Introduction
Esophageal carcinoma is foremost for cancer
incidence and mortality rates in males and females in developing
countries (1). In addition, ~50% of
esophageal cancer cases in the world occur in China, and esophageal
squamous cell carcinoma (ESCC) accounts for ~90% of all esophageal
cancers diagnosed in China each year (1). To date, the molecular pathogenesis of
ESCC remains unclear. At present, the focus for research is
transitioning between the cloning of novel tumor-associated genes
and characterizing the biological function of the protein product
(2). As a result, a major research
effort has been directed at expressing and identifying the function
of novel specific esophageal cancer-associated proteins and
elucidating the relevant molecular mechanisms in the carcinogenesis
in ESCC.
Human chromosome 2 open reading frame 40
(C2ORF40) gene, also referred to as ECRG4, is
expressed in various normal tissues, including the esophageal
epithelium, heart, brain, placenta, lung, liver, kidney and
pancreas (3,4). The C2ORF40 gene is important in
processes associated with physiological functional regulation,
including inflammation, injury, senescence, the neuroendocrines
environment, differentiation and apoptosis (5–13).
Notably, previous studies have indicated that C2ORF40 is a
candidate tumor suppressor gene associated with prognosis in a
variety of tumors (4,14–22). In
addition, C2ORF40 has been demonstrated to be chemosensitive
to 5-fluorouracil and cisplatin (23,24).
Previous research by the present authors demonstrated that
C2ORF40 is a candidate tumor suppressor gene and an
independent prognostic factor in ESCC, and C2ORF40 gene
overexpression inhibits tumor cell proliferation and invasion in
ESCC (25–29). Notably, additional bioinformatics
analysis indicated that pro-C2ORF40 protein was a secreted protein
with a signal peptide. In addition, previous studies indicated that
secreted C2ORF40 protein exists in C2ORF40 gene-transfected
esophageal cancer cell medium (30).
However, the exact biological function of secreted C2ORF40 protein
in carcinogenesis has not been thoroughly investigated.
The present study initially expressed and purified
soluble recombinant human C2ORF40 protein (rhC2ORF40), validated
the tumor-suppressing biological activities of rhC2ORF40 protein
in vivo, and explored the possible molecular mechanism of
rhC2ORF40 in ESCC, to the best of our knowledge, for the first
time.
Materials and methods
Production and purification of soluble
rhC2ORF40
The pGEM-T-C2ORF40 vector and pET30a (+) plasmid
used in the present study were constructed at the State Key
Laboratory of Molecular Oncology and Department of Etiology and
Carcinogenesis of the Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China). The shortened
rhC2ORF40 cDNA (with the 1–28 amino acid signal peptide sequence
deleted) was excised from the preserved pGEM-T-C2ORF40 vector and
subcloned into the pET30a (+) plasmid using a previously described
method (30). The resulting product
was an inducible pET30a-C2ORF40 expression vector encoding
His-tagged soluble rhC2ORF40 (without a signal peptide).
The cDNA was amplified by polymerase chain reaction
(PCR) using the GoldScript one-step RT-PCR kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The primers were as
follows: Forward, 5′-TCGGATCCATAAGTGGAAATAACTC-3′ and reverse
5′-TCAAGCTTTTAGTAGTCATCGTAGTT-3′ (Invitrogen™; Thermo Fisher
Scientific, Inc.). The thermal cycling conditions were: 95°C for 5
min; 35 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 45
sec; followed by extension at 72°C for 7 min. The PCR product was
digested by BamHI and HindIII. Subsequently, the
recombinant plasmids were transformed into Escherichia coli
BL21 (DE3) cells (Takara Biotechnology Co., Ltd., Dalian, China),
according to a previous study (30),
to produce N-terminal His-tagged soluble rhC2ORF40.
