Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality worldwide, particularly
in Africa and Asia (1). Despite
extensive studies, the key molecules that control the development
and progression of HCC remain unclear.
Members of the peroxiredoxWin (PRDX) family reduce
hydrogen peroxide (H2O2) and alkyl
hydroperoxides. PRDX2 is a member of the peroxiredoxin family of
antioxidant enzymes. Similar to PRDXs, PRDX2 has been demonstrated
to form disulfide-linked homodimers during its catalytic cycle
(2). The expression of PRDX2 is
unregulated in breast cancer, cervical cancer and colorectal cancer
(3–6).
By contrast, silencing of PRDX2 occurs in acute myeloid leukemia
and malignant melanomas (7,8). Therefore, PRDX2 may serve a cell
type-dependent role in tumorigenesis.
The pro-tumorigenic role of PRDX2 may be attributed
to, at least partially, protecting cells from oxidative stress
(3–6).
Under oxidative stress, reactive oxygen species (ROS) also
contribute to tumor necrosis factor-α (TNF-α)-induced cell death
(9). However, the role of PRDX2 in
TNF-α-induced cell death has not yet been established. Recently,
PRDX2 has been identified to be the novel target of miR-122a, which
has been demonstrated to be frequently downregulated in HCC
(10). Thus, PRDX2 may serve a
pro-tumorigenic role in HCC. Because the role of PRDX2 in HCC has
not been reported, it is of interest to explore how PRDX2 may
affect ROS-mediated cell death in HCC cells. The present study aims
to examine the role of PRDX2 in H2O2- or
TNF-α-induced cell death in HCC SMMC-7721 cells.
Materials and methods
Cell culture and transfection
Cells were purchased from the Shanghai Institutes
for Biological Sciences (Shanghai, China) and were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Hyclone), 100 U/ml penicillin, and 100 µg/ml
streptomycin and were maintained at 37°C with 5% CO2.
Transfection was performed with Lipofectamine 2000 (Invitrogen:
ThermoFisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The transfection procedure used 6-well
plates, and 100 pmol siRNA or 1 µg plasmid was used for each well.
Cells were treated for at least 48 h for siRNA transfection, and 24
h for plasmid transfection. Small interfering RNA (siRNA) targeting
PRDX2 (5′-CCAGTACACAGACGAGCAT-3′) and non-targeting control (NC)
siRNA were obtained from Shanghai GenePharma, Inc. (Shanghai,
China). Mammalian expression vector encoding PRDX2 was generated by
cloning PCR-amplified products into pEGFP-N1 (Sino Biological,
Inc., Beijing, China) vector and confirmed by DNA sequencing. For
PRDX2 siRNA, NC siRNA was used as a control. For pGFP-PRDX2, the
pEGFP-N1 empty vector was used as a control.
Immunoblotting analysis
Cells were washed twice with ice-cold PBS and were
then lysed with 20 mM Tris/HCl (pH 7.6), 250 mM NaCl, 3 mM EDTA, 3
mM EGTA, 0.5% NP40, 1 mM DTT, 5 mM NaF, 2 mM
Na3VO4 and 0.2 µM Aprotinin. The whole cell
extract was clarified at 4°C at 12,000 rpm for 15 min. The amount
of protein recovered was quantified with Bradford protein assay.
Equal amounts of proteins were resolved by sodium dodecy1
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to Hybond-P polyvinylidene difluoride (PVDF) membranes.
Membranes were sequentially incubated with primary antibody over
night at 4°C and horseradish peroxidase-conjugated polyclonal goat
anti-rabbit or anti-mouse secondary antibodies (cat. no. ZB2301 and
ZB2305, respectively; 1:5,000; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 1 h at room
temperature. Bound antibody was detected using ECL
chemiluminescence kit (GE Healthcare, Little Chalfont, UK) and
Kodak X-ray film. Monoclonal mouse anti-human antibody against
PRDX2 was purchased from Proteintech (cat. no. 60202-1-Ig, 1:1,000;
Wuhan, China). Monoclonal mouse anti-human antibody against β-actin
(cat. no. sc-8432; 1:5,000) was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell death assays
Cells were adjusted to a density of 2×105
cells/ml, and 0.5 ml was added to each well of a 24-well plate.
