Introduction
In animal models, consumption of a molecular iodine
(I2) diet has been demonstrated to prevent
N-methyl-N-nitrosourea and dimethylbenz(a)anthracene induction of
breast tumours (1,2) and to reduce breast hyperplasia and
perilobular/ductal fibrosis (3).
Similarly, clinical trials have revealed that I2 has
beneficial effects in fibrocystic breast disease (4) and in cyclic mastalgia (5), suggesting that iodine therapy may
diminish breast disease, including breast cancer progression
(6,7).
Povidone-iodine (PVP-I) is widely used in clinical practice as an
antiseptic agent following tumour surgery. PVP-I has been
demonstrated to induce the death of epithelial HeLa cells and to
reduce oral mucosal tissue in rats (8). Further data supporting a potent
anticancer effect of I2 and of iodolactones has been established
via cell culture investigations, which have revealed a significant
decrease in cell growth in human breast cancer cell lines (9,10).
In a previous study, we showed that, in addition to
breast carcinoma cells, the proliferation of seven other human
malignant cell lines (neuroblastoma, glioma, melanoma, and lung,
colon and pancreas carcinomas) was variably diminished by
I2 and iodolactones (11).
With the intention of developing an iodine-based anticancer
therapy, the present study extended this research by comparing the
antiproliferative/cytotoxic capability of I2, potassium
iodide (KJ), combined KJ + I2, PVP-I and Lugol's
solution on various human carcinoma cell lines. The data suggest
that PVP-I and the combination of iodide and I2 may be potential
tools to directly interfere with tumour cell growth.
Materials and methods
Materials
The following human cell lines were used in the
present study: MCF-7 (breast carcinoma); HS-24, H1299 and A549
(lung carcinoma); Capan-2 and PaTu 8902 (pancreatic carcinoma),
these cells lines were provided by Dr. Margarete Fischer of The
Bosch Institute of Clinical Pharmacology, Stuttgart, Germany, and
the IPC-298 (melanoma) cell line, provided by the Institute for
Radiobiology, SanAk, Munich, Germany). The fluorescent dye Hoechst
33342 was obtained from Calbiochem (Merck Millipore, Darmstadt,
Germany). Gibco Ham's F12 medium, fetal calf serum (FCS),
streptomycin and penicillin were purchased from Thermo Fisher
Scientific, Inc. (Carlsbad, CA, USA). I2, KJ and Lugol's
solution were obtained from Merck Millipore, and PVP-I was obtained
from Mundipharma International, Ltd. (Cambridge, UK).
Cell culture
The cells were seeded at a low density (200,000
cells per well) and cultured in Ham's F12, completed with 10% FCS
and 1% penicillin/streptomycin. After 5 h, the following iodine
compounds were added (final concentrations are given separately):
i) KJ (100 µM), or I2 (20 µM), or combined
KJ/I2 (100 µM/20 µM, respectively) from freshly prepared
stocks dissolved in sterile water (for KJ) or pure ethanol (for
I2); ii) PVP-I corresponding to 20–80 µM I2;
iii) Lugol's solution corresponding to 20–80 µM I2 and
60–240 µM KJ. The cells were then cultured for a further 6 days. In
separate experiments, fresh sodium-heparin human blood (taken from
one of the authors in MCZ Synlab, Leinfelden) was incubated with
PVP-I (0.5, 1 or 2 mM I2) or Lugol's solution (2 mM
I2) or I2 (2 mM) for 30 min and then centrifuged (1,200
rpm, 12 min). Each supernatant (blood plasma; 200 µl) was diluted
with 1,800 ml cell culture medium. MCF-7 cells were cultured with
these plasma-containing medium samples for 6 days, as above.
Staining and counting of cells
In order to determine the total cell densities, cell
cultures were washed twice with fresh medium in order to remove
detached dead cells, stained with Hoechst 33342 (1:5,000 in
phosphate-buffered saline) for 10 min, and washed again. From each
cell culture well, digital images of 26 different areas of 1.5
mm2 were captured, and the densities of stained nuclei
were determined using ImageJ software (11). The results were calculated as relative
values (%) compared to untreated cells (100%).
