Introduction
Lung cancer has become a common malignant tumor in
the past recent years (1). Along with
tobacco smoking and environmental factors, lung adenocarcinoma is
one of the most important pathological causes of lung cancer
(2), and is the first cause of
cancer-associated mortalities in men (3). The occurrence and development of lung
adenocarcinoma is a disease process involving multiple genes
(4). As the occurrence mechanism has
not been clarified thus far, the treatment for lung cancer still
lacks specificity.
Choline kinase α (CHKA) is an important enzyme at
the crossroads of the main growth factor-triggered survival
signaling pathways such as Ras activation, and is constitutively
activated in certain human tumor cells and tissues (5). In addition, a large number of studies in
cancer cells suggest that CHKA expression and activity are directly
associated not only with increased cancer cell proliferation but
also with malignancy and metastasis (6), making it a potential prognostic
biomarker of certain common cancers such as hepatocellular
carcinoma (7) and ovarian cancer
(8), as well as a potential novel
target for cancer therapy. However, as an important oncogene, its
association with lung adenocarcinoma carcinogenesis and development
has not been reported thus far.
In the present study, the role of CHKA in lung
adenocarcinoma and its correlation with the prognosis of lung
adenocarcinoma was investigated. The present findings may provide a
prognostic indicator and underlying target for lung adenocarcinoma
prevention and therapy.
Materials and methods
Microarray tissue samples
A tissue microarray block containing 119 surgically
resected lung adenocarcinoma specimens and paired non-cancerous
surrounding tissues was constructed using a tissue microarrayer
(Shanghai Outdo Biotech Co. Ltd., Shanghai, China).
Patients and lung adenocarcinoma
samples
A total of 39 cancer samples were randomly retrieved
from lung adenocarcinoma patients who underwent curative resection
at the Provincial Hospital Affiliated to Shandong University
(Jinan, China) from September 2010 to December 2012. The procedure
of human sample collection was approved by the Ethics Committee of
the Provincial Hospital Affiliated to Shandong University. Written
informed consent was obtained from the patients.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using RNA Simple Total RNA
kit (Tiangen Biotech, Co., Ltd., Beijing, China), according to the
manufacturer's protocol. qPCR was performed using a SYBR Green PCR
kit (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in an Applied Biosystems™ 7900HT Fast Real-Time PCR System
(Thermo Fisher Scientific, Inc.). The following thermal cycling
conditions were used: 2 min at 50°C, 1 min at 94°C, 1 min at 55°C
and 1 min at 72°C for 40 cycles, and 5 min at 72°C. The messenger
RNA level of specific genes was normalized against 18S using the
2-∆∆Cq method. The sequences of the primers used in the present
study are listed in Table I.
 | Table I.Sequence of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequence of primers for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) |
|---|
| CHKA forward |
CACTTTCCGAGGCTCATCAC |
| CHKA reverse |
GGACGAGTTCCACATCAGTGT |
| 18S forward |
CGGCTACCACATCCAAGGAA |
| 18S reverse |
GCTGGAATTACCGCGGCT |
Western blotting
Total protein was extracted using Minute TM Total
Protein Extraction Kit for Animal Cultured Cells/Tissues (Invent
Biotechnologies, Inc., Plymouth, MN, USA). The lung cancer tissue
extract was separated on 12% polyacrylamide-sodium dodecyl sulfate
gels, and transferred to polyvinylidene difluoride membranes. The
membranes were blocked with Tris-buffered saline (TBS) with 5% milk
for 2 h. Subsequently, the membranes were washed with TBS with
Tween 20 (TBST) and the membranes were incubated with a rabbit
polyclonal anti-CHKA and anti-GAPDH (catalog nos., ab88053 and
ab9485; 1 µg/ml; Abcam Trading Co., Ltd., Shanghai, China) at 4°C
overnight. Then the membranes were washed with TBST three times and
incubated with Dylight 680, goat anti-rabbit IgG (catalog no.,
A23720; 1:1,000; Abbkine, Inc., Redlands, CA, USA) for 2 h at room
temperature. The protein band, specifically bound to the primary
antibody, was detected with a LI-COR imaging system (LI-COR
Biotechnology, Lincoln, NE, USA).
