Introduction
Ovarian cancer (OC) has the worst prognosis among
the gynecological malignancies (1).
Each year, ~21,980 new cases are diagnosed in the United States and
~14,270 women succumb to OC (1).
During the last decade, novel insights have led to a dualistic
model of the carcinogenesis of OC (2–5). This
model assists in the grouping of various different histological
subtypes into two broad categories (3,4). Low-grade
serous OC, endometrioid OC, clear cell OC and mucinous OC represent
type I OC (3–5). These conditions generally present as a
large tumor that is confined to a single ovary. Mutations in
isoform b of rapidly accelerated fibrosarcoma protein (BRAF),
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PI3KCA), catenin β-1 (CTNNB1), phosphatase and tensin homolog,
Kirsten rat sarcoma viral oncogene homolog, and AT-rich interactive
domain-containing protein 1A are responsible for a step-wise
progression from normal epithelium through differing degrees of
atypia to non-invasive and then invasive type I carcinoma (3–5). Type II
OC mainly consists of high-grade serous OC and presents as advanced
disease with a poor prognosis (3–5). In the
majority of type II OCs, alterations in p53 are responsible for
genetic instability, with marked chromosomal aberrations (6).
Hepatocyte growth factor/scatter factor (HGF/SF) and
its receptor tyrosine kinase, c-met, the product of the c-met
proto-oncogene, provide vital signals for survival and
long-distance epithelial and myogenic precursor cell migration
during embryogenesis (7). Cancer
cells are able to use HGF/SF-c-met for invasion and metastasis
(7). Aberrant c-met activation
results from various mechanisms and occurs in a variety of
different types of cancer, including renal cancer, hepatocellular
cancer, basal-like breast cancer, lung cancer and OC (8). The intracellular signaling cascades
activated by c-met include the phosphoinositide 3-kinase-protein
kinase B (PI3K-PKB) and the RAS-mitogen-activated protein kinase
(MAPK) pathways (7,9). A complex cross-signaling network
involving the c-met epidermal growth factor receptor,
c-met-vascular endothelial growth factor receptor (VEGFR) and
c-met-Wingless-related integration site-CTNBB1 pathways has also
emerged (10,11). According to the results of a phase II
trial, cabozantinib, a potent inhibitor of c-met and VEGFR-2,
exhibited clinical activity in 68 OC patients, as suggested by the
recorded response rates (12).
However, the prognostic impact of c-met in OC remains controversial
(13–15).
Therefore, the present study examined the expression
of c-met and its prognostic effect in an unselected, consecutive
cohort of patients with OC.
Materials and methods
Patients and tissue samples
The archives were searched for all patients with OC
who underwent primary surgery at University Medical Center Mainz
between 2004 and 2011. Patients entered the study only if
formalin-fixed, paraffin-embedded (FFPE) tissue samples were
available. Follow-up was performed by writing letters to patients
or their physicians and by checking the patient records until May
2013. Mortality from OC or other reasons unrelated to cancer, and
recurrence of disease, which included metastasis and local relapse,
was documented. Patient charts were reviewed to collect data
regarding age at diagnosis, histological grade, tumor stage of
disease in accordance to the International Federation of Gynecology
and Obstetrics guidelines, as previously reported (16,17).
Briefly, the amount of residual disease subsequent to primary
surgery was registered as no tumor burden (R0), or as residual
disease <2 cm (R1) or >2 cm (R2). Completed chemotherapy was
defined as 6 courses of platinum-based monotherapy in early OC or
as platinum-based combination therapy with paclitaxel for patients
with advanced OC. Pathological review of all cases was performed by
one individual in order to determine the histological type: In
accordance with the current FIGO classification from 2014, all
serous OC were classified as high-grade or low-grade serous OC as
suggested by Malpica et al (18). In contrast, the current FIGO
classification relies on the traditional three-tier grading system
from 1971 for the mucinous, endometrioid, clear-cell and mixed
subtypes (3–5). The study was approved by the Research
Ethics Committee of the University Medical Centre Mainz (Mainz,
Germany). Informed consent was obtained from all patients. All
specimens were handled according to the ethical and legal
standards.
