Introduction
The development of a vascular network is considered
to be a rate-limiting step for tumor growth, invasion and
metastasis (1). Tumor angiogenesis is
defined as the formation of new capillary from pre-existing
vessels. Early studies demonstrated that microvessel density, a
measure of angiogenesis, was highly correlated with poorer survival
in primary breast, prostate, gastric, non-small cell lung and
cervical cancers (2–4). Therefore, tumor angiogenesis is a
promising target for the treatment of solid cancers. A number of
growth factors and inflammatory cytokines are known to promote
angiogenesis, including vascular endothelial growth factor (VEGF),
angiopoietins (Angs) and interleukin-8 (5–7). Among
these, VEGF is one of the most significant pro-angiogenic factors,
which binds to kinase insert domain-containing receptor
(KDR/FLk-1/VEGFR2), particularly expressed in endothelial cells, to
mediate its angiogenic effect (8).
Accumulating evidence has revealed that VEGF stimulates
autophosphorylation of VEGF receptor 2 (VEGFR2) and then initiates
the signaling cascade involving the PKC-Raf1-MEK-ERK1/2 and
PI-3K/AKT/eNOS pathways to exert angiogenic and pro-survival
effects on endothelial cells (9,10).
Subsequently, inhibition of VEGFR2 signaling by small molecule
inhibitors was demonstrated to significantly suppress the growth of
a variety of solid tumors (11). In
addition, bevacizumab, a neutralizing antibody to VEGF, has been
applied to prolong survival in patients with malignant colon
cancer, and is currently being tested with other tumor types
(12).
However, new blood vessels stimulated by VEGF alone
are not mature. Angiopoietin 1 (Ang1) is another significant
pro-angiogenic factor. Unlike VEGF, Ang1 is required for vascular
remodeling and maturation during angiogenesis. After binding to its
receptor Tie2, Ang1 stimulates autophosphorylation of Tie2 and then
activates downstream signaling molecules, including AKT and ERK,
which promotes stabilization of the immature endothelial cell
network (13). It is generally
accepted that VEGF/VEGFR2 signaling is essential for new blood
vessel formation, while Ang1/Tie2 signaling is critical for blood
vessel maturation and stabilization. Therefore, the inhibition of
the two pathways exhibits additive anti-angiogenic effects.
Accumulating evidence has indicated that masses of
reactive oxygen species (ROS) induce cell death, while low
concentrations of ROS play a crucial role in tumor progression by
acting as a signaling molecule (14,15). ROS
are also involved in the processes of angiogenesis, including
proliferation, degradation of the extracellular matrix, migration,
and differentiation of endothelial cells. A number of antioxidants
exhibit anti-angiogenic activity.
The nitroxides are a group of metal-free, low
molecular weight, water-soluble and stable free radicals which are
widely used in electron paramagnetic resonance spectroscopy
(16,17). Increasing evidence has revealed that
nitroxides have antioxidative and anticancer activity. Gariboldi
et al also demonstrated that treatment with nitroxide
decreased the rate of tumor vascularization and reduced microvessel
density (18).
4-isothiocyanate-2,2,6,6-tetramethyl piperidinooxyl (4-ISO-Tempo)
is one of the nitroxides that exhibits significant antioxidant
activity. However, the anti-neoplastic and anti-angiogenic effect
of 4-ISO-Tempo has yet not been reported. The main aim of the
present study was to investigate the anti-angiogenic activity of
4-ISO-Tempo and its underlying molecular mechanism.
Materials and methods
Materials and reagents
4-ISO-Tempo was a gift from the State Key Laboratory
of Applied Organic Chemistry, Lanzhou University (Lanzhou, China).
It was dissolved in dimethylsulfoxide. MCDB131 medium, epidermal
growth factor (EGF), hydrocortisone, sulforhodamine B (SRB) sodium
salt, gelatin and 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Leibovitz's L-15
medium and Dulbecco's modified Eagle's medium (DMEM) were purchased
from Gibco Life Technologies (Carlsbad, CA, USA). Fetal bovine
serum (FBS) was purchased from Lanzhou HyClone (Lanzhou, China).
Matrigel was purchased from Becton Dickinson (Bedford, MA, USA).
Millicell cell culture inserts were purchased from Millipore
(Bedford, MA, USA). Recombinant human VEGF and recombinant human
Ang1 were purchased from Peprotech (Rocky Hill, NJ, USA).
Antibodies to VEGFR2, Tie2 and actin were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Phospho-VEGFR2 (Tyr 1175)
rabbit mAb and Phospho-Tie2 (Tyr 992) antibody were purchased from
Cell Signaling Technology (Beverly, MA, USA). Other agents were of
analytical purity.