rhC2ORF40 expression in E. coli BL21 cells
was induced with 0.3 mM isopropyl-D-thiogalactopyranoside and
detected by western blotting, according to a previous study
(31). Briefly, total protein was
extracted from E. coli BL21 cells using the Complete
Bacterial Protein Extraction Reagent (cat. no. 89821; Pierce
Biotechnology, Inc., Rockford, IL, USA), and the resulting protein
lysate was separated by 15% SDS-PAGE, followed by transfer onto
polyvinylidene fluoride membranes. The mebranes were blocked with
5% bovine serum albumin (Pierce Biotechnology, Inc.) for 1 h at
room temperature, followed by incubation with rabbit anti-ECRG4
polyclonal antibody (cat. no. sc-135139; 1:150 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 2 h at room temperature.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated chicken anti-rabbit secondary antibody (cat.
no. sc-516087; 1:2,000 dilution; Santa Cruz Biotechnology, Inc.)
for 1 h at room temperature. The membranes were visualized by
enhanced chemiluminiescence to confirm the presence of
rhC2ORF40.
rhC2ORF40 was purified and renatured by affinity
chromatography with nickel-nitrilotriacetic acid resin (Merck
Millipore, Darmstadt, Germany), according to the manufacturer's
protocol. Purified rhC2ORF40 was dialyzed in phosphate-buffered
saline (PBS), 0.1 M sodium phosphate and 0.15 M sodium chloride (pH
7.4) to remove the denaturant. Soluble rhC2ORF40 was used for
additional experiments.
Tumor growth in vivo
A total of 24 six-week-old female BALB/c nude mice
weighing 16–18 g were obtained from Beijing Vital River Laboratory
Animal Technology, Co., Ltd. (Beijing, China). The mice were housed
at four mice per cage and were maintained at 25–27°C and 45–50%
humidity, under a 12-h light/dark cycle. The mice were fed ad
libitum with autoclaved food. Esophageal cancer EC9706 cells
(5×106; Type Culture Collection of the Chinese Academy of Sciences,
Shanghai, China) that had been cultured in RPMI-1640 medium
containing 10% fetal bovine serum (both Invitrogen; Thermo Fisher
Scientific, Inc.) in 5% CO2 at 37°C for 48 h, were
incubated in Trypsin-EDTA (Invitrogen; Thermo Fisher Scientific,
Inc.), washed with PBS, centrifuged at 1,500 × g for 5 min,
resuspended in PBS, and injected subcutaneously into the armpit
region of the nude mice. When the mean tumor volume reached 100
mm3, the nude mice were randomly divided into two groups (eight
mice per group). The rhC2ORF40 treatment group received various
concentrations of rhC2ORF40 (0.1, 1.0 and 10.0 mg/kg) injected
subcutaneously around tumors every other day, and the control group
mice were injected with 200 µl PBS. Tumor volumes were recorded
twice per week thereafter for 14 days. At the end of the 14 days,
the mice were sacrificed by cervical dislocation. Tumor sizes were
estimated from the length (a) and width (b) of the tumors, as
measured using calipers, according to the following formula: Tumor
volume = ab2 / 2. Nude mice experiments were approved by Zhengzhou
University Ethics Committee (Zhengzhou, China; approval no.
U1304817).
Telomerase activity assay
The telomerase activities of EC9706 cells treated
with 10 µg/ml rhC2ORF40 or PBS for 48 h were examined by telomeric
repeat amplification protocol (TRAP)-ELISA kits (JRDUN
Biotechnology (Shanghai), Co., Ltd., Shanghai, China), according to
the manufacturer's protocol. The mean values for statistical
analysis was the average of three independent experiments.
Telomerase-component RNA reverse
transcription PCR (RT-PCR)
The human telomerase-component RNA (hTR) of EC9706
cells, which were treated with 10 µg/ml rhC2ORF40 or PBS for 48 h,
was examined using RT-PCR. Briefly, total RNA was extracted from
EC9706 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and the extracted RNA was treated with DNase
(Invitrogen; Thermo Fisher Scientific, Inc.) to remove
contaminating genomic DNA. The purity and concentration of the
extracted RNA was assessed using an ultraviolet spectrophotometer.
RT-PCR was performed using the GoldScript one-step RT-PCR kit, with
the following primers: hTR, forward 5′-CTAACCCTATCTGAGTTGGGCGTA-3′
and reverse 5′-CAACGGACAGACAGCAGCTGACAT-3′; and GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′
(Invitrogen™; Thermo Fisher Scientific, Inc.). The PCR conditions
were 35 cycles at 95°C for 45 sec, 55°C for 45 sec and 72°C for 65
sec, followed by an extension at 72°C for 10 min. The PCR products
were separated by 1.5% agarose gel electrophoresis and referenced
to a DNA molecular weight marker (Takara Biotechnology Co., Ltd.).