Immunohistochemistry (IHC) and tissue
microarray analysis
IHC analysis of tumor tissue microarray was
performed using an anti-CHKA antibody (catalog no., AP8179b; Abgent
Biotech Co., Ltd., Suzhou, China). The tissue array was incubated
with this primary anti-CHKA antibody at 4°C overnight, followed by
incubation with a horseradish peroxidase-conjugated secondary
antibody at 37°C for 30 min. Next, the tissue array was incubated
with 3,3′-diaminobenzidine and counterstained with hematoxylin for
detection. Assessment of tissue microarray staining was based on
the percentage of positively stained cells, and staining intensity
was measured by Aperio ImageScope software (Leica Microsystems,
Inc., Buffalo Grove, IL, USA).
Statistical analysis
Overall survival was defined as the interval between
the dates of surgery and mortality. All statistical analyses were
performed with SPSS version 18.0 software (SPSS, Inc., Chicago, IL,
USA). Survival curves were calculated using the Kaplan-Meier method
and compared with the log-rank test. The Cox proportional hazards
model was used to determine the independent factors affecting
survival based on variables selected by univariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
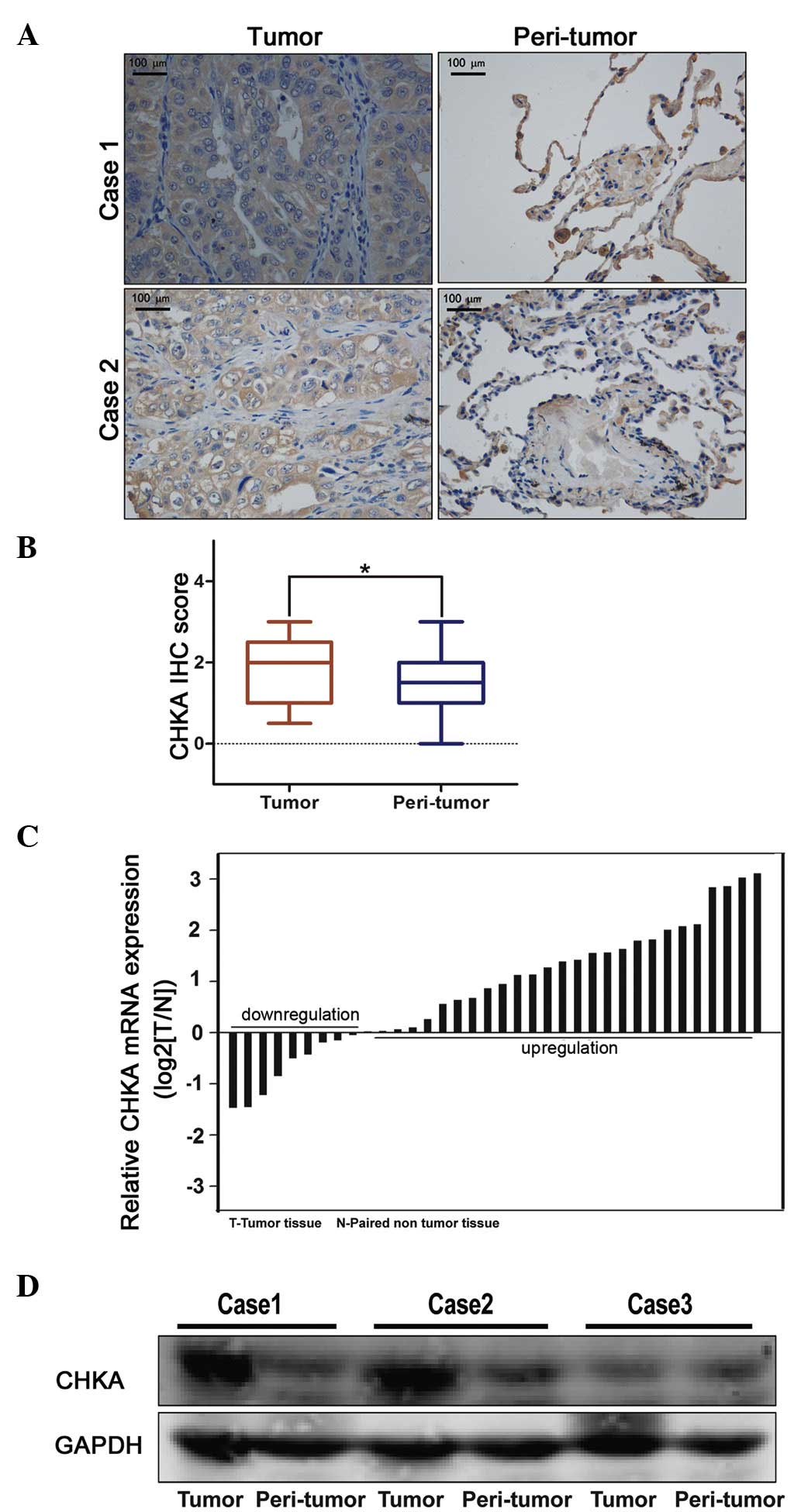
CHKA expression was upregulated in
lung adenocarcinoma