Cell line and cell culture
Human microvascular endothelial cells (HMEC-1) were
cultured in MCDB131 medium containing 20% FBS, 10 ng/ml EGF and 1
µg/ml hydrocortisone. Human colon carcinoma cells (SW620) were
cultured in Leibovitz's L-15 medium supplemented with 10% FBS.
Human lung adenocarcinoma cells (A549) were cultured in DMEM with
10% FBS. Cells were incubated in an atmosphere of 5% CO2
at 37°C.
Cell viability assay
The effect of 4-ISO-Tempo on the viability of
various cell lines was examined with SRB assay (19,20).
Briefly, HMEC-1, SW620 and A549 cells were seeded in 96-well
plates. Following overnight incubation, the cells were treated with
or without 4-ISO-Tempo for 24 and 72 h. The culture medium was then
discarded. 10% trichloroacetic acid (100 µl) was added to each well
and the plates were incubated at 4°C for 1 h. Each well was stained
with 100 µl 0.4% SRB for 30 min, washed with 1% acetic acid to
remove unbound dye and left to dry overnight. The protein-bound SRB
dye was solubilized with 100 µl 10 mM Tris base. The optical
density in each well was read with a microplate reader
(Infinite® 200 PRO series; Tecan Group Ltd., Männedorf,
Switzerland) at 515 nm.
Gelatin zymography
Matrix metalloproteinase (MMP)-2 and MMP-9
gelatinase activity was measured as described in a previous study
(21) with slight modification.
HMEC-1 cells were treated with various concentrations of
4-ISO-Tempo in the absence of serum for 24 h at 37°C. The
conditioned medium was collected and centrifuged. Supernatant was
collected and separated by 7.5% sodium dodecyl sulphate (SDS)
polyacrylamide gel containing 1% gelatin. Then the gels were washed
for 1 h at room temperature to remove SDS and incubated in the
incubation buffer [50 mM Tris-HCl (pH 7.5), 0.2 M NaCl and 5 mM
CaCl2] for 36 h at 37°C. The gel was stained for 1 h
with 0.5% Coomassie brilliant blue R-250 and destained with 25%
methanol and 7.5% acetic acid. Gelatinase activity was detected as
clear bands against a dark blue background.
Cell migration assay
Endothelial cell migration assay was measured as
described previously (19) with
slight modification. Briefly, 2×105 HMEC-1 cells in
serum-free culture medium with or without 4-ISO-Tempo were added
into the upper chamber cell culture inserts. The lower chamber
contained 600 µl MCDB131 medium supplemented with 20% FBS.
Following incubation for 24 h, non-migrated cells were scraped with
a cotton swab. The bottom side of the membrane was fixed by ethanol
and then stained with 0.1% crystal violet. The migrated cells were
then washed three times with phosphate-buffered saline (PBS).
Images of at least five randomly selected microscopic fields were
captured under light microscopy. The number of migrated cells was
counted using ImagePro Plus software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Tube formation assay
Tube formation assay was measured as described
previously (19) with slight
modification. HMEC-1 cells at a density of 8×104/well
were seeded in Matrigel-coated 96-well plates in MCDB131 medium
containing various concentrations of 4-ISO-Tempo. After 8 h, images
were captured under an inverted microscope (Olympus IX41; Olympus
Corporation, Tokyo, Japan) and the tubular length was measured
using ImagePro Plus software.
Western blot assay
Western blot assay was assessed as previously
described (22) with slight
modification. HMEC-1 cells were pretreated with various
concentrations of 4-ISO-Tempo for 24 h and then stimulated with 50
ng/ml VEGF for 10 min or 200 ng/ml Ang1 for 30 min. Cells were
lysed in lysis buffer with proteinase inhibitors (50 mM NaF, 0.2 mM
Na3VO4, 1 mM phenylmethane sulfonyl fluoride,
2 µg/ml aprotinin and 2 µg/ml leupeptin). Equal amounts of protein
for each sample were separated by SDS polyacrylamide and
transferred onto the polyvinylidene fluoride membrane. The membrane
was then blocked and probed with specific antibodies. Horseradish
peroxidase-conjugated secondary antibodies were used to probe the
antibody. Immunoreactive bands were detected with
chemiluminescence.
Intracellular ROS measurement
The intracellular ROS were measured as described
previously (23) with slight
modification. HMEC-1 cells were seeded in a six-well plate and
treated with 4-ISO-Tempo in serum-free medium for 4 h. Then the
cells were left untreated or treated with 50 ng/ml VEGF for 10 min
and incubated with 10 µM DCFH-DA for 30 min. The cells were washed
twice with PBS and the fluorescent images were captured under a
contrast fluorescence microscope (Olympus BX51; Olympus
Corporation). The fluorescent intensity was measured using ImagePro
Plus software.