Immunohistochemistry and evaluation of
c-met staining
Immunohistochemical analyses were performed on 4-µm
thick sections according to standard procedures. Serial sections of
FFPE tumor tissues were stained with polyclonal anti-HGF R/c-met
goat anti-human antibodies (catalog no. AF276; R&D Systems
GmbH, Wiesbaden, Germany). The immunoreaction was visualized using
Histofine® Simple Stain MAX peroxidase anti-goat
antibody (catalog no. 414162F; Medac, Tornesch, Germany).
Immunohistochemistry assays were performed in accordance with the
manufacturer's protocols. All series included appropriate positive
(human liver tissues) and negative (distilled water used as a
substitute for anti-HGF-R antibodies in human liver tissues)
controls with adequate results.
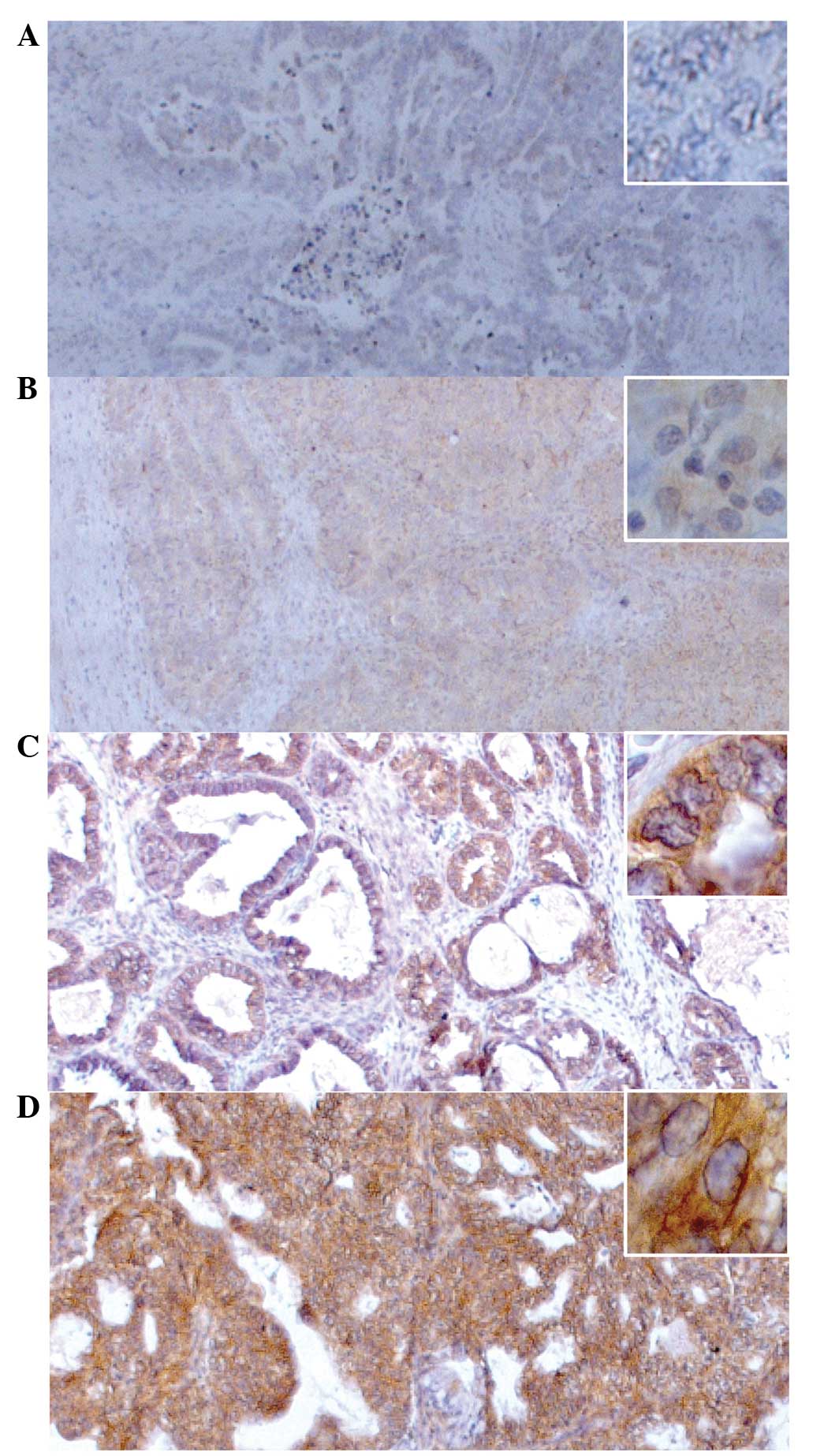
c-met was assessed using a four-tier system, as
previously described by Yamamoto et al for c-met in OC
(13). Briefly, no discernible
staining or background type staining was considered as negative
(score of 0); definite cytoplasmic staining and/or equivocal
discontinuous membrane staining scored 1+; unequivocal membrane
staining with mild to moderate intensity scored 2+; and strong and
complete membrane staining scored 3+ (Fig. 1). c-met was assessed as 2+ or 3+ only
if at least 10% of the tumor cells showed complete membrane
staining (13). A score of 2+ or 3+
was defined as c-met overexpression in accordance to the criteria
reported by Yamamoto et al (13). The immunohistochemical evaluation was
performed independently by two individuals trained in histological
and immunohistochemical diagnostics, who were unaware of the
clinical outcome. Slides with a different assessment were discussed
until a consensus was reached.
Statistical analysis
Statistical analyses were performed using the SPSS
statistical software program, version 23.0 (IMB SPSS, Armonk, NY,
USA). Patient characteristics are presented in absolute and