The GDS-8000 Gel Image Analysis system (UVP, LLC, Upland, CA, USA)
was used to quantify the band intensities.
Statistical analysis
All statistical analysis was performed with SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). Statistical
significance was determined using Student's t test and analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Soluble purified rhC2ORF40 was
obtained for in vivo functional experiments
The present authors previously demonstrated that
C2ORF40 protein was secreted from a sub-cellular location in
esophageal cells (30). The Universal
Protein Resource (http://web.expasy.org) predicted that C2ORF40 protein
was a secreted protein with a signal peptide. In addition, it was
revealed that secreted C2ORF40 protein existed in esophageal cancer
EC9706 cell medium transfected with a C2ORF40 expression
plasmid compared with an empty plasmid control group, as shown by
western blotting (30). Therefore,
C2ORF40 protein was a secreted protein, which is secreted into the
extracellular environment where it exhibits biological
functions.
In the present study, the signal peptide sequence of
the C2ORF40 cDNA was removed to produce soluble secreted
rhC2ORF40. The constructed expression plasmid pET30a-C2ORF40 was
identified by PCR restrictive enzyme digestion and DNA sequencing.
Subsequently, recombinant E. coli BL21 strains, which
expressed soluble rhC2ORF40, were obtained. Soluble rhC2ORF40 was
specifically recognized by anti-His and anti-C2ORF40 antibodies
(data not shown). Soluble rhC2ORF40 was purified with a purity of
>95%. Therefore, soluble rhC2ORF40 with a high purity was
successfully obtained for in vivo functional experiments by
the present study.
rhC2ORF40 suppresses tumor growth in
vivo in ESCC
The effect of rhC2ORF40 on tumor growth in
vivo was additionally evaluated in ESCC. Esophageal cancer
EC9706 cells were subcutaneously injected into athymic nude mice.
One week following tumor cell injection, the experimental group of
mice received various doses of rhC2ORF40 (0.1, 1.0 and 10.0 mg/kg)
and the control group was treated with 200 µl PBS. The mice were
sacrificed 2 weeks following rhC2ORF40 protein treatment and tumor
volumes were measured. The results revealed that purified rhC2ORF40
inhibited the growth of EC9706 tumor xenografts in nude mice in a
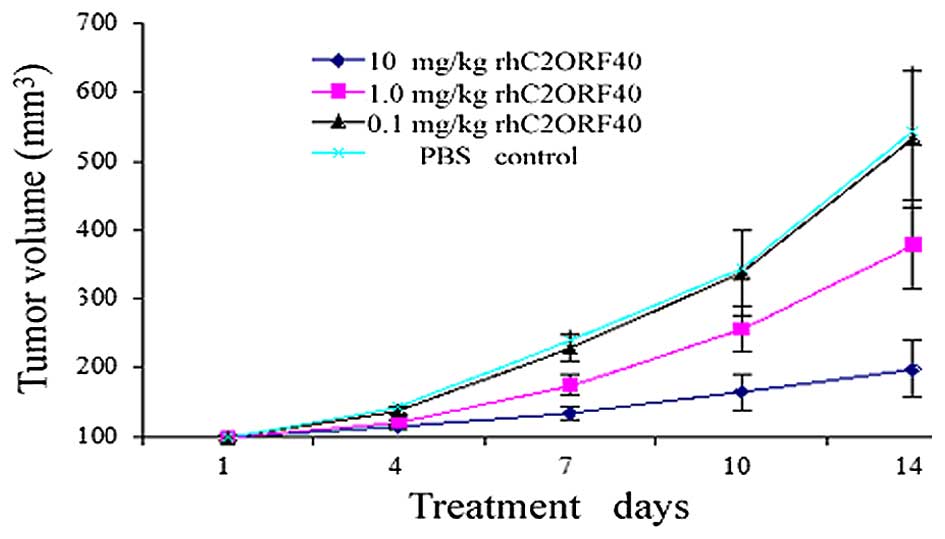
dose-dependent manner. Compared with the PBS control group,
rhC2ORF40 treatment (1 and 10 mg/kg) significantly inhibited the
development of xenograft growth in vivo (P=0.014 and P=0.001
for 1 and 10 mg/kg rhC2ORF40, respectively); however, 0.1 mg/kg
rhC2ORF40 treatment did not demonstrate a suppressive effect
compared with the control mice (P=0.86; Fig. 1). Therefore, the results suggest that
soluble rhC2ORF40 inhibits tumor cell growth in vivo in ESCC
in a dose-dependent manner.