To examine the clinical relevance of expression
deregulation of CHKA in lung adenocacinoma patients, two lung
cancer tissue microarrays containing 119 lung adenocarcinoma
samples were used for IHC staining. It was noticed that the
expression of CHKA was significantly elevated in lung adenocacinoma
samples compared with peri-tumor tissues (Fig. 1A and B). These results were further
confirmed by RT-qPCR and western blot analyses (Fig. 1C and D). This finding prompted us to
speculate whether CHKA is important in lung adenocarcinoma.
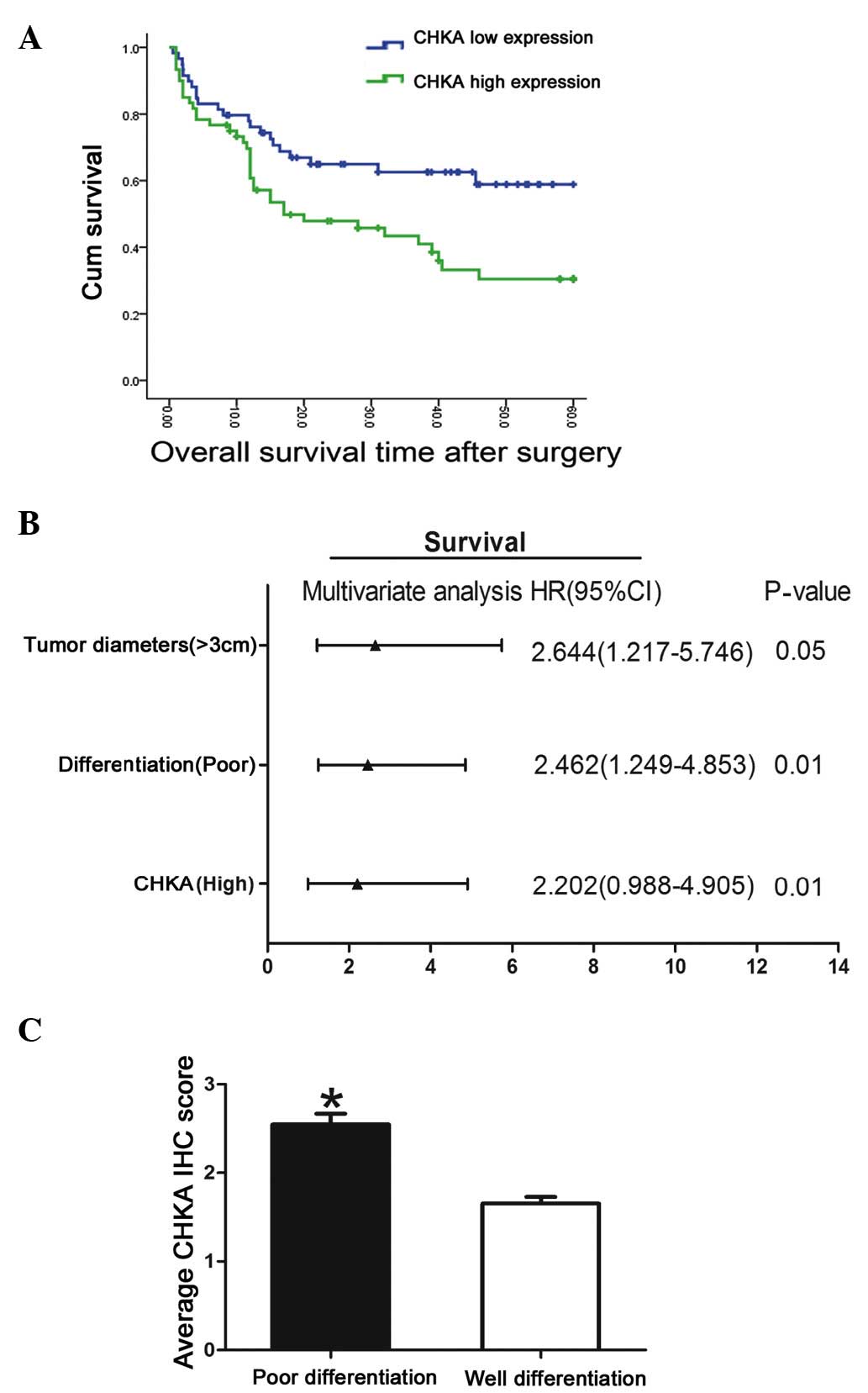
Clinical significance of CHKA
expression in lung adenocarcinoma
The clinical implication of CHKA expression in lung
adenocarcinoma was analyzed. High levels of CHKA were observed to
be associated with poor survival in lung adenocarcinoma patients
(Fig. 2A). The association between
CHKA expression in tumor tissues and the clinicopathological
characteristics of the 119 patients was examined (Table II). Correlation regression analysis
indicated that prognosis was correlated with several individual
parameters, including CHKA expression, tumor diameter, tumor stage
and differentiation (Table III).
These individual parameters were further analyzed with a
multivariate Cox proportional hazards model. The results indicated
that CHKA expression, tumor diameter and differentiation were
significant and independent factors that affected the survival of
lung adenocarcinoma patients (Table
III and Fig. 2B). Among these
factors, the significant hazard ratio (HR) value for the cumulative
survival of CHKA expression levels was 2.644 [95% confidence
interval (CI), 1.217–5.746; P=0.05]. Tumor diameter and
differentiation also exhibited a significant HR value for
cumulative survival. For tumor diameter, the HR value was
determined to be 2.462 (95% CI, 1.249–4.853; P=0.01). Poor
differentiation displayed the greatest HR value (HR, 2.644; 95% CI,
1.217–5.746; P=0.01). Since poor differentiated lung cancer
exhibited the greatest HR value for cumulative survival, the
expression level of CHKA in poor- vs. well-differentiated cancer
was further analyzed. Patients with poor differentiation tended to
have high CHKA expression levels (Fig.
2C). As known, lung cancer patients with poor differentiation
are more inclined to bad prognosis compared with high
differentiation group (9); therefore,
this result suggested that CHKA may act as an oncogene and mediate
the poor prognosis and poor differentiation of lung cancer.
 | Table II.Association between CHKA expression
and clinicopathological characteristics of lung adenocarcinoma
patients. |
Table II.