Statistical analysis
All experiments were performed in duplicate and
repeated (n=4–6). Results are presented as the means ± standard
deviations. In all cases, statistical significance comparing
experimental values with corresponding controls was estimated by
means of student t-test (for details see figure legends).
Results
Antiproliferative/cytotoxic effects of
I2, KJ and the combination of the two in different carcinoma cell
lines
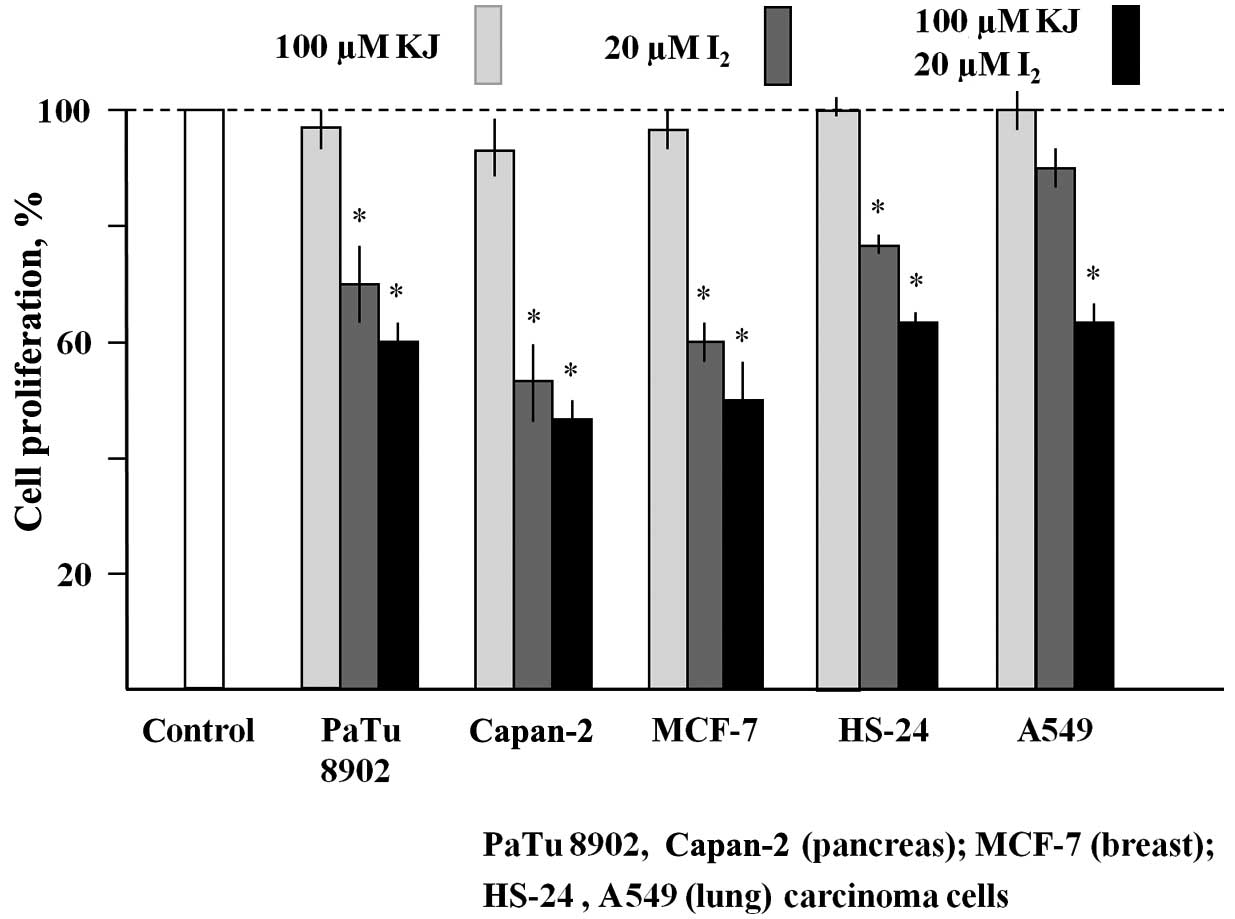
Various human carcinoma cell lines (PaTu 8902,
Capan-2, MCF-7, HS-24 and A549) were cultured in the presence of
100 µM KJ or 20 µM I2, or a combination of the two (KJ +
I2) for 6 days (Fig. 1).