rhC2ORF40 decreases telomerase
activity
The telomerase activity of EC9706 cells was also
examined, in order to investigate the molecular mechanism of the
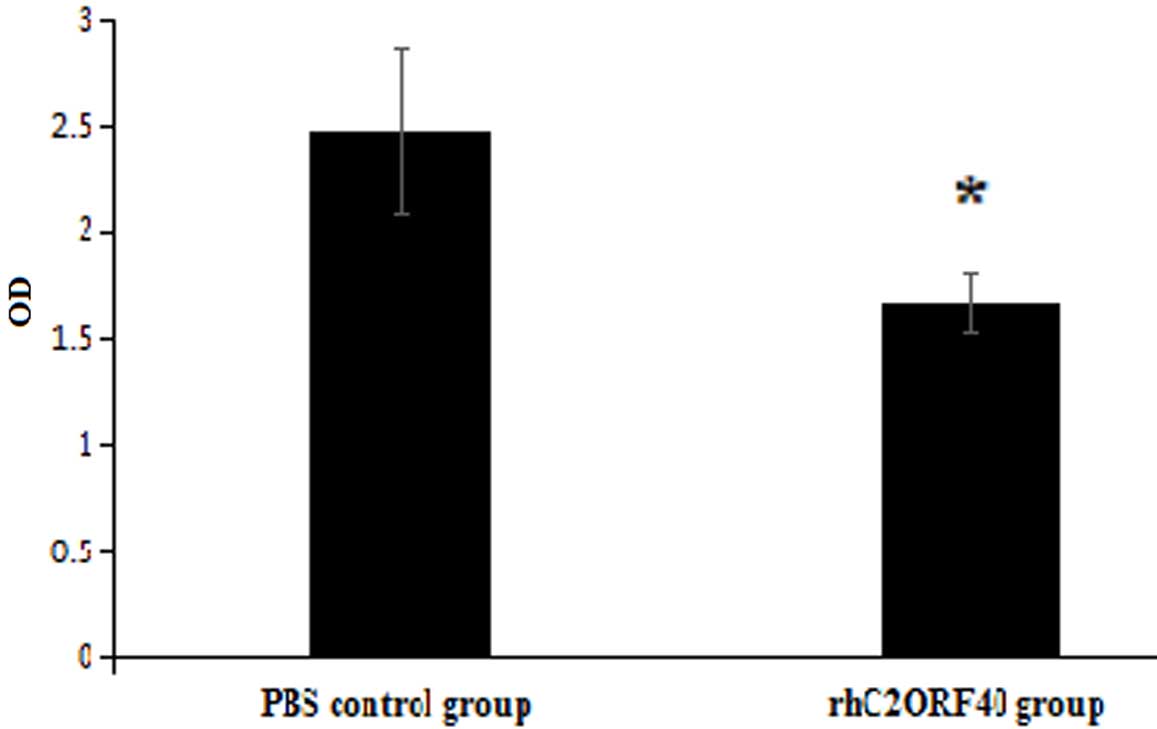
tumor-suppressive function exhibited by rhC2ORF40. A TRAP-ELISA
revealed that the telomerase activities in the control cell group
and rhC2ORF40 treatment (10 µg/ml) cell group were 2.475±0.395 and
1.672±0.138, respectively (P=0.017; Fig.
2). This result indicated that rhC2ORF40 decreased the
telomerase activity in EC9706 cells.