Cells were treated with 10 ng/ml TNF-α and 1 µg/ml cycloheximide
(CHX, Sigma-Aldrcih, St. Louis, MO, USA) or 300 µM
H2O2 for 24 h. Cells were washed with PBS
twice and stained with Annexin V-PE/7-aminoactinomycin D (7AAD) or
Annexin V-FITC/propidium iodide (PI) (Nanjing KeyGen Biotech,
Nanjing, Jiangsu, China) for 15 min at room temperature in the
dark. The level of cell death was determined by measuring the
fluorescence of the cells with a flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA).
ROS production assays
A LIVE Green Reactive Oxygen Species Detection Kit
(Molecular Probes, Eugene, OR, USA) was used for detecting the
generation of ROS. Briefly, cells were incubated in serum-free RPMI
medium containing 2 µM carboxy-H2DCFDA (Molecular
Probes) at 37°C for 30 min. Cells were washed with PBS and were
immediately subjected to flow cytometry to analyse the intensity of
green fluorescence at a 488 nm excitation wavelength.
Statistical analysis
Statistically significant differences between groups
were identified using 2-tailed Student's t test. P<0.05
was considered to indicate a statistically significant difference.
Statistical analysis was conducted using SPSS version 13.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Validation of anti-PRDX2 antibody and
PRDX2 siRNA
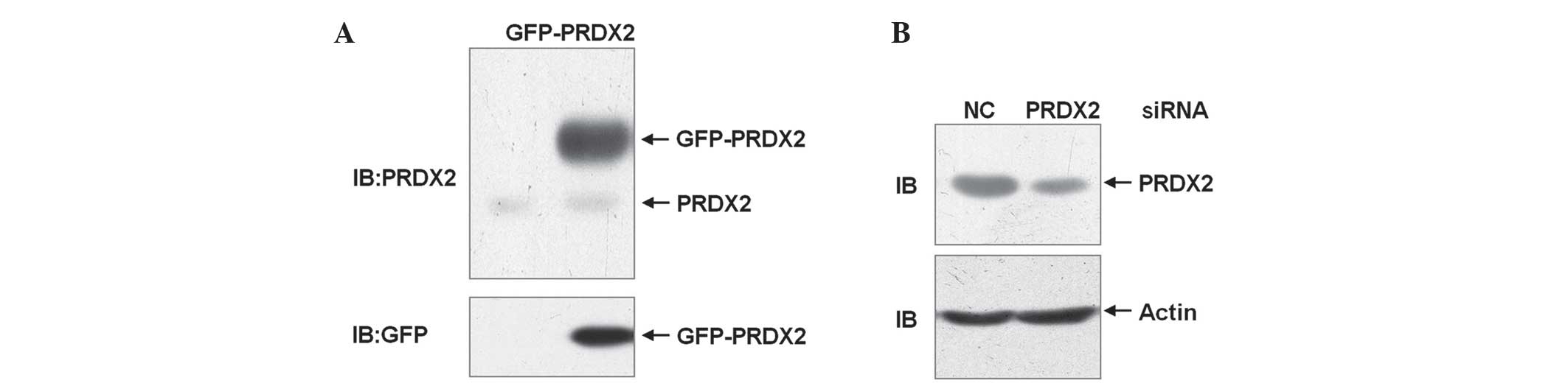
To explore the role of PRDX2 in ROS-mediated cell
death in HCC SMMC-7721 cells, a mammalian expression vector
encoding GFP-PRDX2 was constructed and a PRDX2 siRNA was designed.
SMMC-7721 cells were transfected with the mammalian expression
vector encoding GFP-PRDX2 or left untreated. 24 h later, cell
lysates were harvested and subjected to immunoblotting analysis.