relative numbers or as the median and quartiles. Comparison between
clinicopathological factors and the overexpression of c-met was
calculated using the χ2 test. The Cox proportional
hazard regression model was used to evaluate the effect of
investigative variables on progression-free survival (PFS) and
disease-specific survival (DSS). PFS was defined as the time-frame
between the date of the first histological proof of OC and the date
of the first progression. DSS was defined as the time-frame between
the date of the first histological proof of OC and the date of
death due to OC. Univariate Cox-regression analysis was performed
for every single variable. Variables with a P-value of <0.05
entered the multivariate Cox-regression analysis with a variable
selection via backward elimination. All associations were reported
as hazard ratios (HRs) with 95% confidence interval (CIs) and
P-values. Kaplan-Meier estimations were performed to describe
survival rates. As this was an investigative study, no adjustments
for multiple testing were made. The statistical tests were
performed for illustrative purposes only. P-values were awarded for
descriptive reasons only and should be interpreted with caution and
in connection with effect estimates.
Results
A total of 146 patients were screened. Of these, 8
and 29 patients were excluded due to missing tissue samples and
incomplete follow-up information, respectively. A further 3
patients suffered from an ovarian borderline tumor. Therefore, 106
patients were enrolled in the study. The median follow-up time was
28.2 months. In this time, 69 (65.1%) cases of recurrence and 44
(41.5%) mortalities due to OC occurred. A total of 58 (54.7%)
patients exhibited high-grade serous OC, and 48 (45.3%) patients
exhibited type I OC. The characteristics of the patients are
presented in Table I.
 | Table I.Patient characteristics (n=106). |
Table I.