Association between CHKA expression
and clinicopathological characteristics of lung adenocarcinoma
patients.
|
|
| CHKA expression, N
(%) |
|
|---|
|
|
|
|
|
|---|
| Characteristics | N | Low N=59 | High N=60 | P-value |
|---|
| Age |
|
|
| 0.650 |
| ≤65
years | 63 | 30 (47.6) | 33 (52.4) |
|
| >65
years | 56 | 29 (51.8) | 27 (48.2) |
|
| Gender |
|
|
| 0.230 |
| Male | 61 | 27 (44.3) | 34 (55.7) |
|
|
Female | 58 | 32 (55.2) | 26 (44.8) |
|
| Smoking |
|
|
| 0.770 |
| Yes | 69 | 35 (50.7) | 34 (49.3) |
|
| No | 50 | 24 (48.0) | 26 (52.0) |
|
| Tumor
diametera |
|
|
| 0.020 |
| ≤3
cm | 54 | 33 (61.1) | 21 (38.9) |
|
| >3
cm | 65 | 26 (40.0) | 39 (60.0) |
|
| Lymph metastasis |
|
|
| 0.530 |
| Yes | 72 | 34 (47.2) | 38 (52.8) |
|
| No | 47 | 25 (53.2) | 22 (46.8) |
|
|
Differentiationa |
|
|
| 0.001 |
| Well | 64 | 43 (67.2) | 21 (32.8) |
|
| Poor | 55 | 16 (29.1) | 39 (70.9) |
|
| TNM
stagea |
|
|
| 0.050 |
| T1/2 | 66 | 38 (57.6) | 28 (42.4) |
|
| T3/4 | 53 | 21 (39.6) | 32 (60.4) |
|
 | Table III.Univariate and multivariate analyses
of the primary cohort. |
Table III.
Univariate and multivariate analyses
of the primary cohort.
| Variable | N | Time, monthsb | Univariate
P-value | Multivariate
P-value |
|---|
| CHKA
expressiona |
|
| 0.003 | 0.05 |
| High | 60 | 27.117±6.103 |
|
|
| Low | 59 | 38.186±4.296 |
|
|
| Lymph metastasis |
|
| 0.159 | 0.25 |
|
Yes | 26 | 43.094±3.829 |
|
|
| No | 33 | 37.750±16.277 |
|
|
| Tumor
diametera |
|
| 0.022 | 0.01 |
| ≤3
cm | 54 | 40.074±3.839 |
|
|
| >3
cm | 65 | 23.389±8.494 |
|
|
| TNM stage |
|
| 0.050 | 0.07 |
|
1/2 | 72 | 41.194±3.986 |
|
|
|
3/4 | 47 | 29.989±4.901 |
|
|
| Smoking |
|
| 0.368 | 0.42 |
|
Yes | 69 | 35.225±4.776 |
|
|
| No | 50 | 31.007±6.400 |
|
|
| Age |
|
| 0.482 | 0.53 |
| ≤65
years | 63 | 34.000±4.786 |
|
|
| >65
years | 56 | 30.604±6.271 |
|
|
|
Differentiationa |
|
| 0.000 | 0.01 |
|
Poor | 22 | 21.409±11.274 |
|
|
|
Well | 97 | 36.743±3.636 |
|
|
Discussion
Lung cancer is one of the malignant tumors that
severely threat people's health and life (10). It has a high malignant degree and a
high mortality rate, and its 5-year survival rate is just 10–15%
(11–13). In recent years, its morbidity and
mortality rate have markedly increased (14). The occurrence and development of lung
cancer is a disease process involving multiple genes (15). As the occurrence mechanism has not
been clarified thus far, the treatment for lung cancer still lacks
specificity (16). The biological
characteristics of lung cancer are complicated, with different
types of tumors being driven by different genes and exhibiting
expression of different proteins (17).
