPX2) is a microtubule-associated protein, and functions to
regulate the construction of the mitotic spindle by promoting
microtubule nucleation from chromatin and stabilization of the
spindle microtubules (7,8). It has been demonstrated that TPX2
overexpression induces the amplification of centrosomes and results
in DNA polyploidy (9). TPX2 also
serves a crucial role in the proliferation of various tumor cells
(10,11). TPX2 expression is closely regulated by
the cell cycle, and exhibits particularly high expression in
proliferating cells transitioning between the G1 and S
phase (12–14). Certain studies have demonstrated that
the TPX2 gene, in addition to elevated gene copy numbers, is
frequently identified in various human malignancies, including lung
carcinoma (15), salivary gland
cancer (16), esophageal cell
carcinoma (17) and breast cancer
(18). Furthermore, the upregulated
expression of TPX2 has been observed in a number of different
tumors, including bladder and colon cancer (19,20). Such
results have revealed the correlation between TPX2 and the
tumorigenesis of cancer. In addition, it has been demonstrated that
the overexpression of TPX2 serves an important role in the
progression and invasion of human malignancies (21), and that its expression is associated
with the aggressiveness of ovarian cancer (22). Previous studies have reported that
TPX2 is involved in cell proliferation and associated with poor
prognosis in patients with esophageal cell carcinoma (23,24),
supporting the hypothesis that TPX2 functions as a tumor promoter
in this particular type of cancer.
When combined, the aforementioned reports suggest
that TPX2 may be involved in the progression of malignant tumors;
however, the function of TPX2 expression in human RCC remains
unknown. Thus, the present study investigated the effect of TPX2
expression on the proliferation and invasion of RCC cells. The
results indicated that the expression of TPX2 is upregulated in
RCC, and that TPX2 provokes the invasion and proliferation of RCC
cells through the suppression of Akt/mammalian target of rapamycin
(mTOR) signaling. Such findings indicate that TPX2 may serve as an
independent prognostic factor and a therapeutic target in human
clear cell RCC.
Materials and methods
Patients and tissue specimens
Tissue samples from 286 patients with RCC were
collected (36 patients succumbed to disease and 15 patients were
lost to follow-up, therefore a total of 51 patients were not
evaluated in the survival analysis), and all of the tumor samples
were diagnosed as conventional clear cell RCC. Neoplasms were
surgically resected in the Department of Urology, The Second
Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China)
between January 2003 and May 2012. The study was approved by the
Ethics Committee of The Second Affiliated Hospital of Xi'an
Jiaotong University. The tumors were graded according to the
criteria of the Fuhrman grading system of malignant tumors, and the
clinical tumor stage was classified according to the tumor, node,
metastasis classification system (25,26). RCC
specimens and corresponding normal healthy kidney tissues located
as far as possible from the tumor were obtained. The samples were
then fixed in formalin, dehydrated and embedded in paraffin. All
the RCC specimens were frozen in liquid nitrogen subsequent to
surgical resection, and were maintained at −90°C for protein and
mRNA extraction.
Cell culture
A total of 4 RCC cell lines (ACHN, Caki-1, Caki-2
and NC65) were obtained from the American Type Culture Collection
(Manassas, VA, USA), and were cultured with complete medium
composed of RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 2 mM L-glutamine, 1% non-essential amino
acids, 25 mM HEPES, 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 µg/ml streptomycin (all purchased from
Sigma-Aldrich, St. Louis, MO, USA). All RCC cell lines were
maintained as monolayers in 10-cm plastic dishes and were incubated
in a humidified atmosphere containing 5% CO2 at
37°C.
Animal RCC xenograft experiments
A total of 30 BALB/c nude mice (age, 3–4 weeks) were
kept in specific pathogen-free conditions at a temperature of
26–28°C and humidity of 30–40%. The light/dark cycle was 12 h, and
food and water were supplied. The mice were randomly divided into
the following two groups: TPX2 vector group and control group. The
RCC cells (4×107) were seeded into the back region of
each mouse via subcutaneous injection. All of the mice were
observed, with observations recorded for 5 continuous weeks and the
size of the xenograft measured once a week (calculated using the
formula v = ab2π / 6, where a is the longest diameter
and b is the longest diameter perpendicular to the tumor). At 5
weeks post-injection, all mice were sacrificed under deep
anesthesia via intraperitoneal injection with pentobarbital sodium
(80 mg/kg; Roche Diagnostics GmbH, Penzberg, Germany) and the final
size of each xenograft was recorded.
Immunohistochemistry (IHC)
Following the replacement of paraffin with xylene
the tissue samples were rehydrated via a graded alcohol series
(Sigma-Aldrich). Endogenous peroxidase activity was quenched with
0.3% hydrogen peroxide (Sigma-Aldrich) for 15 min, and the sections
were blocked with 5% bovine serum albumin (Sigma-Aldrich) for 1 h.
The sections were then incubated at 37°C for 1 h with rabbit
anti-human TPX2 polyclonal antibody (dilution, 1:1,000; catalog no.
ab71816; Abcam, Cambridge, UK) diluted in phosphate-buffered
saline. Following incubation at 37°C for 2 h with a biotinylated
goat anti-rabbit immunoglobulins polyclonal secondary antibody
(dilution, 1:2,000; catalog no. E0432; Dako, Glostrup, Denmark),
all sections were treated with an avidin-biotin peroxidase complex
(Dako). IHC staining was assessed, and the results were scored
using a light microscope (BH-2; Olympus Corporation, Tokyo, Japan).
The intensity of TPX2 immunostaining was semi-quantified using the
following scale: -, negative; +, weak; ++, moderate; and +++,
strong staining.