Patient characteristics (n=106).
| Parameter | Value |
|---|
| Age, years |
|
| Mean
(±SD) | 59.08 (±12.60) |
|
Interquartile range | 51.44–70.36 |
| Tumor stage (FIGO), n
(%) |
|
| I | 22 (20.8) |
| II | 6 (5.7) |
| III | 63 (59.4) |
| IV | 15 (14.2) |
| Histological grade, n
(%)a |
|
| G1 | 3 (12.5) |
| G2 | 11 (45.8) |
| G3 | 10 (41.7) |
| Histological type, n
(%) |
|
| Type
I | 48 (45.3) |
| Type
II | 58 (54.7) |
|
Serous | 82 (77.4) |
|
High-grade
serous | 58 (54.7) |
|
Low-grade
serous | 24 (22.6) |
|
Mucinous | 14 (13.2) |
|
Endometrioid | 5 (4.7) |
| Clear
cell | 2 (1.9) |
|
Mixed | 3 (2.8) |
| Post-operative
residual tumor burden, n (%) |
|
| R0 | 62 (58.5) |
| R1 | 33 (31.1) |
| R2 | 11 (10.4) |
| Chemotherapy, n
(%) |
|
|
Complete | 82 (77.4) |
|
Incomplete | 13 (12.3) |
| Missing
data | 11 (10.4) |
| Events, n (%) |
|
|
Relapse | 69 (65.1) |
| Mortality
due to OC | 44 (41.5) |
|
c-met-positive | 22 (20.8) |
Overexpression of c-met was observed in 22 (20.8%)
cases. The characteristics of the patients with overexpression of
c-met are presented in Table II. It
became evident that 8 out of 14 (57.1%) cases with mucinous OC and
5 out of 24 (20.8%) cases with low-grade serous OC showed
overexpression of c-met. In total, 17 out of 48 (35.4%) cases
displayed overexpression of c-met among the patients with type I
OC, whereas only 5 out of 58 (8.6%) of the cases with type II OC
displayed c-met overexpression (P=0.001) (Table III and Fig. 2). Overexpression of c-met was not
associated with any other clinicopathological features (Table III and Fig. 2).
 | Table II.Patients' characteristics with c-met
positive ovarian cancer. |
Table II.
Patients' characteristics with c-met
positive ovarian cancer.
| Case no. | Expression of
c-met | Age, years | Histotype | Grading | Stage | Post-operative tumor
burden, cm | Completeness of
chemotherapy | Follow-up + time
after diagnosis, years |
|---|
| 1 | 2+ | 47 | HGS | – | I | 0 | Yes | ALI, +6.9 |
| 2 | 2+ | 72 | HGS | – | III | <2 | Yes | DOD, +1.2 |
| 3 | 2+ | 75 | HGS | – | IV | <2 | No | DOD, +2.4 |
| 4 | 2+ | 63 | HGS | – | III | <2 | Yes | AWD, +3.5 |
| 5 | 2+ | 66 | HGS | – | III | <2 | Yes | DOD, +8.5 |
| 6 | 2+ | 55 | LGS | – | I | 0 | Yes | ALI, +1.4 |
| 7 | 2+ | 54 | LGS | – | III | >2 | Yes | DOD, +5.0 |
| 8 | 2+ | 65 | LGS | – | III | >2 | N/A | DOD, +4.1 |
| 9 | 2+ | 68 | LGS | – | III | 0 | Yes | DOD, +0.4 |
| 10 | 2+ | 77 | Mucinous | 1 | III | 0 | Yes | AWD, +1.4 |
| 11 | 2+ | 69 | Mucinous | 2 | III | 0 | Yes | DOD, +7.6 |
| 12 | 2+ | 48 | Mucinous | 2 | IV | <2 | Yes | ALI, +0.5 |
| 13 | 2+ | 36 | Mucinous | 2 | I | 0 | Yes | ALI, +4.1 |
| 14 | 2+ | 73 | Mucinous | 2 | I | 0 | Yes | DOD, +1.2 |
| 15 | 2+ | 24 | Mucinous | 2 | III | 0 | Yes | AWD, +1.0 |
| 16 | 2+ | 40 | Mucinous | 3 | III | <2 | Yes | DOD, +0.5 |
| 17 | 2+ | 65 | Endometrioid | 2 | IV | <2 | N/A | DOD, +0.5 |
| 18 | 2+ | 40 | Endometrioid | 2 | I | 0 | Yes | ALI, +4.0 |
| 19 | 2+ | 48 | Clear cell | 3 | III | 0 | Yes | ALI, +4.0 |
| 20 | 2+ | 71 | Mixed | 3 | III | >2 | Yes | DOD, +4.0 |
| 21 | 3+ | 52 | LGS | – | I | 0 | Yes | DOD, +4.9 |
| 22 | 3+ | 57 | Mucinous | 2 | I | 0 | Yes | ALI, +5.7 |
 | Table III.Clinicopathological factors with
regard to the overexpression of c-met. |
Table III.