While KJ had no antiproliferative effect, the presence of
I2 inhibited the cell growth of all cell lines tested.
The antiproliferative activity was pronounced in the pancreas
carcinoma line Capan-2 and the breast carcinoma line MCF-7
(reduction of cell densities by 55% and 50%, respectively, compared
with untreated cells). Fig. 1 also
shows that, in all cell lines, the inhibitory effect of 20 µM
I2 was enhanced in the presence of 100 µM KJ.
Antiproliferative/cytotoxic effects of
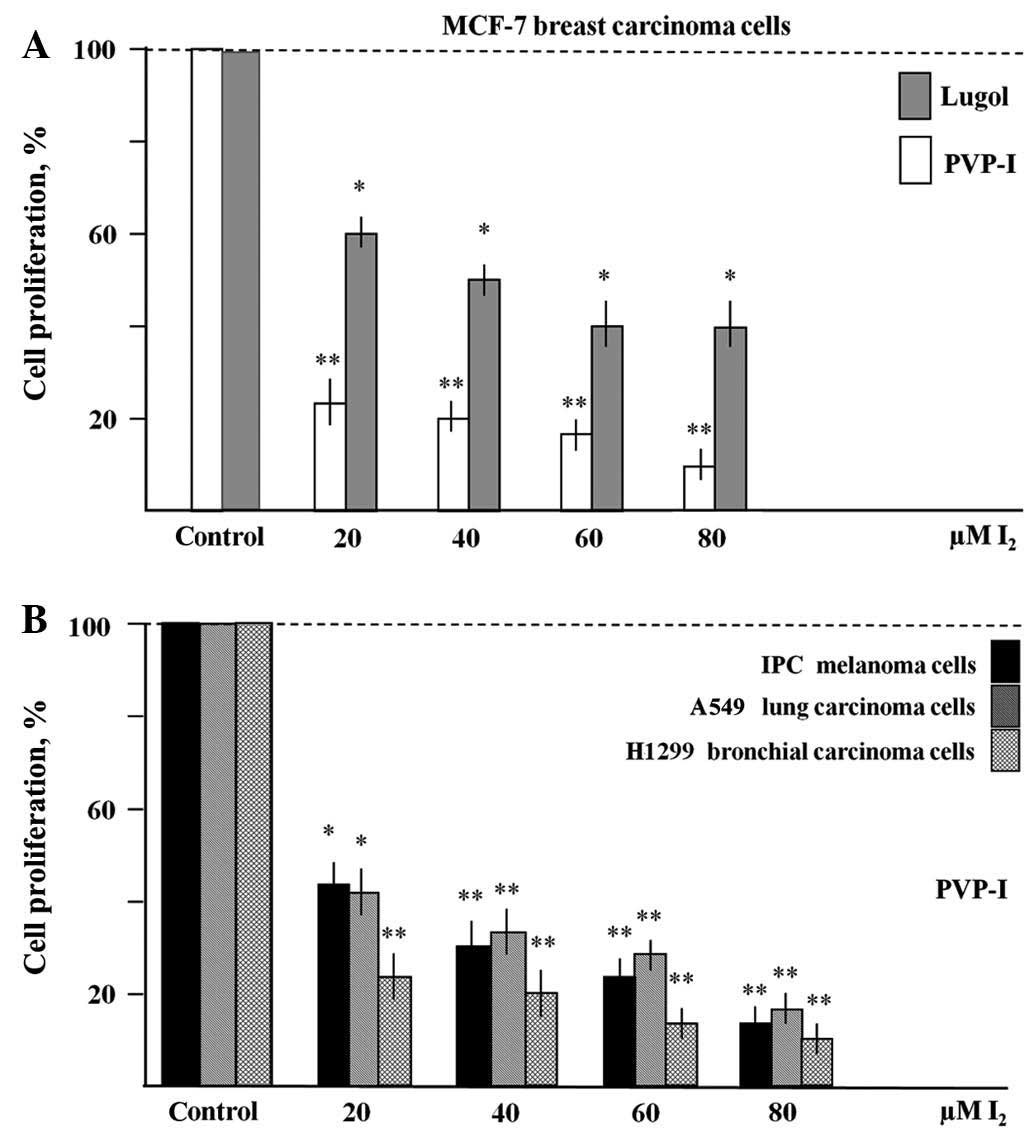
PVP-I and Lugol's solution on MCF-7, IPC, A549 and H1299 cells
MCF-7 breast carcinoma, IPC melanoma, A549 lung
carcinoma and H1299 bronchial carcinoma cells were cultured in the
presence of the iodine compounds for 6 days and analysed as
described. Fig. 2A shows that
treatment with PVP-I (20–80 µM I2) resulted in a
significant reduction (by 75–85%) of total MCF-7 cell numbers when
compared to untreated controls within 6 days. Similarly, Lugol's
solution (20–80 µM I2 and 60–240 µM KJ) inhibited the
growth of MCF-7 cells by 40–60%. Fig.