Cell proliferation assay
Cell proliferation was analyzed using a WST-1 assay,
with 2×103 exponentially-growing RCC cells seeded in a
96-well microtiter plate. Following continuous incubation for 1, 2
and 3 days, 10 µl WST-1 (Roche Diagnostics GmbH) was added to each
well and the incubation was continued for an additional 1 h. The
absorbance, representing the RCC cell count in each well, was
measured using a microculture plate reader (NJ-2000 Immunoreader;
Intermed Japan Co., Ltd., Tokyo, Japan) at an optical density of
450 nm.
Invasion assay
RCC cells (2×104) were harvested and
seeded in the upper chamber of a 24-Multiwell BD FluoroBlok insert
(8.0 µm pore size; BD Biosciences, Franklin Lakes, NJ, USA) with
no-FBS medium (Gibco; Thermo Fisher Scientific, Inc.). The
chemotaxis gradient was performed using medium containing 10% FBS
in the lower chamber. Following a 24-h incubation, the RCC cells
that had migrated to the lower chamber were stained with
hematoxylin (Sigma-Aldrich) and counted using ImageJ software
version 1.45 (www.imagej.nih.gov/ij; National Institutes of Health,
Bethesda, MD, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA isolation was performed using TRIzol®
reagent, according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.), and the total RNA was subjected to
first-strand cDNA synthesis (Promega Corporation, Madison, WI,
USA). All reagents used were from the GoScript™ Reverse
Transcription system (Promega Corporation, Madison, WI, USA)
RT-qPCR was performed using a Mastercycler® Nexus
(Eppendorf, Hamburg, Germany) with an IQ™ SYBR® Green
Supermix kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). TPX2
was amplified using the following primers: Forward,
5′-AGGGGCCCTTTGAACTCTTA-3′ and reverse, 5′-TGCTCTAAACAAGCCCCATT-3′.
GAPDH was used as an endogenous control with the following primers:
Forward, 5′-ATCAAGAAGGTGGTGAAGCAGG-3′ and reverse,
5′-GTGGAGGAGTGGGTGTCGC-3′. PCR was performed under the following
conditions: Initial denaturation was performed at 95°C for 5 min,
followed by 35 cycles of 95°C for 30 sec, 60°C for 30 sec, 72°C for
1 min, followed by a final extension step at 72°C for 10 min. The
PCR products were quantified using the LightCycler® 480
Real-Time PCR system (Roche Diagnostics GmbH) and TPX2 expression
was normalized to GAPDH. Each RT-qPCR experiment was repeated three
times.
Western blotting
Protein was isolated using lysis buffer (Abcam), and
the total protein concentration was evaluated using the Quick
Start™ Bradford Protein assay (Bio-Rad Laboratories, Inc.). Western
blot analysis was performed by loading 50 µg protein onto an 8%
sodium dodecyl sulfate polyacrylamide gel for electrophoresis. The
polyvinylidene fluoride membranes were blocked with 0.5% bovine
serum albumin, then incubated with the rabbit anti-human TPX2
polyclonal antibody (catalog no., ab71816; dilution, 1:1,000;
Abcam) at 37°C for 1 h; a mouse anti-human β-actin monoclonal
antibody (mAb; catalog no. ab6276; dilution, 1:5,000; Abcam) was
also used as a loading control. Other antibodies used for western
blotting were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), as follows: Rabbit anti-human Akt (pan)
(catalog no. 4685)/phosphorylated-Akt (p-Akt; Ser473) monoclonal
antibody (mAb; catalog no. 4060); rabbit anti-human mTOR (catalog
no. 2983)/p-mTOR (Ser2481) mAb (catalog no. 2974); and rabbit
anti-human p70S6K (catalog no. 2708)/p-p70S6K (Thr421) rabbit mAb
(catalog no. 9204). All antibodies were diluted at 1:2,000. The
immune complexes were visualized using the Amersham ECL Prime
Western Blotting Detection reagent (GE Healthcare Life Sciences,
Chalfont, UK) and quantified using Image J software (online
version; imagej.nih.gov/ij/; National Institutes
of Health).
Small interfering RNA (siRNA) and
transfection
siRNA oligonucleotide sequences for TPX2 were
designed using siDirect software (www.sidirect2.rnai.jp). The sequences were as follows:
Forward, 5′-CCAUUAACCUGCCAGAGAAT-3′; and reverse,
5′-UUCUCUGGCAGGUUAAUGGT-3′. The RCC cells were seeded into plastic
culture dishes and incubated with complete medium without
antibiotics until the cells reached 40% confluence. The cells were
transfected with siRNA oligonucleotides using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following continuous incubation at 37°C for 2
days, TPX2 expression was analyzed by western blotting. The cDNA of
TPX2 was cloned using the HK-2 normal human kidney cell line as a
substrate, and the PCR products were subcloned into the pcDEF3
vector (Sigma-Aldrich). The RCC cell lines were stably transfected
using Lipofectamine 2000 with the expression vector containing
full-length TPX2 cDNA. The monoclonal cell line was selected using
G418 (Sigma-Aldrich) and TPX2 expression, and evaluated by western
blotting.
Statistical analysis
SPSS software (version 19.0; IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. All data are expressed as
the mean ± standard deviation. Comparisons between groups were made
using Student's t-test, and the χ2 test was used to
analyze the association between TPX2 expression and the
clinicopathological parameters. A total of 51 patients succumbed to
the disease or were lost to follow up and thus survival could only
be analyzed in 235 patients. Survival curves were estimated by
Kaplan-Meier analysis. All experiments were performed in
triplicate, and a P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The study enrolled 192 males (61.1±16.3 years) and
94 females (62.5±17.4 years). The tumor diameter range was 3.8–14.7
cm (median size, 6.9 cm). Tumor grading identified 124, 97 and 65
patients with Fuhrman grades 1, 2 and 3, respectively. Tumor
staging identified 147, 73, 51 and 25 patients with cancer stages
I, II, III and IV, respectively. RCC was an incidental finding
during routine examination in 209 patients. The presenting symptoms
for RCC included flank pain (33 patients), a palpable mass (21
patients) and hematuria (21 patients). Laboratory examination
detected an increased sedimentation rate in 42 patients, anemia in
31 patients, thrombocytopenia in 14 patients and erythrocytosis in
7 patients. A total of 49 patients had one or more complicated
diseases, including urolithiasis, angina pectoris, valvular heart
disease and diabetes mellitus. In addition, 10 patients had
previously undergone radical nephrectomies and 25 patients had
metastatic disease at the time of diagnosis.