Effect of rhC2ORF40 on
telomerase-component RNA transcription
The GC-rich region of telomerase-component RNA is
essential for ribonucleoprotein enzyme biological function
(1). To investigate the detailed
molecular mechanisms of the decrease in telomerase activity induced
by rhC2ORF40, the present study evaluated the transcriptional
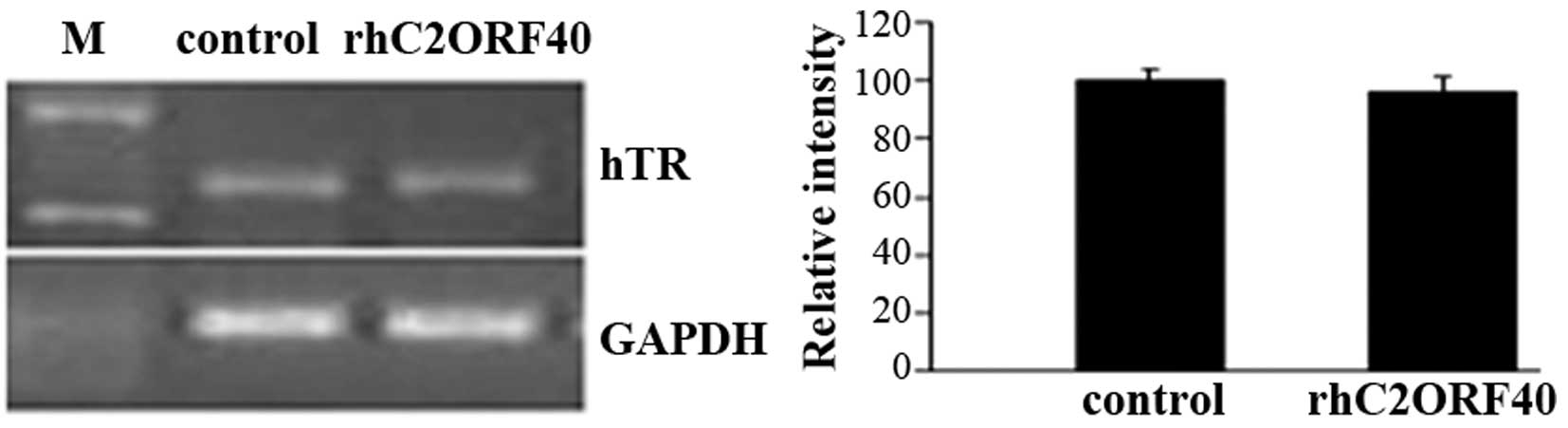
alteration of telomerase-component RNA in EC9706 cells. There was
no apparent alteration in telomerase-component RNA levels in the
rhC2ORF40 treatment group (10 µg/ml) compared with the control
group (P=0.98; Fig. 3). This result
demonstrated that rhC2ORF40 does not affect telomerase-component
RNA transcription; however, it may post-transcriptionally modulate
telomerase activity in ESCC.
Discussion
ESCC is a highly invasive and clinically challenging
cancer in China. Despite advances in clinical comprehensive
treatment, ESCC prognosis remains poor, due to its diffuse and
invasive nature (32). Novel
biological therapy drugs with high anti-tumor efficacy are being
constantly sought to improve the survival of ESCC patients. A
previous study by the present authors revealed that C2ORF40 was
secreted into cell medium, and rhC2ORF40 inhibits EC9706 cell
proliferation in vitro (30).
In the present study, soluble secreted rhC2ORF40 was expressed and
purified, and subsequent experiments revealed for the first time,
to the best of our knowledge, that rhC2ORF40 inhibits ESCC tumor
cell growth in vivo in a dose-dependent manner. Although all
the mice treated with rhC2ORF40 possessed tumors at the end of the
experimental phase, mice treated with 1 and 10 mg/kg rhC2ORF40 had
tumors that grew more slowly compared with those in the control
group (P<0.05). In addition, compared with the control group,
the rhC2ORF40 treatment group (1 and 10 mg/kg) had significantly
inhibited xenograft tumor growth (P<0.05); the tumor weights of
the control group mice were increased compared with those of the
rhC2ORF40 treatment group (P<0.05). Therefore, soluble rhC2ORF40
with high purity and biological activity was obtained by the
present study. Furthermore, the in vivo tumor-suppressing
functional experiments by the present study demonstrated that
soluble rhC2ORF40 may be a candidate biological therapy for
ESCC.