2B shows comparable antiproliferative effects of PVP-I in IPC
melanoma, A549 and H1299 cells (reduction of cell densities by
60–90%). In all experiments shown in Fig.
2, the inhibitory effects of the iodine compounds were clearly
dose-dependent and significant.
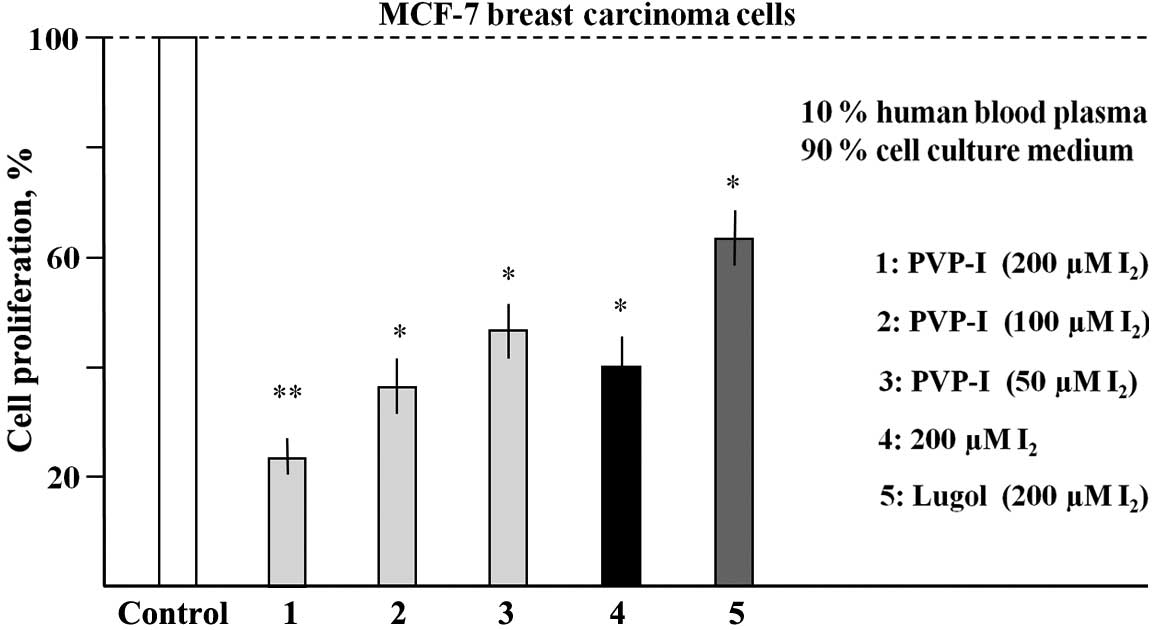
Effects of iodine-containing (PVP-I,
Lugol's solution, I2) human blood samples on the proliferation of
MCF-7 cells
Fresh heparinised human blood was incubated with
PVP-I, Lugol's solution or with I2 for 30 min and centrifuged to
obtain blood plasma supernatants. MCF-7 cells were cultured in
medium containing 10% of the plasma supernatants for 6 days. In
Fig. 3, for each of these different
samples a final concentration of I2 is given, which
would be expected if there were no loss of I2 by blood cell binding
or by other components removed by centrifugation.