TPX2 protein expression in RCC
tissue
TPX2 protein expression was examined in the human
RCC and normal kidney tissue samples by IHC and RT-qPCR. Generally,
it was observed that TPX2 was overexpressed in the RCC tissues when
compared with the corresponding normal kidney tissues. TPX2
expression was detected in 262/286 patients with RCC (91.6%). By
contrast, TPX2 expression was detected in 130/286 normal kidney
tissues (36.3%). Regarding the association between TPX2 expression
and clinicopathological parameters in RCC, χ2 testing
determined that TPX2 expression was significantly increased in
advanced tumor stages, higher histological grades and tumors >7
cm in size (P<0.05). None of the other parameters, including
gender and age, demonstrated a significant association with TPX2
expression (Table I). RT-qPCR was
also used to assess TPX2 expression in the human RCC and normal
kidney tissues. The relative level of TPX2 expression was
determined with reference to GAPDH. TPX2 was significantly
overexpressed in the RCC tissues when compared with the
corresponding normal kidney tissues. Similar expression levels were
detected by IHC (Table I). These
findings suggest that TPX2 may function in the carcinogenesis and
progression of human RCC.
 | Table I.Characteristics of 286 patients with
RCC, including TPX2 expression detected by reverse
transcription-quantitative polymerase chain reaction and
immunohistochemistry. |
Table I.
Characteristics of 286 patients with
RCC, including TPX2 expression detected by reverse
transcription-quantitative polymerase chain reaction and
immunohistochemistry.
|
|
|
|
| TPX2 protein
expression, n |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Patients, n | TPX2 mRNA
expressiona | P-value | − | + | ++ | +++ | P-value |
|---|
| RCC | 286 | 3.31±0.34 |
| 24 | 61 | 112 | 89 |
|
| Healthy kidney | 286 | 0.89±0.15 | <0.05 | 156 | 97 | 33 | 0 | <0.05 |
| Gender |
|
|
|
|
|
|
|
|
|
Male | 192 | 3.34±0.35 |