The data revealed that exogenous GFP-PRDX2 could be detected by an
anti-PRDX2 antibody as well as by an anti-GFP antibody (Fig. 1A). Therefore, the anti-PRDX2 antibody
detected PRDX2 protein successfully. SMMC-7721 cells were
transfected with PRDX2 siRNA or non-targeting control siRNA. Cell
lysates were harvested 72 h later. Immunoblotting analysis with the
anti-PRDX2 antibody confirmed that PRDX2 expression was efficiently
knocked down (Fig. 1B). Thus, the
mammalian expression vector encoding GFP-PRDX2 and PRDX2 siRNA are
useful tools to explore the role of PRDX2 in oxidative
stress-mediated cell death in HCC SMMC-7721 cells.
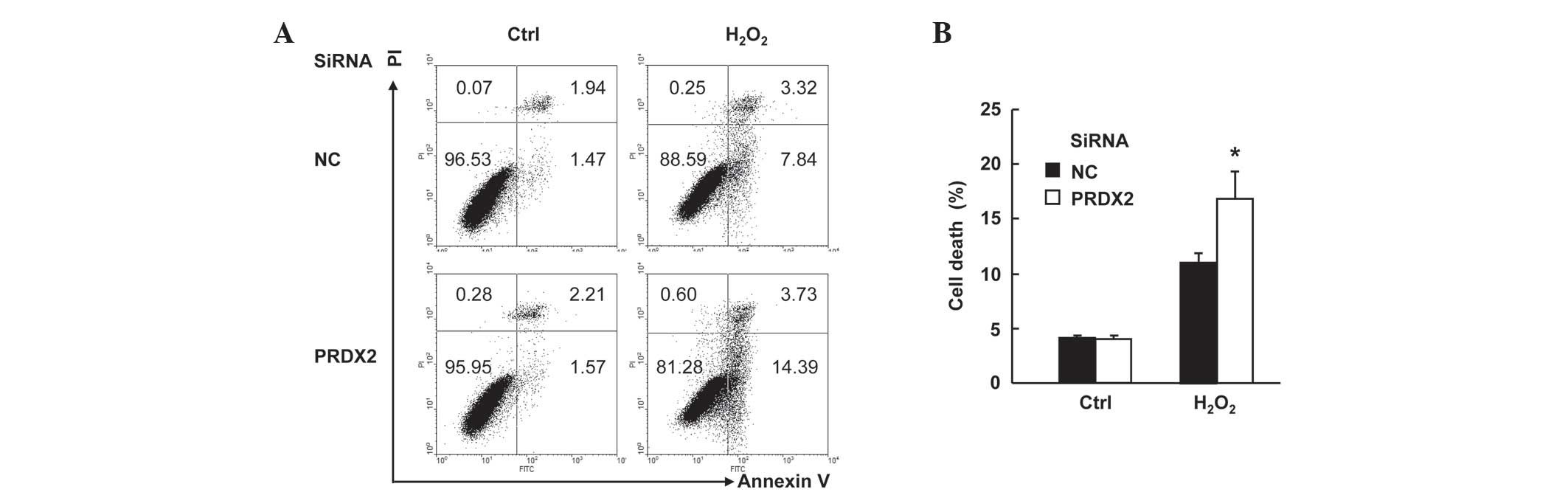
PRDX2 knockdown augmented
H2O2-induced cell death in SMMC-7721
cells
SMMC-7721 cells were transfected with PRDX2 siRNA or
non-targeting control siRNA. A total of 48 h later, cells were
treated with or without 300 µM H2O2 for 24 h.
Cell death assays with Annexin V-FITC/PI staining revealed that
H2O2-induced total cell death (apoptosis plus
necrosis, Fig. 2A) increased form ~11
to ~17% upon PRDX2 knockdown (Fig.
2B; P<0.05). However, PRDX2 knockdown showed no effects on
basal level of cell death (Fig. 2A and
B). Taken together, these data suggest that PRDX2 antagonizes
H2O2-induced cell death in SMMC-7721
cells.