As shown in Fig. 3,
PVP-I treatment resulted in a dose-dependent inhibition of
proliferation of MCF-7 cells. At calculated (maximal) final
concentrations of 200, 100 and 50 µM I2, the reductions
in proliferation were 71.6, 63.1 and 56.5%, respectively. A
calculated (maximal) concentration of 200 µM molecular iodine led
to a growth inhibition of 69.5%. Lugol's solution, however,
exhibited a markedly less effective inhibition (26.3%) in this type
of experiment. These data clearly indicate that I2
(PVP-I or molecular iodine) in human blood retains its
antiproliferative/cytotoxic activity to a significant degree.
Discussion
In accordance with our previous results (11), the present data strongly confirm I2 as
a potent inhibitor of carcinoma growth. KJ was found to have no
effect, but at high concentrations enhances the
antiproliferative/cytotoxic effect of molecular iodine. Although
the mechanisms of action were not analysed here, it is most likely
that mitochondrial-mediated apoptosis pathways are involved as
previously described for I2 and iodolactones (6,9–13).
The present results also revealed a highly
significant dose-dependent antiproliferative/cytotoxic effect of
PVP-I in cell cultures of MCF-7 breast carcinoma, IPC melanoma,
A549 lung carcinoma and H1299 bronchial carcinoma cells. At a
concentration corresponding to 20 µM I2, within 6 days
the growth of MCF-7, IPC melanoma, A549 and H1299 cells was reduced
by 76, 57, 59 and 77%, respectively. This is consistent with
previous data demonstrating cytotoxic activity of PVP-I on CT26
colon cancer and H-22 ascites cells in vitro and in
vivo (14). In the present study,
Lugol's solution was also revealed to reduce the growth of MCF-7
cells (40% at a concentration corresponding to 20 µM I2
and 60 µM KJ).
However, with respect to a therapeutic application,
further knowledge concerning the resorption, systemic distribution,
organ-specific uptake and metabolism of iodine compounds is
necessary. A number of reports have indicated that I2 exerts
antineoplastic effects in thyroid, mammary and prostate glands, all
of which were shown to take up I2, whereas iodide
supplementation had no effect (2,15,16). This is in line with the observation
that I2 treatment of patients with benign breast disease
led to a bilateral reduction in breast size and a remission of
disease symptoms, which was not observed when iodide was
administered (17). Other authors
have proposed that iodine, if ingested as I2 at concentrations
higher than 3 mg per day, acts as an antioxidant and prevents
lipoperoxidation in various organs, including the brain (18). In a recent study, Delgado et al
(19) supplemented Sprague-Dawley
rats with I2 and KJ in drinking water. They found that
iodine ingestion and intestinal uptake was similar for both forms
of iodine compounds. Following systemic distribution, iodide was
preferentially taken up and retained by the thyroid, lactating
mammary gland and breast milk, whereas pituitary, ovary and virgin
mammary gland were found to take up both iodine compounds, but
preferentially I2. Taken together, it appears that I2 is
taken up, distributed systemically and preserved in blood for
tissue absorption. This is in agreement with the data obtained in
the present study with fresh human blood, indicating that the
antiproliferative activity of PVP-I, I2 and also, to a certain
degree, Lugol's solution, is preserved in fresh human blood.
As PVP-I is already widely used in clinical practice
as an antiseptic and flushing agent following tumour surgery, and
PVP has even been used intravenously as a plasma expander in
emergency medicine, the present data strongly suggest that PVP-I
and possibly also Lugol's solution or I2 may be potent agents for
the development of potential antitumour strategies in humans,
either by systemic or local stereotactic application.
Acknowledgements
The authors would like to thank Dr Heiko van der
Kuip, (Dr Margarete Fischer-Bosch Institute of Clinical
Pharmacology, Stuttgart, Germany) for providing human carcinoma
cell lines, and Dr John M. Lindquist (Surgeon and General
Practitioner and native speaker) for language correction and
proofreading.