| 16 | 42 | 74 | 60 |
|
|
Female | 94 | 3.23±0.39 | >0.05 | 8 | 19 | 38 | 29 | >0.05 |
| Age, years |
|
|
|
|
|
|
|
|
|
<60 | 159 | 3.34±0.33 |
| 13 | 34 | 62 | 50 |
|
|
≥60 | 127 | 3.26±0.44 | >0.05 | 11 | 27 | 50 | 39 | >0.05 |
| Tumor diameter,
cm |
|
|
|
|
|
|
|
|
| ≤7 | 147 | 2.59±0.21 |
| 18 | 53 | 50 | 26 |
|
|
>7 | 139 | 4.06±0.32 | <0.05 | 6 | 8 | 62 | 63 | <0.05 |
| Histological
grade |
|
|
|
|
|
|
|
|
| G1 | 124 | 2.31±0.15 |
| 21 | 42 | 59 | 2 |
|
| G2 | 97 | 3.45±0.37 |
| 3 | 17 | 35 | 42 |
|
| G3 | 65 | 4.98±0.46 | <0.05 | 0 | 2 | 18 | 45 | <0.05 |
| Clinical stage |
|
|
|
|
|
|
|
|
| I | 147 | 2.59±0.21 |
| 18 | 53 | 50 | 26 |
|
| II | 73 | 3.43±0.22 |
| 6 | 7 | 38 | 22 |
|
|
III | 41 | 4.34±0.37 |
| 0 | 1 | 17 | 23 |
|
| IV | 25 | 5.44±0.48 | <0.05 | 0 | 0 | 7 | 18 | <0.05 |
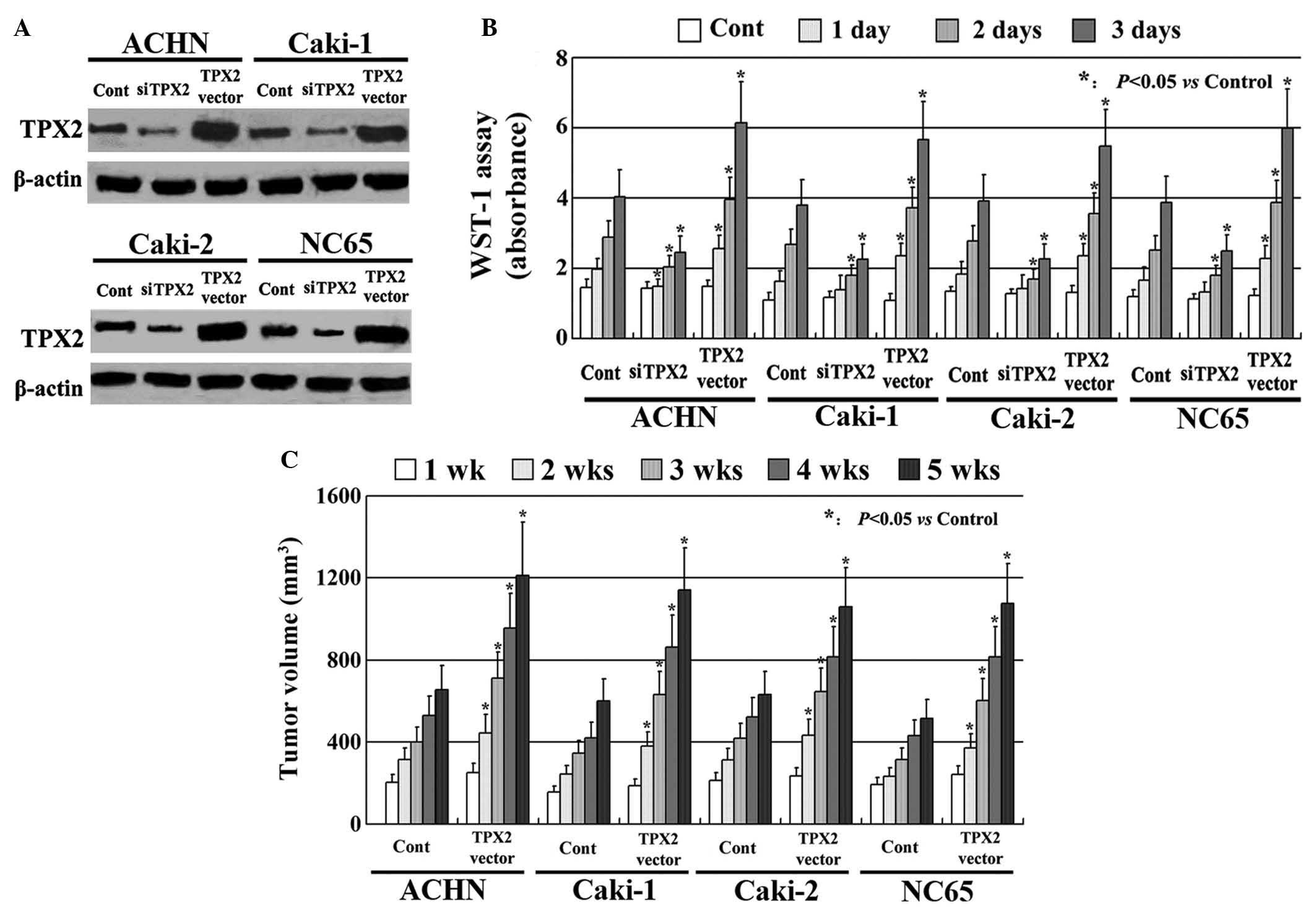
TPX2 increases the proliferation of
RCC cells
TPX2 expression was increased using an expression
vector containing full-length TPX2 cDNA. The vector was stably
transfected into the ACHN, Caki-1, Caki-2 and NC65 cell lines. TPX2
expression was also decreased using siRNA. Following transfection,
all experiments were evaluated by western blotting. It was observed
that TPX2 expression was markedly upregulated by the TPX2 vector
and downregulated by the siRNA compared with the control (Fig. 1A). The effect of TPX2 expression on
the proliferation of the RCC cells in vitro was investigated
using a WST-1 assay, which indicated that the RCC cells expressing
high levels of TPX2 had markedly increased proliferation when
compared with the untreated control cells. The RCC cells with low
TPX2 expression had a significantly reduced proliferative ability
(Fig. 1B; P<0.05). Similar results
were confirmed by the animal xenograft model (P<0.05; Fig. 1C; P<0.05).
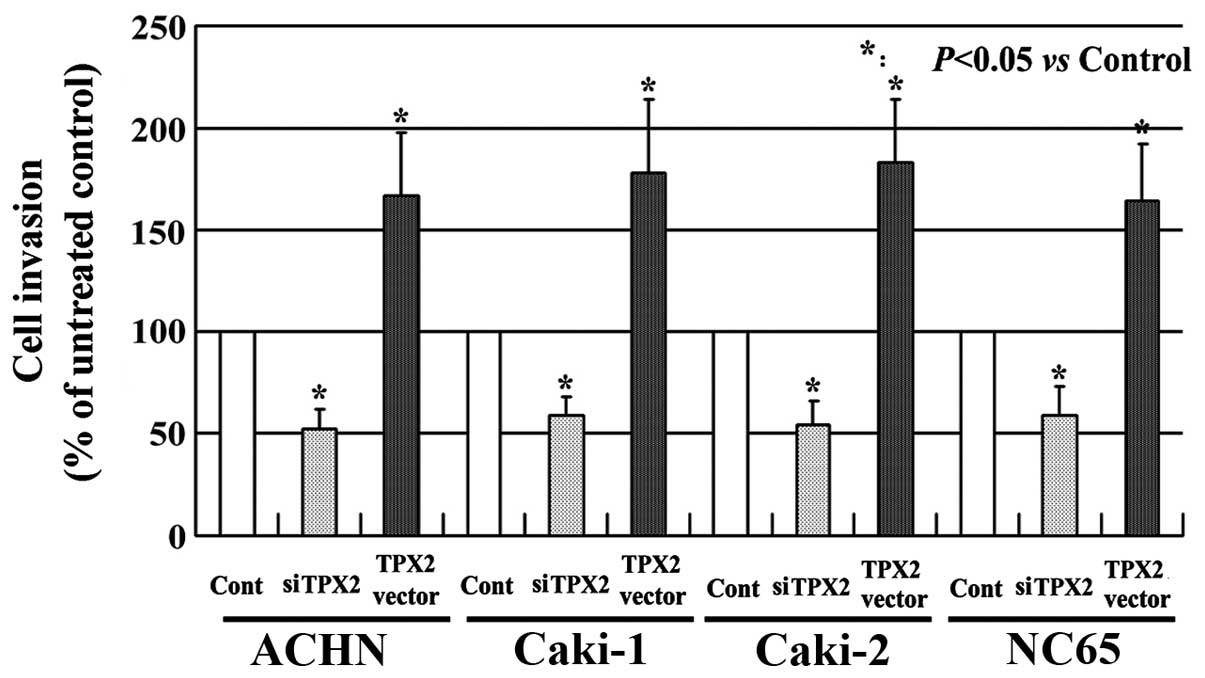
TPX2 increases the invasion of RCC
cells
The effects of TPX2 on the invasion of the RCC cells
was also evaluated. As presented in Fig.