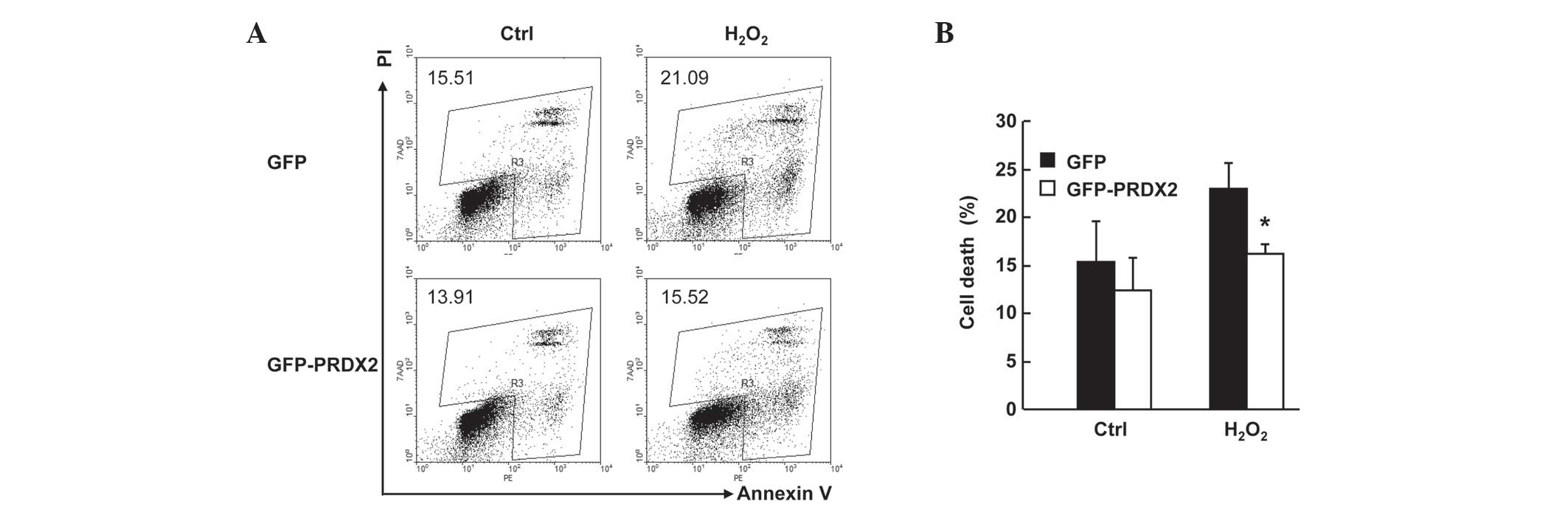
PRDX2 overexpression inhibited
H2O2-induced cell death in SMMC-7721
cells
SMMC-7721 cells were transfected with the mammalian
expression vectors encoding GFP or GFP-PRDX2. A total of 24 h
later, cells were treated with or without 300 µM
H2O2 for 24 h. Cell death assays with Annexin
V-PE/7AAD staining revealed that H2O2-induced
total cell death (Fig. 3A) in GFP+
population decreased ~6% upon PRDX2 overexpression (Fig. 3B; P<0.05) despite that PRDX2
overexpression showed no effects on basal level of cell death
(Fig. 3A and B). Together, these data
confirm that PRDX2 antagonizes H2O2-induced
cell death in SMMC-7721 cells.