Clinicopathological factors with
regard to the overexpression of c-met.
| Clinicopathological
factor | Negative, n | Positive, n
(%) |
P-valuea |
|---|
| Ageb |
|
| 0.406 |
|
<59.08 | 41 | 12 (22.6) |
|
|
>59.08 | 43 | 10 (18.9) |
|
| Tumor stage
(FIGO) |
|
| 0.344 |
| I | 15 | 7
(31.8) |
|
| II | 6 | 0 (0.0) |
|
|
III | 51 | 12 (19.0) |
|
| IV | 12 | 3
(20.0) |
|
| Histological grade
(n=24)c |
|
| 0.122 |
| 1 | 2 | 1
(33.3) |
|
| 2 | 3 | 8
(72.7) |
|
| 3 | 7 | 3
(30.0) |
|
| Histological
typeb |
|
| 0.001d |
| Type
I | 31 | 17 (35.4) |
|
| Type
II | 53 | 5 (8.6) |
|
| Post-operative
residual tumor burden |
|
| 0.834 |
| R0 | 50 | 12 (19.4) |
|
| R1 | 26 | 7
(21.2) |
|
| R2 | 8 | 3
(27.3) |
|
| Completeness of
chemotherapy (n=105)b |
|
| 0.186 |
|
Yes | 12 | 1 (7.7) |
|
| No | 63 | 19 (23.2) |
|
According to univariate Cox-regression analysis,
c-met was not associated with PFS or DSS (P=0.835 and 0.414,
respectively) (Table IV). According
to multivariate Cox-regression analysis, only tumor stage and
post-operative tumor burden retained significance in terms of PFS
(P=0.001 and P<0.001, respectively), with HRs of 1.608 (95% CI,
1.210–2.138) and 1.915 (95% CI, 1.345–2.728), respectively. In
terms of DSS, post-operative tumor burden and completeness of
chemotherapy retained their significance (P=0.003 and P=0.012,
respectively), with HRs of 2.077 (95% CI, 1.284–3.360) and 0.321
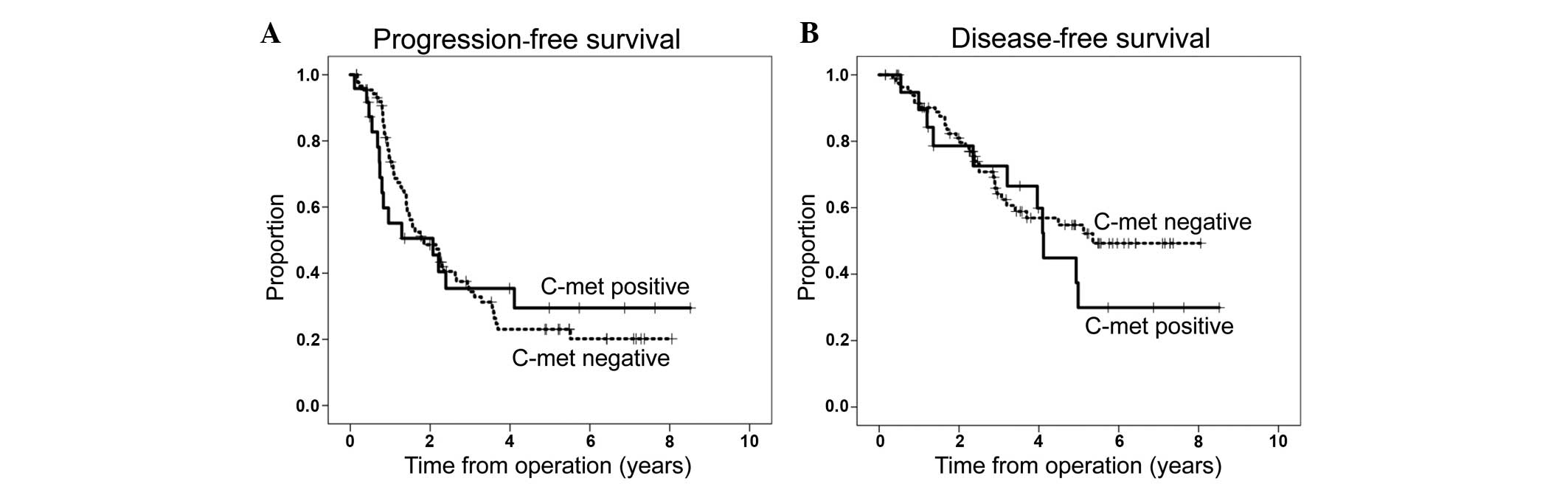
(95% CI, 0.133–0.776), respectively (Table IV). Accordingly, Kaplan-Meier plots
failed to demonstrate an effect of c-met, with no significant
difference between c-met-positive and c-met-negative cases,
respectively, in terms of 5-year PFS rate (24.2 vs. 32.5%, P=0.938)
and 5-year DSS rate (54.8 vs. 29.9%, P=0.412) (Fig. 3A and B). Furthermore, c-met expression
did not alter the 5-year PFS and DSS rates among any subgroups,
including the high-grade serous OC or non-high-grade serous OC