CHKA has been identified as the initial enzyme,
playing a regulatory role, in the biosynthesis of
phosphatidylcholine, which occurs via the cytidine
diphosphate-choline pathway (18).
Besides its regular function (19),
CHKA was reported to be important in cancer carcinogenesis and
development (8,20). Granata et al reported that CHKA
could act as a potential druggable target for ovarian cancer by
regulating cell aggressiveness and drug sensitivity (21). In addition, the study by de la Cueva
et al revealed that a CHKA inhibitor combined with
fluorouracil displayed a synergistic antitumoral effect in
colorectal cancer therapy (22).
Furthermore, previous studies on the human triple negative breast
cancer cell line MDA-MB-231 demonstrated that CHKA knockdown
significantly decreased the proliferation and malignance of cancer
cells, supporting CHKA downregulation as a cancer-specific
treatment (23).
In the present study, CHKA expression was observed
to be increased in lung adenocarcinoma, and this elevated
expression was associated with malignant clinicopathological
characteristics. It was observed that the tumor survival rate
substantially differed between patients with high and low
expression levels of CHKA in tumor tissues. Multivariate analysis
revealed that CHKA expression was an independent and significant
risk factor affecting the survival rate subsequent to curative
resection, with the greatest HR value identified for survival.
Importantly, CHKA overexpression displayed enhanced accuracy in
predicting poor outcome in patients with lung adenocarcinoma at
early stages. Furthermore, CHKA expression exhibited a close
correlation with poor differentiation in lung adenocarcinoma; thus,
further analysis of CHKA expression would help to assess the
prognosis of lung adenocarcinoma patients.
In conclusion, the present data provided a new
insight into the prognosis of lung adenocarcinoma and the
underlying target therapy of patients. Therefore, the authors
propose that CHKA functions as a proto-oncogene and
contributes to malignant progression.
Acknowledgements
The authors would like to thank Dr Qi Wang
(Department of Respiratory Medicine, Provincial Hospital Affliated
to Shandong University, Jinan, China) for his technical assistance.
The present study was supported by a grant from the Technology
Development Schedule of Shandong Province (Jinan, China; grant
number 2010G0020227).
References
|
1
|
Quoix E and Lemarié E: Epidemiological
novelties in lung cancer. Rev Mal Respir. 28:1048–1058. 2011.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasaki H, Maekawa M, Tatematsu T, Okuda K,
Moriyama S, Yano M and Fujii Y: Increased BRAF copy number in lung
adenocarcinoma. Oncol Lett. 9:709–712. 2015.PubMed/NCBI
|
|
3
|
Shields PG: Molecular epidemiology of
smoking and lung cancer. Oncogene. 21:6870–6876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woo HI, Kim JA, Jung HA, Kim KK, Lee JY,
Sun JM, Ahn JS, Park K, Lee SY and Ahn MJ: Correlation of genetic
polymorphisms with clinical outcomes in pemetrexed-treated advanced
lung adenocarcinoma patients. Pharmacogenomics. 16:383–391. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janardhan S, Srivani P and Sastry GN:
Choline kinase: An important target for cancer. Curr Med Chem.
13:1169–1186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clem BF, Clem AL, Yalcin A, Goswami U,
Arumugam S, Telang S, Trent JO and Chesney J: A novel small
molecule antagonist of choline kinase-α that simultaneously
suppresses MAPK and PI3K/AKT signaling. Oncogene. 30:3370–3380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwee SA, Hernandez B, Chan O and Wong L:
Choline kinase alpha and hexokinase-2 protein expression in
hepatocellular carcinoma: Association with survival. PLoS One.