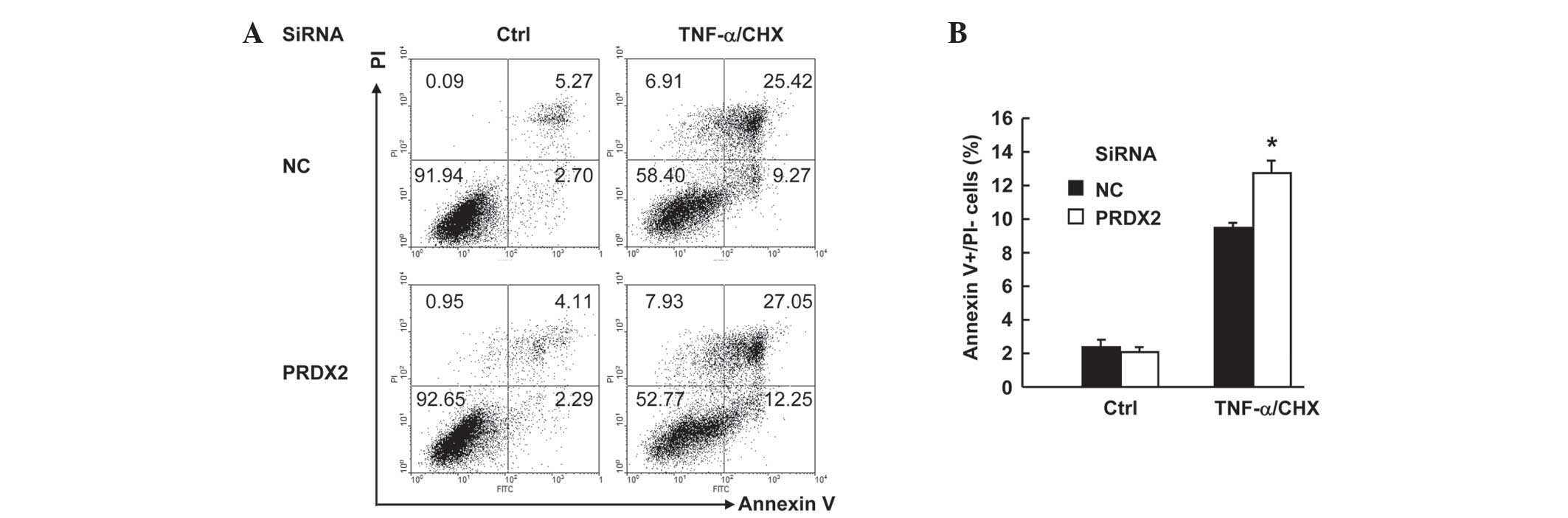
PRDX2 knockdown augmented
TNF-α-induced apoptosis in SMMC-7721 cells
TNF-α usually does not induce cell death unless de
novo protein synthesis is blocked (11). In this regard, protein synthesis
inhibitor cloheximide (CHX) is widely used to facilitate
TNF-α-induced cell death, which includes both apoptosis and
necrosis (12). SMMC-7721 cells were
transfected with PRDX2 siRNA or non-targeting control siRNA. Then
48 h later, cells were treated with or without 10 ng/ml TNF-α plus
1 µg/ml CHX for 24 h. Cell death assays with Annexin V-FITC/PI
staining revealed that TNF-α-induced total cell death (apoptosis
plus necrosis, Fig. 4A) increased
marginally upon PRDX2 knockdown. More careful examination of the
data revealed the enhancement of cell death occurred only in
apoptosis, namely Annexin V+PI- part. Annexin V+PI- cells increased
from ~9 to ~12% upon PRDX2 knockdown (Fig. 4B). However, PRDX2 knockdown showed no
effects on the basal level of cell death (Fig. 4A and B). Taken together, these data
suggest that PRDX2 antagonizes TNF-α-induced apoptosis in SMMC-7721
cells.