groups (data not shown).
 | Table IV.Univariate and multivariate
Cox-regression analysis for progression-free and disease-specific
survival. |
Table IV.
Univariate and multivariate
Cox-regression analysis for progression-free and disease-specific
survival.
| A, Progression-free
survival |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 0.730
(0.455–1.172) | 0.192 | – |
|
| Tumor stage
(FIGO) | 1.825
(1.406–2.368) |
<0.001a | 1.608
(1.210–2.138) | 0.001a |
| Histological
grade | 1.518
(0.482–4.782) | 0.476 | – |
| Histological
type | 0.692
(0.423–1.132) | 0.142 | – |
| Post-operative
residual tumor burden | 2.420
(1.744–3.358) |
<0.001a | 1.915
(1.345–2.728) |
<0.001a |
| Completeness of
chemotherapy | 0.547
(0.278–1.077) | 0.081 | – |
| c-met | 0.938
(0.511–1.719) | 0.835 | – |
|
|
| B, Disease-specific
survival |
|
|
| Univariate | Multivariate |
|
|
|
|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
| Age, years | 0.569
(0.311–1.040) | 0.067 | – |
|
| Tumor stage
(FIGO) | 1.986
(1.387–2.843) |
<0.001a | 1.396
(0.898–2.170) | 0.138 |
| Histological
grade | 1.251
(0.329–4.764) | 0.742 | – |
|
| Histological
type | 1.002
(0.551–1.821) | 0.996 | – |
|
| Post-operative
residual tumor burden | 2.135
(1.440–3.168) |
<0.001a | 2.077
(1.284–3.360) | 0.003a |
| Completeness of
chemotherapy | 0.312
(0.141–0.688) | 0.004a | 0.321
(0.133–0.776) | 0.012a |
| c-met | 1.330
(0671–2.637) | 0.414 | – |
|
Discussion
To the best of our knowledge, the present study
reports for the first time that the overexpression of c-met is
significantly associated with type I OC in comparison with type II
OC. The study results support the hypothesis that the
overexpression of c-met is involved in the carcinogenesis of type I
OC. This may be due to the fact that c-met is involved in
cross-talks with several pathways, such as RAS-MAPK and PI3K-AKT
(7,9),
known to impact the development of type I OC subtypes (3–5).