Transformed cells acquire a series of malignant
traits, during ESCC development and progression. Among them, escape
from cellular death by constitutive telomerase activation, or
alternative telomere maintenance, represents a trait that tumor
cells acquire for indefinite growth during carcinogenesis (32). The present study revealed that
rhC2ORF40 decreased telomerase activity in ESCC cells (P<0.05).
However, there was no apparent alteration in telomerase RNA level
(P>0.05). Therefore, the present study hypothesizes that C2ORF40
post-transcriptionally modulates telomerase, either by direct
protein interaction or by indirect protein signal transduction.
Cell cycle alteration is also a key phase in
carcinogenesis. Once cell cycle regulation is broken, tumorigenesis
may result. In addition, recent studies have revealed that a
decrease in telomerase activity correlates closely with cell cycle
arrest (33,34). The present authors previously
demonstrated that rhC2ORF40 causes a cell cycle G1/S phase block in
esophageal cancer cells in vitro (30). In the present study, rhC2ORF40 was
shown to decrease the telomerase activity in EC9706 cells, which
may underlie the mechanism of rhC2ORF40-mediated inhibition of ESCC
tumor cell growth in vivo.
Numerous oncogenes and tumor suppressor genes are
directly involved in the regulation of the cell cycle. Among them
p21 and p16 genes, critical cyclin-dependent kinase inhibitors, are
hypothesized to be functionally relevant to the regulation of cell
cycle G1 phase. Previous results have revealed that C2ORF40
transfection induces the upregulation of p21 expression in
ESCC (25–27). However, there is no significant
upregulation of p16 expression. Therefore, the increased
expression of p21, not p16, is likely to be responsible for
the cell cycle G1 phase block induced by C2ORF40 in ESCC. An
additional study also demonstrated that C2ORF40 causes cell cycle
G1 phase block via interaction with Transmembrane Protease, Serine
11A in ESCC (27). Previous studies
have indicated that C2ORF40 may be processed and released from the
cell surface to function biologically (35,36).
However, a detailed molecular mechanism for the tumor suppressing
function of C2ORF40 protein remains to be clarified in ESCC.
Overall, the present study revealed that soluble
rhC2ORF40 inhibits tumor cell growth in vivo by decreasing
telomerase activity in ESCC. Soluble rhC2ORF40 was purified by the
present study with a high purity and had a biological activity that
may be a potential biological therapy for esophageal cancer in the
future.
Acknowledgements
The present study was supported by the Chinese
National Natural Science Foundation (Beijing, China; grant no.
U1304817) and the Zhengzhou City Science Research Project
(Zhengzhou, China; grant no. 141PPTGHG298).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eisenberg D, Marcotte EM, Xenarios I and
Yeates TO: Protein function in the post-genomic era. Nature.
405:823–826. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steck E, Breit S, Breusch SJ, Axt M and
Richter W: Enhanced expression of the human chitinase 3-like 2 gene
(YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic
cartilage. Biochem Biophys Res Commun. 299:109–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue CM, Deng DJ, Bi MX, Guo LP and Lu SH:
Expression of ECRG4, a novel esophageal cancer-related gene,
downregulated by CpG island hypermethylation in human esophageal
squamous cell carcinoma. World J Gastroenterol. 9:1174–1178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kao S, Shaterian A, Cauvi DM, Dang X, Chun
HB, De Maio A, Costantini TW, Coimbra R, Eliceiri BP and Baird A:
Pulmonary preconditioning, injury and inflammation modulate
expression of the candidate tumor suppressor gene ECRG4 in lung.
Exp Lung Res. 41:162–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Podvin S, Dang X, Meads M, Kurabi A,
Costantini T, Eliceiri BP, Baird A and Coimbra R: Esophageal
cancer-related gene-4 (ECRG4) interactions with the innate immunity
receptor complex. Inflamm Res. 64:107–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huh YH, Ryu JH, Shin S, Lee DU, Yang S, Oh
KS, Chun CH, Choi JK, Song WK and Chun JS: Esophageal cancer
related gene 4 (Ecrg4) is a marker of articular chondrocyte
differentiation and cartilage destruction. Gene. 448:7–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kujuro Y, Suzuki N and Kondo T: Esophageal
cancer-related gene 4 is a secreted inducer of cell senescence
expressed by aged CNS precursor cells. Proc Natl Acad Sci USA.