2, the RCC cells with high TPX2 expression exhibited a higher
level of invasion when compared with the untreated cells
(P<0.05). By contrast, the RCC cells with low TPX2 expression
had a markedly reduced invasive ability compared with the untreated
controls (P<0.05). These results indicate that TPX2 enhances the
invasion of the RCC cells and that TPX2 may serve a crucial role in
RCC progression.
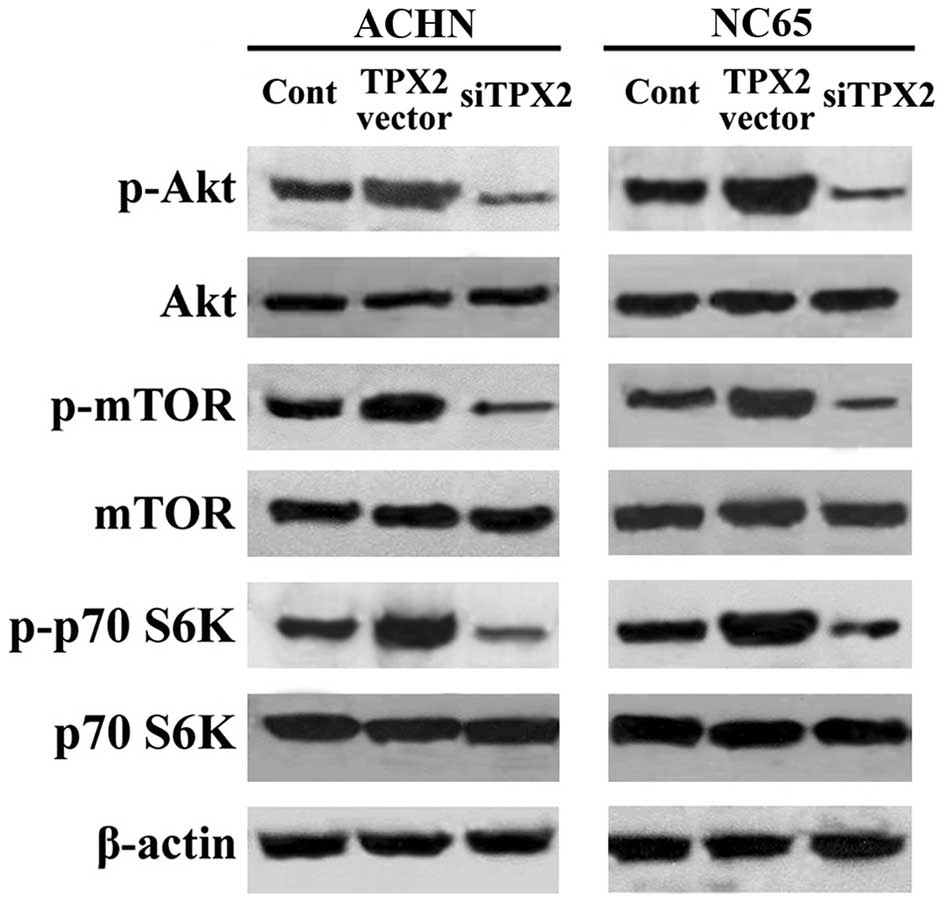
TPX2 increases phosphoinositide
3-kinase (PI3K)/mTOR activity in RCC cells
To clarify how the Akt/mTOR pathway is involved in
TPX2-induced proliferation and invasion, phosphorylation of the
Akt/mTOR pathway was analyzed. TPX2 expression was upregulated by
transfection with the pcDEF3 vector containing the full length TPX2
cDNA, and TPX2 expression was downregulated using siRNA. In the
ACHN and NC65 cell lines, despite TPX2 expression having no affect
on the expression of the Akt and mTOR/p70S6K proteins, the
increased expression of TPX2 upregulated the phosphorylation of Akt
and mTOR/p70S6K compared with the control, as observed by western
blotting. By contrast, decreased expression of TPX2 downregulated
the phosphorylation of Akt and mTOR/p70S6K compared with the
control (Fig. 3). These findings
indicate that the Akt/mTOR pathway is regulated by TPX2, and may
therefore serve a key role in TPX2-induced proliferation and
invasion of RCC cells.