Results of studies by Yamamoto et al
(13) and Sawada et al
(14) are in agreement with the
present study observations. To the best of our knowledge, Yamamoto
et al (13) reported the
largest study of c-met overexpression in non-serous OC: In a
consecutive cohort of 201 OC patients, a large subset of 90
patients exhibited clear-cell OC. Not unexpectedly, the reported
c-met protein levels were comparable with the present study
results: C-met overexpression was detected in 22% of clear-cell OC
but not in any non-clear cell OC, and copy number alterations were
detectable in 24% of the clear cell OC and in 3% of the non-clear
cell OC, respectively (13). In 2007,
Sawada et al (14) published
the results of c-met overexpression in a cohort of 138 OC patients.
Within this cohort, 82.6% patients exhibited serous OC and 69.6%
exhibited poorly-differentiated OC. Comparable with the present
results, the study reported c-met overexpression in only 11% of
cases (15/138). Notably, a higher proportion of patients with
mucinous and clear cell OC showed overexpression of c-met (3/10 and
1/3, respectively) (14). However,
potential limitations of these two studies result from the fact
that no further differentiation between high-grade and low-grade
serous OC was performed. Furthermore, the latter study had only a
small proportion of patients with non-serous OC. By contrast, Goode
et al (15) reported a notably
high rate of 97.8% for c-met overexpression in a cohort of >320
consecutive OC patients (moderate staining in 38.9% and strong
staining in 58.9% of the entire cohort). However, no further
differentiation into the various histological subtypes of OC was
provided. In summary, the available literature on the pattern of
expression of c-met in OC is partially contradictory (13–15).
The present study performed Cox-regression analyses
and Kaplan-Meier estimations to investigate the potential
prognostic impact of c-met. According to the data, overexpression
of c-met failed to exhibit any prognostic impact in the entire
cohort, as well as in the subsets of type I or type II OC,
respectively. In line with this analysis, Goode et al
(15) were not able to detect an
association of genotype, protein expression and mortality within a
consecutive cohort of >320 patients. Conversely, Yamamoto et
al (13) reported an independent
negative prognostic effect of c-met, as evaluated by
immunohistochemistry, on overall survival in the subset of clear
cell carcinoma (P=0.018). Supporting the present results, the study
failed to demonstrate any further prognostic impact of c-met in the
entire cohort or in the subset of non-clear cell OC in terms of PFS
(13). Sawada et al (14) showed an independent impaired effect of
c-met overexpression in terms of overall survival (P=0.005), but
not in terms of PFS. Within this cohort, a notably high rate of
82.6% of serous OC cases and a high rate of 69.6% of histological
poorly-differentiated OC patients were included (14). Arguably, potential limitations of
these two studies arise from the fact that no further
differentiation between high-grade and low-grade serous OC was
performed and that the number of patients presenting with
non-serous OC was notably low or was not reported.
Overall, in the present study, the prognostic effect
of the post-operative tumor burden, the tumor stage and the
completeness of chemotherapy, as well as the low rate of c-met
positive cases, are in line with the published literature. However,
limitations result from the retrospective design of the study. In
order to reduce a bias resulting from incomplete follow-up,
patients with incomplete follow-up were excluded from the
study.
In conclusion, to the best of our knowledge, the
present study shows for the first time that c-met was predominantly
overexpressed in type I OC, supporting the hypothesis that c-met
has a role in the development of type I OC. However, c-met
overexpression was not associated with prognosis in this
disease.