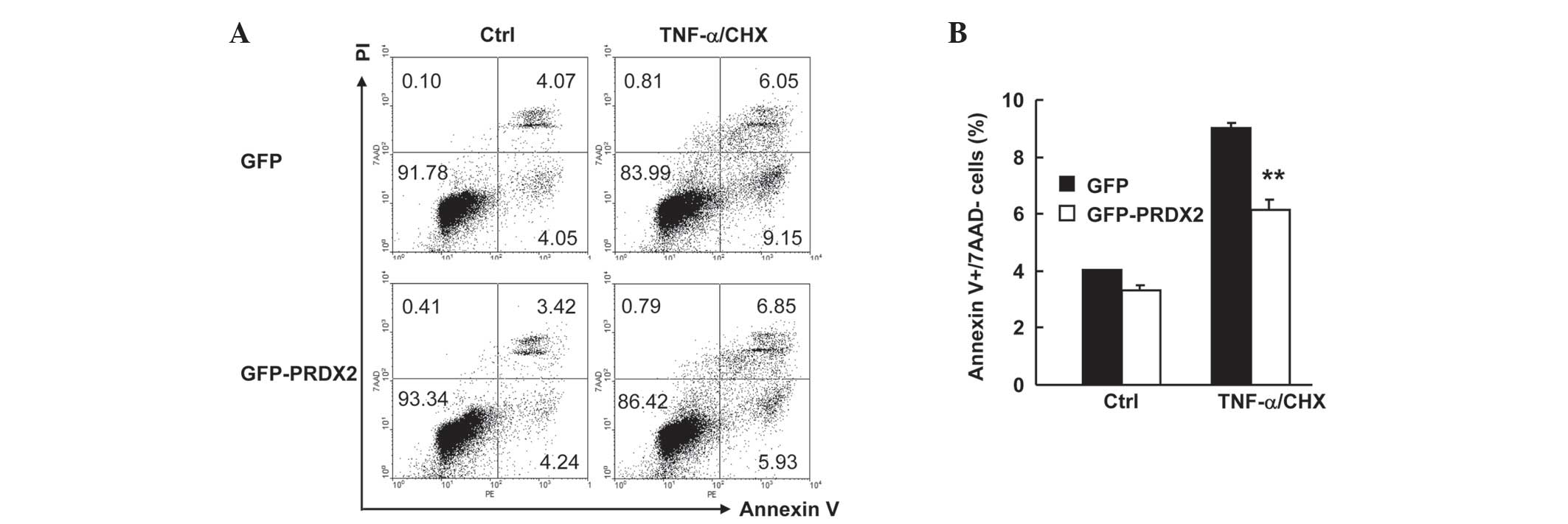
PRDX2 overexpression inhibited
TNF-α-induced apoptosis in SMMC-7721 cells
It is possible that 24 h treatment of SMMC-7721 cell
with 10 ng/ml TNF-α plus 1 µg/ml CHX is too long a time to observe
the effect of PRDX2 on necrosis. It may therefore be better to
shorten the treatment time. SMMC-7721 cells were transfected with
the mammalian expression vectors encoding GFP or GFP-PRDX2. A total
of 24 h later, cells were treated with or without TNF-α plus CHX
for 12 h. Cell death assays with Annexin V-PE/7AAD staining
revealed that TNF-α-induced apoptosis (Fig. 5A) in GFP+ population decreased ~3%
upon PRDX2 overexpression (Fig. 5B;
P<0.01). However, PRDX2 overexpression showed no effects on
TNF-α-induced necrosis and basal level of cell death (Fig. 5A and B). Together, these data confirm
that PRDX2 antagonizes TNF-α-induced apoptosis, but not necrosis,
in SMMC-7721 cells.