References
|
1
|
Funahashi H, Imai F, Tanaka Y, Tobinaga J,
Wada M, Morita T, Yamada F, Tsukamura K, Oiwa M, Kikumori T, et al:
Suppressive effect of iodine on DMBA-induced breast tumour growth
in the rat. J Surg Oncol. 61:209–213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
García-Solís P, Alfaro Y, Anguiano B,
Delgado G, Guzman RC, Nandi S, Díaz-Muñoz M, Vázquez-Martínez O and
Aceves C: Inhibition of N-methyl-N-nitrosourea-induced mammary
carcinogenesis by molecular iodine (I2) but not by iodide (I-)
treatment evidence that I2 prevents cancer promotion. Mol Cell
Endocrinol. 236:49–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eskin BA, Grotowski CE, Conolly CP and
Ghent WR: Different tissue responses for iodine and iodide in rat
thyroid and mammary glands. Biol Trace Elem Res. 49:9–19. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghent WR, Eskin BA, Low DA and Hill LP:
Iodine replacement in fibrocystic disease of the breast. Can J
Surg. 36:453–460. 2004.
|
|
5
|
Kessler JH: The effect of supraphysiologic
levels of iodine on patients with cyclic mastalgia. Breast J.
10:328–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aceves C, Anguiana B and Delgado G: Is
iodine a gatekeeper of the integrity of the mammary gland? J
Mammary Gland Biol Neoplasia. 10:189–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torremante P: Mastopathie, breast cancer
and iodolactone. Dtsch Med Wochenschr. 129:641–645. 2004.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato S, Miyake M, Hazama A and Omori K:
Povidone-iodine- induced cell deathin cultured human epithelial
Hela cells and rat oral mucosa tissue. Drug Chem Toxicol.
37:269–275. 2014. View Article : Google Scholar
|
|
9
|
Arroyo-Helguera O, Anguiano B, Delgado G
and Aceves C: Uptake and antiproliferative effect of molecular
iodine in the MCF-7 breast cancer cell line. Endocr Relat Cancer.
13:1147–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shrivastava A, Tiwari M, Sinha RA, Kumar
A, Balapure AK, Bajpai VK, Sharma R, Mitra K, Tandon A and Godbole
MM: Molecular iodine induces caspase-independent apoptosis in human
breast carcinoma cells involving mitochondria-mediated pathway. J
Biol Chem. 28:19762–19771. 2006. View Article : Google Scholar
|
|
11
|
Rösner H, Torremante P, Möller W and
Gärtner R: Antiproliferative/cytotoxic activity of molecular iodine
and iodolactones in various human carcinoma cell lines. No
interfering with EGF-signaling, but evidence for apoptosis. Exp
Clin Endocrinol Diabetes. 118:410–419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nava-Villalba M, Nuñez-Anita RE, Bontempo
A and Aceves C: Activation of peroxisome proliferator-activated
receptor gamma is crucial for the antitumor effect of
6-iodolactone. Mol Cancer. 14:1682015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arroyo-Helguera O, Rojas E, et al:
Signaling pathways involved in the antiproliferative effect of
molecular iodine in normal and tumoral breast cells: Evidence that
6-iodolactone mediates apoptotic effects. Endocr Relat Cancer.
15:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun P, Zhao J, Luo ZC, Zhang P, Chen P,
Zhang XL, Luo S, Yang DB, Tan J, Zhou Y, et al: Diluted
povidone-iodine inhibits tumour growth through apoptosis-induction
and suppression of SOD activity. Oncol Rep. 27:383–388.
2012.PubMed/NCBI
|
|
15
|
Ghent WR, Eskin BA, Low DA and Hill LP:
Iodine replacement in fibrocystic disease of the breast. Cancer J
Surg. 36:453–460. 1993.
|
|
16
|
Aceves C, Anguiano B and Delgado G: The
extrathyronine actions of iodine as antioxidant, apoptotic, and
differentiation factor in various tissues. Thyroid. 23:938–946.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kessler JH: The effect of
supraphysiological levels of iodine on patients with cyclic
mastalgia. Breast J. 10:328–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winkler R, Griebenow S and Wonisch W:
Effect of iodide on total antioxidant status of human serum. Cell
Biochem Funct. 18:143–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delgado G, Muñoz-Torres C, Orozco-Esquivel
T, Anguiano B and Aceves C: Total iodine quantification in fluids
and tissues from iodine- or iodide-supplemented rats by ion
chromatography following microwave-assisted digestion. Thyroid.
25:352–360. 2015. View Article : Google Scholar : PubMed/NCBI
|