107:8259–8264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuzaki J, Torigoe T, Hirohashi Y,
Kamiguchi K, Tamura Y, Tsukahara T, Kubo T, Takahashi A, Nakazawa
E, Saka E, et al: ECRG4 is a negative regulator of
caspase-8-mediated apopotosis in human T-leukemia cells.
Carcinogenesis. 33:996–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaterian A, Kao S, Chen L, DiPietro LA,
Coimbra R, Eliceiri BP and Baird A: The candidate tumor suppressor
gene Ecrg4 as a wound terminating factor in cutaneous injury. Arch
Dermatol Res. 305:141–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Podvin S, Gonzalez AM, Miller MC, Dang X,
Botfield H, Donahue JE, Kurabi A, BoissaudCooke M, Rossi R,
Leadbeater WE, et al: Esophageal cancer related gene-4 is a choroid
plexus-derived injury response gene: Evidence for a biphasic
response in early and late brain injury. PLoS One. 6:e246092011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonzalez AM, Podvin S, Lin SY, Miller MC,
Botfield H, Leadbeater WE, Roberton A, Dang X, Knowling SE,
CardenasGalindo E, et al: Ecrg4 expression and its product augurin
in the choroid plexus: Impact on fetal brain development,
cerebrospinal fluid homeostasis and neuroprogenitor cell response
to CNS injury. Fluids Barriers CNS. 8:62011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurabi A, Pak K, Dang X, Coimbra R,
Eliceiri BP, Ryan AF and Baird A: Ecrg4 attenuates the inflammatory
proliferative response of mucosal epithelial cells to infection.
PLoS One. 8:e613942013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Götze S, Feldhaus V, Traska T, Wolter M,
Reifenberger G, Tannapfel A, Kuhnen C, Martin D, Müller O and
Sievers S: ECRG4 is a candidate tumor suppressor gene frequently
hypermethylated in colorectal carcinoma and glioma. BMC Cancer.
9:4472009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Wen M, Huang Y, He X, Wang Y, Wu Q,
Li Z, CastellanosMartin A, Abad M, CruzHernandez JJ, et al: C2ORF40
suppresses breast cancer cell proliferation and invasion through
modulating expression of M phase cell cycle genes. Epigenetics.
8:571–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mori Y, Ishiguro H, Kuwabara Y, Kimura M,
Mitsui A, Kurehara H, Mori R, Tomoda K, Ogawa R, Katada T, et al:
Expression of ECRG4 is an independent prognostic factor for poor
survival in patients with esophageal squamous cell carcinoma. Oncol
Rep. 18:981–985. 2007.PubMed/NCBI
|
|
17
|
Li W, Liu X, Zhang B, Qi D, Zhang L, Jin Y
and Yang H: Overexpression of candidate tumor suppressor ECRG4
inhibits glioma proliferation and invasion. J Exp Clin Cancer Res.
29:892010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuzaki J, Torigoe T, Hirohashi Y,
Tamura Y, Asanuma H, Nakazawa E, Saka E, Yasuda K, Takahashi S and
Sato N: Expression of ECRG4 is associated with lower proliferative
potential of esophageal cancer cells. Pathol Int. 63:391–397. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu T, Xiao D and Zhang X: ECRG4 inhibits
growth and invasiveness of squamous cell carcinoma of the head and
neck in vitro and in vivo. Oncol Lett. 5:1921–1926. 2013.PubMed/NCBI
|
|
20
|
Wang YB and Ba CF: Promoter methylation of
esophageal cancer-related gene 4 in gastric cancer tissue and its
clinical significance. Hepatogastroenterology. 59:1696–1698.