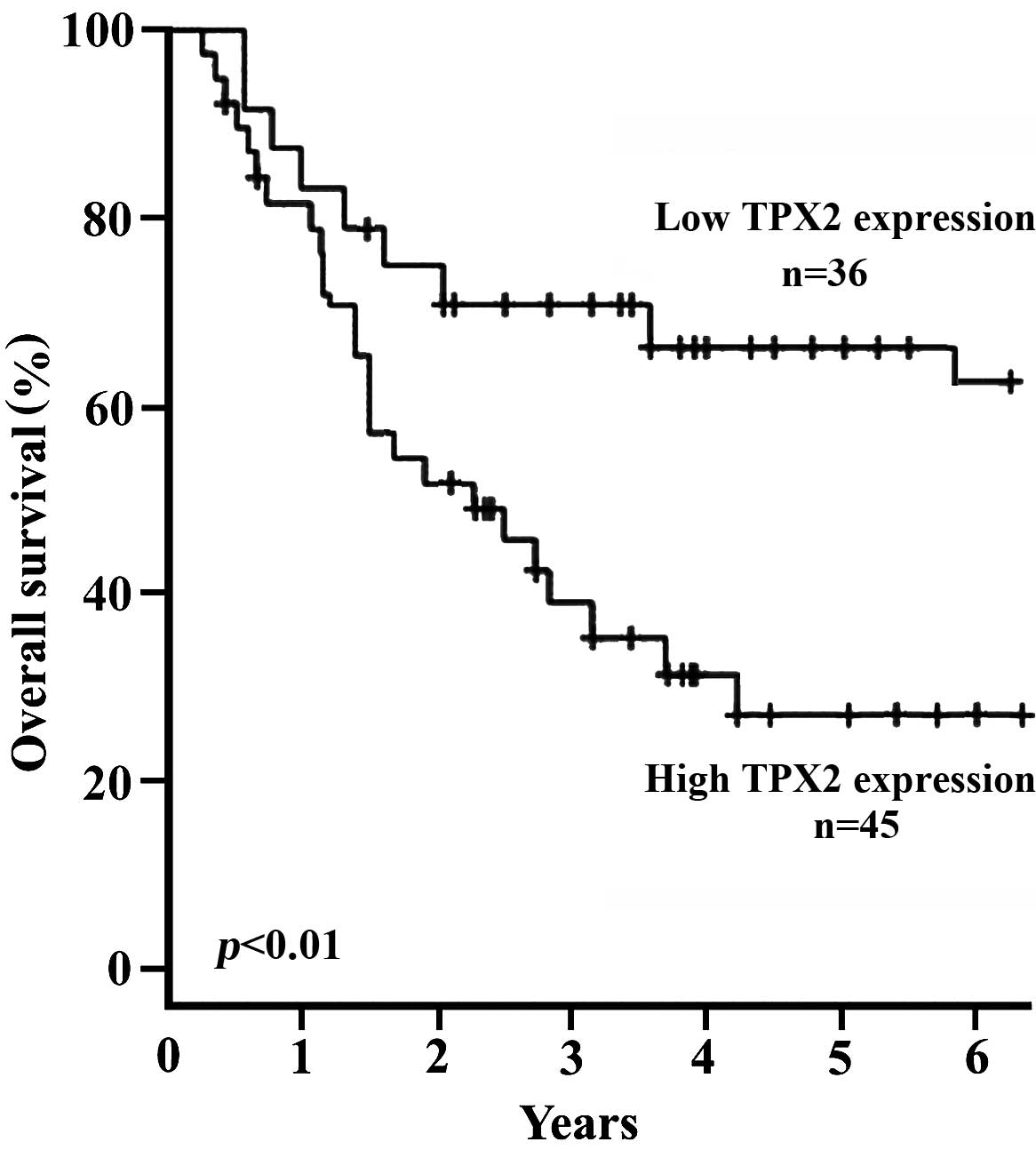
Prognostic significance of TPX2
expression
As a correlation was identified between TPX2
expression, and RCC stage and grade, it was then investigated
whether or not TPX2 functions as a prognostic factor in human RCC.
Kaplan-Meier analysis was performed to calculate the correlation
between TPX2 expression and the survival time of patients with RCC
(Fig. 4). A total of 19 patients
succumbed to a myocardial infarction, 17 patients succumbed to
disseminated malignant disease and 15 patients were lost to
follow-up. The results also demonstrated that the survival time was
significantly different between the high and the low TPX2
expression group (P<0.01 for time points from 2–6 years). After
6 years of follow-up, 36/56 patients (64.3%) who had low TPX2
expression (IHC, - and +), were alive and disease-free compared
with 45/179 patients (25.1%) with high TPX2 expression (IHC, ++ and
+++; Fig. 4). These results indicate
that TPX2 may function as an independent factor in predicting the
prognosis of human RCC.
Discussion
TPX2 was initially reported to be a Xenopus
microtubule-associated protein (27).
TPX2 is a cell cycle protein and serves a key role in the stability
of the mitotic spindle (18). As a
microtubule-associated protein, TPX2 tightly adheres to the mitotic
spindle in mitotic cells (28). In
certain tumors, overexpression of TPX2 results in centrosome
amplification and heteroploidy formation (29,30). TPX2
has been demonstrated to be upregulated in salivary gland
carcinoma, lung carcinoma and ovarian cancer, with its abnormal
expression involved in tumorigenesis and malignant progression
(15,16,31).
Furthermore, overexpression of TPX2 can suppress the completion of
mitosis and disturb the physiological activation of its own
channels (32).
Matrix metalloproteases (MMPs) are known to degrade
the components of the extracellular matrix, and are also
particularly important for tumor invasion and metastasis (33). A previous study reported that TPX2
upregulates MMP levels by activating Akt signaling in colon cancer
(20). Akt is a prominent
serine/threonine kinase that affects important cellular processes
by regulating its downstream effectors (34,35);
however, it remains unclear whether high levels of TPX2 expression
enhances the proliferation and invasion of RCC cells by activating
Akt signaling. Recently, gene target therapy has been the primary
focus of research in the treatment of malignant tumors (36,37). Thus,
the investigation of novel gene therapy methods for RCC is
important.
In the current study, the expression of TPX2 in
human RCC tissue was analyzed. The IHC results indicated that TPX2
expression was significantly upregulated in the RCC tissues
compared with the normal kidney tissues. Furthermore, TPX2
expression was significantly associated with clinical stage,
histological grade and tumor volume. In addition, the expression of
TPX2 in the RCC tissues was examined by RT-qPCR; this demonstrated
consistent data to that produced by IHC, suggesting that TPX2
serves an important role in tumorigenesis and is a key gene in the
progression of RCC. The effect of TPX2 on the proliferation and
invasion of the RCC cells was also evaluated. The results indicated
that TPX2 significantly increased the proliferative ability of the
RCC cells in vitro. The same results were observed in
vivo in the animal xenograft model with BALB/c nude mice.
Additionally, high expression levels of TPX2 significantly
increased the invasive ability of the RCC cells.