Acknowledgements
The abstract was presented at the Annual Meeting of
Deutsche Krebsgesellschaft Feb 2016 in Berlin and published as
abstract no. 0194 in Oncol Res Treat (Suppl 1): 2016. This study
was supported in part by a grant from the University of Heidelberg
(Heidelberg, Germany; grant no. 20080715). The authors would like
to thank Ms. Susanne Gebhard and Ms. Martina Seehase (Department of
Gynecology and Obstetrics, University Medical Center Mainz, Mainz,
Germany) for providing technical assistance and support in data
management.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
Statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piek JM, Kenemans P and Verheijen RH:
Intraperitoneal serous adenocarcinoma: A critical appraisal of
three hypotheses on its cause. Am J Obstet Gynecol. 191:718–732.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levanon K, Crum C and Drapkin R: New
insights into the pathogenesis of serous ovarian cancer and its
clinical impact. J Clin Oncol. 26:5284–5293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol.
24(Suppl 10): x16–x21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Landen CN Jr, Birrer MJ and Sood AK: Early
events in the pathogenesis of epithelial ovarian cancer. J Clin
Oncol. 26:995–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed AA, Etemadmoghadam D, Temple J,
Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio
A, et al: Driver mutations in TP53 are ubiquitous in high grade
serous carcinoma of the ovary. J Pathol. 221:49–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma PC, Tretiakova MS, Mackinnon AC,
Ramnath N, Johnson C, Dietrich S, Seiwert T, Christensen JG,
Jagadeeswaran R, Krausz T, et al: Expression and mutational
analysis of MET in human solid cancers. Genes Chromosomes Cancer.
47:1025–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai AZ, Abella JV and Park M: Crosstalk in
Met receptor oncogenesis. Trends Cell Biol. 19:542–551. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trusolino L, Bertotti A and Comoglio PM:
MET signalling: Principles and functions in development, organ
regeneration and cancer. Nat Rev Mol Cell Biol. 11:834–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buckanovich R, Berger R, Sella A, Sikic B,
Shen X, Ramies D, Smith DC and Vergote IB: Activity of cabozantinib
(XL184) in advanced ovarian cancer patients (pts): Results from a
phase II randomized discontinuation trial (RDT). J Clin Oncol.
29(suppl): abstract 5008. 2011.
|
|
13
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Gene amplification and protein
overexpression of MET are common events in ovarian clear-cell
adenocarcinoma: Their roles in tumor progression and
prognostication of the patient. Mod Pathol. 24:1146–1155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sawada K, Radjabi AR, Shinomiya N, Kistner
E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande
Woude GF, et al: c-Met overexpression is a prognostic factor in
ovarian cancer and an effective target for inhibition of peritoneal
dissemination and invasion. Cancer Res. 67:1670–1679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goode EL, Chenevix-Trench G, Hartmann LC,
Fridley BL, Kalli KR, Vierkant RA, Larson MC, White KL, Keeney GL,
Oberg TN, et al: Assessment of hepatocyte growth factor in ovarian
cancer mortality. Cancer Epidemiol Biomarkers Prev. 20:1638–1648.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Battista MJ, Mantai N, Sicking I, Cotarelo
C, Weyer V, Lebrecht A, Solbach C and Schmidt M: Ki-67 as an
independent prognostic factor in an unselected cohort of patients
with ovarian cancer: Results of an explorative, retrospective
study. Oncol Rep. 31:2213–2219. 2014.PubMed/NCBI
|
|
17
|
Battista MJ, Cotarelo C, Jakobi S,
Steetskamp J, Makris G, Sicking I, Weyer V and Schmidt M:
Overexpression of epithelial cell adhesion molecule protein is
associated with favorable prognosis in an unselected cohort of
ovarian cancer patients. J Cancer Res Clin Oncol. 140:1097–1102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malpica A, Deavers MT, Lu K, Bodurka DC,
Atkinson EN, Gershenson DM and Silva EG: Grading ovarian serous
carcinoma using a two-tier system. Am J Surg Pathol. 28:496–504.
2004. View Article : Google Scholar : PubMed/NCBI
|