2012.PubMed/NCBI
|
|
21
|
Sabatier R, Finetti P, Adelaide J, Guille
A, Borg JP, Chaffanet M, Lane L, Birnbaum D and Bertucci F:
Down-regulation of ECRG4, a candidate tumor suppressor gene, in
human breast cancer. PLoS One. 6:e276562011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baird A, Lee J, Podvin S, Kurabi A, Dang
X, Coimbra R, Costantini T, Bansal V and Eliceiri BP: Esophageal
cancer-related gene 4 at the interface of injury, inflammation,
infection, and malignancy. Gastrointest Cancer. 2014:131–142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
You Y, Yang W, Qin X, Wang F, Li H, Lin C,
Li W, Gu C, Zhang Y and Ran Y: ECRG4 acts as a tumor suppressor and
as a determinant of chemotherapy resistance in human nasopharyngeal
carcinoma. Cell Oncol (Dordr). 38:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang CP, Wu BH, Wang BQ, Fu MY, Yang M,
Zhou Y and Liu F: Overexpression of ECRG4 enhances chemosensitivity
to 5-fluorouracil in the human gastric cancer SGC-7901 cell line.
Tumour Biol. 34:2269–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LW, Yu XY, Yang Y, Zhang CP, Guo LP and
Lu SH: Expression of esophageal cancer related gene 4 (ECRG4), a
novel tumor suppressor gene, in esophageal cancer and its
inhibitory effect on the tumor growth in vitro and in vivo. Int J
Cancer. 125:1505–1513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Zhang C, Li X, Lu S and Zhou Y: The
candidate tumor suppressor gene ECRG4 inhibits cancer cells
migration and invasion in esophageal carcinoma. J Exp Clin Cancer
Res. 29:1332010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li LW, Li YY, Li XY, Zhang CP, Zhou Y and
Lu SH: A novel tumor suppressor gene ECRG4 interacts directly with
TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal
carcinoma. BMC Cancer. 11:522011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li LW, Yang Y, Li XY, Guo LP, Zhou Y and
Lu SX: Tumor-suppressing function of human esophageal cancer
related gene 4 in esophageal squamous cell carcinoma. Zhonghua Yi
Xue Za Zhi. 90:2713–2717. 2010.(In Chinese). PubMed/NCBI
|
|
29
|
Li LW, Yu XY, Li XY, Guo LP, Zhou Y and Lu
SX: Mechanism of loss of human esophageal cancer-related gene 4
(ECRG4) gene expression in esophageal squamous cell carcinoma cell
line EC9706. Zhonghua Zhong Liu Za Zhi. 33:570–573. 2011.(In
Chinese). PubMed/NCBI
|
|
30
|
Li X, Li L, Wang W, Yang Y, Zhou Y and Lu
S: Soluble purified recombinant C2ORF40 protein inhibits esophageal
cancer cells proliferation by inducing cell cycle G1 phase block.
Oncol Lett. 10:1593–1596. 2015.PubMed/NCBI
|
|
31
|
Wang W, Fan H, Li X, Wu G, Zhao W, Zhang
G, Zhao G and Li LW: Beclin 1 promotes apoptosis and decreases
invasion by upregulating the expression of ECRG4 in A549 human lung
adenocarcinoma cells. Mol Med Rep. 14:355–360. 2016.PubMed/NCBI
|
|
32
|
Fleisig HB and Wong JMY: Telomerase
promotes efficient cell cycle kinetics and confers growth advantage
to telomerase-negative transformed human cells. Oncogene.
31:954–965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rana C, Piplani H, Vaish V, Nehru B and
Sanyal SN: Downregulation of telomerase activity by diclofenac and
curcumin is associated with cell cycle arrest and induction of
apoptosis in colon cancer. Tumour Biol. 36:5999–6010. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu L, Li S and Stohr BA: The role of
telomere biology in cancer. Annu Rev Pathol. 8:49–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baird A, Coimbra R, Dang X, Lopez N, Lee
J, Krzyzaniak M, Winfield R, Potenza B and Eliceiri BP: Cell
surface localization and release of the candidate tumor suppressor
ECRG4 from polymorphonuclear cells and monocytes activate
macrophages. J Leukoc Biol. 91:773–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dang X, Podvin S, Coimbra R, Eliceiri B
and Baird A: Cell-specific processing and release of the
hormone-like precursor and candidate tumor suppressor gene product,
ECRG4. Cell Tissue Res. 348:505–514. 2012. View Article : Google Scholar : PubMed/NCBI
|