A previous study by Engelman et al (38) reported that TPX2 can stimulate Akt
signaling. Akt is a target protein of PI3K and phosphorylates
several downstream mediators to enhance cell proliferation and
survival (38). Akt activity can also
regulate the phosphorylation of mTOR. It has been reported that
p-mTOR and p-S6 are strongly coexpressed in RCC (39), and that p-mTOR increases the
phosphorylation of p70S6K/S6, subsequently enhancing cell
proliferation (40). An inhibitor of
mTOR has been regarded as a novel therapeutic target for advanced
RCC (41,42). Despite the present study investigating
TPX2 expression and its functions in RCC, the underlying molecular
mechanism of TPX2 in RCC remains unclear. The results of the
present study indicated that despite TPX2 not altering the protein
expression of components in the Akt/mTOR pathway, TPX2 increased
the phosphorylation of Akt/mTOR/p70S6K in the RCC cells. These
findings suggest that the Akt/mTOR pathway is a potential target of
TPX2, and serves a key role in TPX2-induced proliferation and
invasion of RCC cells.
In the current study, the association between TPX2
expression and the survival time of patients with RCC was evaluated
by Kaplan-Meier analysis. The results demonstrated that low TPX2
expression may be regarded as a valuable indicator of positive
prognosis. Thus, TPX2 may function as an independent factor for
predicting prognosis, aiding the follow-up of patients with RCC.
TPX2 serves an important function in tumorigenesis of the kidney
and the overexpression of TPX2 can promote the progression of RCC.
Thus, it is possible that patients with RCC exhibiting high TPX2
expression may be vulnerable to RCC morbidities, with TPX2 serving
as a prognostic factor. However, further investigation of the
detailed TPX2 molecular mechanisms underlying human RCC should be
performed.
In conclusion, the present study demonstrated that
TPX2 is overexpressed in human RCC tissue, and can increase the
proliferation and invasion of RCC cells. This indicates that TPX2
has an important function in the tumorigenesis and progression of
human RCC, with the silencing of TPX2 expression possibly serving
as a novel treatment strategy in the future.
References
|
1
|
Arai E and Kanai Y: Genetic and epigenetic
alterations during renal carcinogenesis. Int J Clin Exp Pathol.
4:58–73. 2010.PubMed/NCBI
|
|
2
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature rereview. Cancer Treat
Rev. 34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng FM and Melamed J: Histologic variants
of renal cell carcinoma: Does tumor type influence outcome? Urol
Clin North Am. 39:119–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartmann JT and Bokemeyer C: Chemotherapy
for renal cell carcinoma. Anticancer Res. 19(2C): 1541–1543.
1999.PubMed/NCBI
|
|
5
|
Yu DS, Chang SY and Ma CP: The expression
of mdr-1-related gp-170 and its correlation with anthracycline
resistance in renal cell carcinoma cell lines and
multidrug-resistant sublines. Br J Urol. 82:544–547. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin JA, Fang SU, Su CL, Hsiao CJ, Chang
CC, Lin YF and Cheng CW: Silencing glucose-regulated protein 78
induced renal cell carcinoma cell line G1 cell-cycle arrest and
resistance to conventional chemotherapy. Urol Oncol.
32:29.e1–29.e11. 2014. View Article : Google Scholar
|
|
7
|
Vos JW, Pieuchot L, Evrard JL, Janski N,
Bergdoll M, de Ronde D, Perez LH, Sardon T, Vernos I and Schmit AC:
The plant TPX2 protein regulates prospindle assembly before nuclear
envelope breakdown. Plant Cell. 20:2783–2797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trieselmann N, Armstrong S, Rauw J and
Wilde A: Ran modulates spindle assembly by regulating a subset of
TPX2 and Kid activities including Aurora-A activation. J Cell Sci.
116:4791–4798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gruss OJ, Wittmann M, Yokoyama H,
Pepperkok R, Kufer T, Silljé H, Karsenti E, Mattaj IW and Vernos I:
Chromosome induced microtubule assembly mediated by TPX2 is
required for spindle formation in HeLa cells. Nat Cell Biol.
4:871–879. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anderson MR, Harrison R, Atherfold PA,
Campbell MJ, Darnton SJ, Obszynska J and Jankowski JA: Met receptor
signaling: A key effector in esophageal adenocarcinoma. Clin Cancer
Res. 12:5936–5943. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kan T, Sato F, Ito T, Matsumura N, David
S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, et al: The
miR-106b-25 polycistron, activated by genomic amplification,
functions as an oncogene by suppressing p21 and Bim.
Gastroenterology. 136:1689–1700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tonon G, Wong KK, Maulik G, Brennan C,
Feng B, Zhang Y, Khatry DB, Protopopov A, You MJ, Aguirre AJ, et
al: High-resolution genomic profiles of human lung cancer. Proc
Natl Acad Sci USA. 102:9625–9630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gruss OJ, Wittmann M, Yokoyama H,
Pepperkok R, Kufer T, Silljé H, Karsenti E, Mattaj IW and Vernos I:
Chromosome-induced microtubule assembly mediated by TPX2 is
required for spindle formation in HeLa cells. Nat Cell Biol.