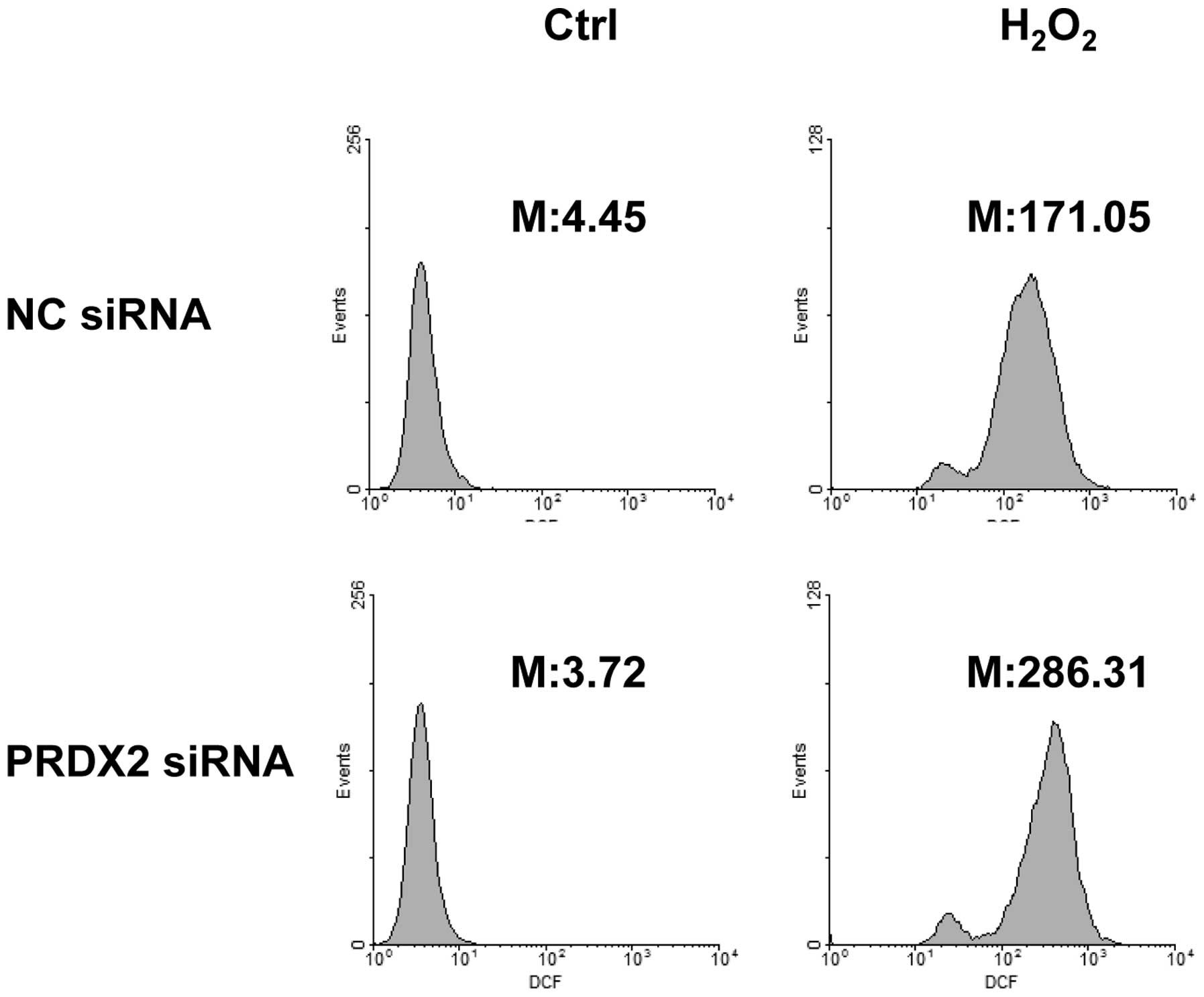
PRDX2 knockdown led to enhanced ROS
generation in response to H2O2
As a member of the peroxiredoxin family of
antioxidant enzymes, PRDX2 should exert its protective effects
under oxidative stress by reducing ROS generation (4). In this regard, SMMC-7721 cells were
transfected with PRDX2 siRNA or non-targeting control siRNA. A
total of 72 h later, cells were treated with or without 300 µM
H2O2 for 30 min. ROS generation was measured
by incubating cells with carboxy-H2DCFDA simultaneously.
Flow cytometry revealed that H2O2
significantly induced ROS generation (Fig. 6). As expected, PRDX2 knockdown led to
enhanced ROS generation in response to H2O2
(Fig. 6).