7:e465912012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Granata A, Nicoletti R, Perego P, Iorio E,
Krishnamachary B, Benigni F, Ricci A, Podo F, Bhujwalla ZM,
Canevari S, et al: Global metabolic profile identifies choline
kinase alpha as a key regulator of glutathione-dependent
antioxidant cell defense in ovarian carcinoma. Oncotarget.
6:11216–11230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen H, Shen J, Wang L, Shi Z, Wang M,
Jiang BH and Shu Y: Low miR-145 expression level is associated with
poor pathological differentiation and poor prognosis in non-small
cell lung cancer. Biomed Pharmacother. 69:301–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wood SL, Pernemalm M, Crosbie PA and
Whetton AD: Molecular histology of lung cancer: From targets to
treatments. Cancer Treat Rev. 41:361–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riquet M, Mordant P, Pricopi C, Legras A,
Foucault C, Dujon A, Arame A and Le Pimpec-Barthes F: A review of
250 ten-year survivors after pneumonectomy for non-small-cell lung
cancer. Eur J Cardiothorac Surg. 45:876–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wao H, Mhaskar R, Kumar A, Miladinovic B
and Djulbegovic B: Survival of patients with non-small cell lung
cancer without treatment: A systematic review and meta-analysis.
Syst Rev. 2:102013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vyas R, Haque S, Mital SP and Srinivasan
S: Analysis of incidence of lung cancer in various states of the
USA. International Medicine Engineering and Informatics. 7:36–45.
2015. View Article : Google Scholar
|
|
15
|
Quintans JS, Antoniolli AR, Onofre FM and
Onofre AS: Detection of lung cancer using multiple genetic markers
- a systematic review. Diagn Cytopathol. 41:834–842. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gottschling S, Jensen K, Herth FJ, Thomas
M, Schnabel PA and Herpel E: Lack of prognostic significance of
neuroendocrine differentiation and stem cell antigen co-expression
in resected early-stage non-small cell lung cancer. Anticancer Res.
33:981–990. 2013.PubMed/NCBI
|
|
17
|
Korpanty GJ, Graham DM, Vincent MD and
Leighl NB: Biomarkers that currently affect clinical practice in
lung cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol.
4:2042014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morton CC, Aitchison AJ, Gehrig K and
Ridgway ND: A mechanism for suppression of the CDP-choline pathway
during apoptosis. J Lipid Res. 54:3373–3384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serran-Aguilera L, Nuti R, López-Cara LC,
Ríos-Marco P, Carrasco MP, Marco C, Entrena A, Macchiarulo A and
Hurtado-Guerrero R: Choline kinase active site provides features
for designing versatile inhibitors. Curr Top Med Chem.
14:2684–2693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lacal JC and Campos JM: Preclinical
characterization of RSM-932A, a novel anticancer drug targeting the
human choline kinase alpha, an enzyme involved in increased lipid
metabolism of cancer cells. Mol Cancer Ther. 14:31–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granata A, Nicoletti R, Tinaglia V, De
Cecco L, Pisanu ME, Ricci A, Podo F, Canevari S, Iorio E, Bagnoli M
and Mezzanzanica D: Choline kinase-alpha by regulating cell
aggressiveness and drug sensitivity is a potential druggable target
for ovarian cancer. Br J Cancer. 110:330–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de la Cueva A, Ramírez de Molina A,
Alvarez-Ayerza N, Ramos MA, Cebrián A, Del Pulgar TG and Lacal JC:
Combined 5-FU and ChoKα inhibitors as a new alternative therapy of
colorectal cancer: Evidence in human tumor-derived cell lines and
mouse xenografts. PLoS One. 8:e649612013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gadiya M, Mori N, Cao MD, Mironchik Y,
Kakkad S, Gribbestad IS, Glunde K, Krishnamachary B and Bhujwalla
ZM: Phospholipase D1 and choline kinase-α are interactive targets
in breast cancer. Cancer Biol Ther. 15:593–601. 2014. View Article : Google Scholar : PubMed/NCBI
|