4:871–879. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kufer TA, Silljé HH, Körner R, Gruss OJ,
Meraldi P and Nigg EA: Human TPX2 is required for targeting
Aurora-A kinase to the spindle. J Cell Biol. 158:617–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin DM, Ma Y, Xiao T, Guo SP, Han NJ, Su
K, Yi SZ, Fang J, Cheng SJ and Gao YN: TPX2 expression and its
significance in squamous cell carcinoma of lung. Zhonghua Bing Li
Xue Za Zhi. 35:540–544. 2006.(In Chinese). PubMed/NCBI
|
|
16
|
Shigeishi H, Ohta K, Hiraoka M, Fujimoto
S, Minami M, Higashikawa K and Kamata N: Expression of TPX2 in
salivary gland carcinomas. Oncol Rep. 21:341–344. 2009.PubMed/NCBI
|
|
17
|
Liu HC, Li YY, Liu YH and Liu HY:
Expression of TPX2 mRNA and its correlation with clinical pathology
in esophageal cancer. J Clin Exp Pathol. 26:151–153. 2010.
|
|
18
|
Colak D, Nofal A, Albakheet A, Nirmal M,
Jeprel H, Eldali A, Al-Tweigeri T, Tulbah A, Ajarim D, Malik OA, et
al: Age-specific gene expression signatures for breast tumors and
cross-species conserved potential cancer progression markers in
young women. PLoS One. 8:e632042013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan L, Li S, Xu C, Zhao X, Hao B, Li H and
Qiao B: Target protein for Xklp2 (TPX2), a microtubule-related
protein, contributes to malignant phenotype in bladder carcinoma.
Tumour Biol. 34:4089–4100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y,
Li D and Cai S: TPX2 is a novel prognostic marker for the growth
and metastasis of colon cancer. J Transl Med. 11:3132013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB, Davila JA, Petersen NJ and
McGlynn KA: The continuing increase in the incidence of
hepatocellular carcinoma in the United States: An update. Ann
Intern Med. 139:817–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cáceres-Gorriti KY, Carmona E, Barrès V,
Rahimi K, Létourneau IJ, Tonin PN, Provencher D and Mes-Masson AM:
RAN nucleo-cytoplasmic transport and mitotic spindle assembly
partners XPO7 and TPX2 are new prognostic biomarkers in serous
epithelial ovarian cancer. PLoS One. 9:e910002014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu HC, Zhang Y, Wang XL, Qin WS, Liu YH,
Zhang L and Zhu CL: Upregulation of the TPX2 gene is associated
with enhanced tumor malignance of esophageal squamous cell
carcinoma. Biomed Pharmacother. 67:751–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu HC, Liu YH, Li SL, Gao DL, Zhang L,
Pang X, Zheng XY and Zhang YH: Expression of TPX2 and Aurora A in
esophageal squamous carcinoma and their significance in clinical
pathology. Cancer Res Prev Treat. 36:932–935. 2009.
|
|
25
|
Elmore JM, Kadesky KT, Koeneman KS and
Sagalowsky AI: Reassessment of the 1997 TNM classification system
for renal cell carcinoma. Cancer. 98:2329–2234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong SK, Jeong CW, Park JH, Kim HS, Kwak
C, Choe G, Kim HH and Lee SE: Application of simplified Fuhrman
grading system in clear-cell renal cell carcinoma. BJU Int.
107:409–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shigeishi H, Fujimoto S, Hiraoka M, Ono S,
Taki M, Ohta K, Higashikawa K and Kamata N: Overexpression of the
receptor for hyaluronan-mediated motility, correlates with
expression of microtubule-associated protein in human oral squamous
cell carcinomas. Int J Oncol. 34:1565–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pascreau G, Eckerdt F, Lewellyn AL,
Prigent C and Maller JL: Phosphorylation of p53 is regulated by
TPX2-Aurora A in xenopus oocytes. J Biol Chem. 284:5497–5505. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart S and Fang G: Anaphase-promoting
complex/cyclosome controls the stability of TPX2 during mitotic
exit. Mol Cell Biol. 25:10516–10527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozlü N, Srayko M, Kinoshita K, Habermann
B, O'toole ET, Müller-Reichert T, Schmalz N, Desai A and Hyman AA:
An essential function of the C. elegans ortholog of TPX2 is to
localize activated aurora A kinase to mitotic spindles. Dev Cell.
9:237–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scharer CD, Laycock N, Osunkoya AO, Logani
S, McDonald JF, Benigno BB and Moreno CS: Aurora kinase inhibitors
synergize with paclitaxel to induce apoptosis in ovarian cancer
cells. J Transl Med. 6:792008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crane R, Gadea B, Littlepage L, Wu H,
Ruderman JV and Aurora A: meiosis and mitosis. Biol Cell.
96:215–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leng J, Han C, Demetris AJ, Michalopoulos
GK and Wu T: Cyclooxygenase-2 promotes hepatocellular carcinoma
cell growth through Akt activation: Evidence for Akt inhibition in
celecoxib induced apoptosis. Hepatology. 38:756–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YH, Dong YY, Wang WM, Xie XY, Wang
ZM, Chen RX, Chen J, Gao DM, Cui JF and Ren ZG: Vascular
endothelial cells facilitated HCC invasion and metastasis through
the Akt and NF-κB pathways induced by paracrine cytokines. J Exp
Clin Cancer Res. 32:512013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsubara N, Mukai H, Naito Y, Itoh K,
Komai Y and Sakai Y: First experience of active surveillance before
systemic target therapy in patients with metastaticrenal cell
carcinoma. Urology. 82:118–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waalkes S, Kramer M, Herrmann TR, Schrader
AJ, Kuczyk MA and Merseburger AS: Present state of target therapy
for disseminated renal cell carcinoma. Immunotherapy. 2:393–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robb VA, Karbowniczek M, Klein-Szanto AJ
and Henske EP: Activation of the mTOR signaling pathway in renal
clear cell carcinoma. J Urol. 177:346–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manning BD and Cantley LC: United at last:
The tuberous sclerosis complex gene products connect the
phosphoinositide 3-kinase/Akt pathway to mammalian target of
rapamycin (mTOR) signalling. Biochem Soc Trans. 31:573–578. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dasanu CA, Clark BA III and Alexandrescu
DT: mTOR-blocking agents in advanced renal cancer: An emerging
therapeutic option. Expert Opin Investig Drugs. 18:175–187. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hudes GR: mTOR as a target for therapy of
renal cancer. Clin Adv Hematol Oncol. 5:772–774. 2007.PubMed/NCBI
|