Discussion
The role of PRDX2 in HCC remains unknown. The
present study demonstrated that PRDX2 is expressed in HCC SMMC-7721
cells and prevents ROS generation and cell death under oxidative
stress. These findings imply a pro-tumorigenic role for PRDX2 in
HCC.
Besides preventing
H2O2-induced cell death, PRDX2 also serves a
role in TNF-α-induced cell death. It is known that TNF-α induces
both apoptosis and programmed necrosis, which are mediated by
distinct signaling pathways (12).
The present study further supports this notion: It was demonstrated
that PRDX2 only inhibits TNF-α-induced apoptosis, but does not
affect TNF-α-induced necrosis even at early time point. Thus, ROS
contributes to apoptosis pathway, but not the necrosis pathway, in
response to TNF-α.
In addition, it should be noted that the effects of
PRDX2 on ROS-mediated cell death in HCC SMMC-7721 cells are weak.
It is possible that other members of the peroxiredoxin family of
antioxidant enzymes serve more important roles. It is also possible
antioxidant enzymes of this type are not major players in cells of
liver origin.
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon JC, Hah YS, Kim WY, Jung BG, Jang HH,
Lee JR, Kim SY, Lee YM, Jeon MG, Kim CW, et al: Oxidative
stress-dependent structural and functional switching of a human
2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance
to H2O2-induced cell death. J Biol Chem. 280:28775–28784. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu W, Fu Z, Wang H, Feng J, Wei J and Guo
J: Peroxiredoxin 2 is upregulated in colorectal cancer and
contributes to colorectal cancer cells' survival by protecting
cells from oxidative stress. Mol Cell Biochem. 387:261–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stresing V, Baltziskueta E, Rubio N,
Blanco J, Arriba MC, Valls J, Janier M, Clézardin P, Sanz-Pamplona
R, Nieva C, et al: Peroxiredoxin 2 specifically regulates the
oxidative and metabolic stress response of human metastatic breast
cancer cells in lungs. Oncogene. 32:724–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA
and Chae HZ: Overexpression of peroxiredoxin in human breast
cancer. Anticancer Res. 21:2085–2090. 2001.PubMed/NCBI
|
|
6
|
Kim K, Yu M, Han S, Oh I, Choi YJ, Kim S,
Yoon K, Jung M and Choe W: Expression of human peroxiredoxin
isoforms in response to cervical carcinogenesis. Oncol Rep.
21:1391–1396. 2009.PubMed/NCBI
|
|
7
|
Agrawal-Singh S, Isken F, Agelopoulos K,
Klein HU, Thoennissen NH, Koehler G, Hascher A, Bäumer N, Berdel
WE, Thiede C, et al: Genome-wide analysis of histone H3 acetylation
patterns in AML identifies PRDX2 as an epigenetically silenced
tumor suppressor gene. Blood. 119:2346–2357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuta J, Nobeyama Y, Umebayashi Y, Otsuka
F, Kikuchi K and Ushijima T: Silencing of Peroxiredoxin 2 and
aberrant methylation of 33 CpG islands in putative promoter regions
in human malignant melanomas. Cancer Res. 66:6080–6086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delhalle S, Deregowski V, Benoit V,
Merville MP and Bours V: NF-kappaB-dependent MnSOD expression
protects adenocarcinoma cells from TNF-alpha-induced apoptosis.
Oncogene. 21:3917–3924. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diao S, Zhang JF, Wang H, He ML, Lin MC,
Chen Y and Kung HF: Proteomic identification of microRNA-122a
target proteins in hepatocellular carcinoma. Proteomics.
10:3723–3731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin S, Ray RM and Johnson LR:
TNF-alpha/cycloheximide-induced apoptosis in intestinal epithelial
cells requires Rac1-regulated reactive oxygen species. Am J Physiol
Gastrointest Liver Physiol. 294:G928–G937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christofferson DE, Li Y and Yuan J:
Control of life-or-death decisions by RIP1 kinase. Annu Rev
Physiol. 76:129–150. 2014. View Article : Google Scholar : PubMed/NCBI
|