Statistical analysis
Data are expressed as the means ± standard error.
Statistical significance was evaluated using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of 4-ISO-Tempo on tumor cell
viability
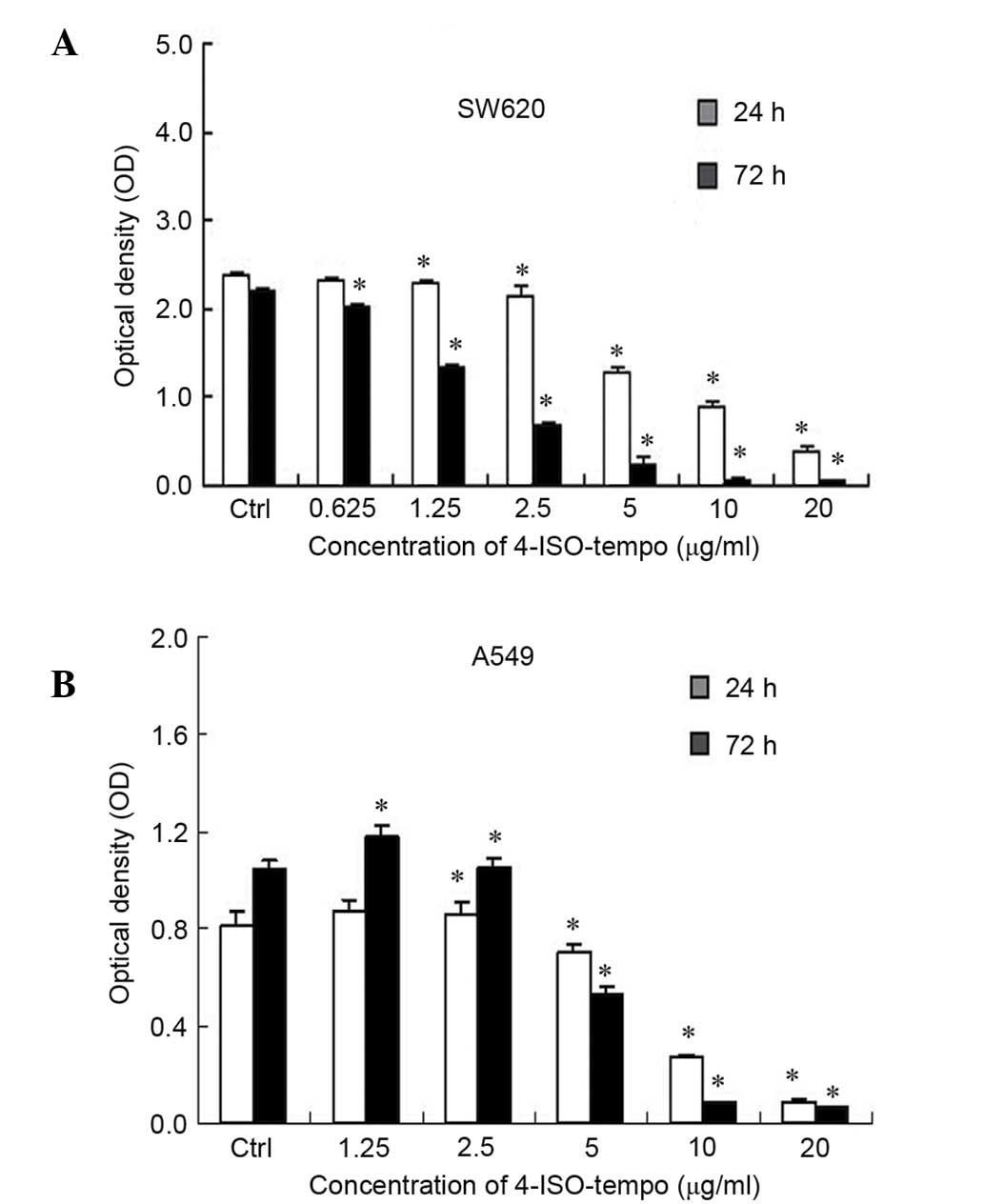
SW620 and A549 cells were sensitive to 4-ISO-Tempo.
As shown in Fig. 1, 4-ISO-Tempo
demonstrated a significant inhibitory effect on the viability of
SW620 and A549 cells in a dose-dependent manner following 72 h
treatment, with an IC50 value of 1.742 µg/ml for SW620
cells and 5.550 µg/ml for A549 cells.
Effect of 4-ISO-Tempo on endothelial
cell viability
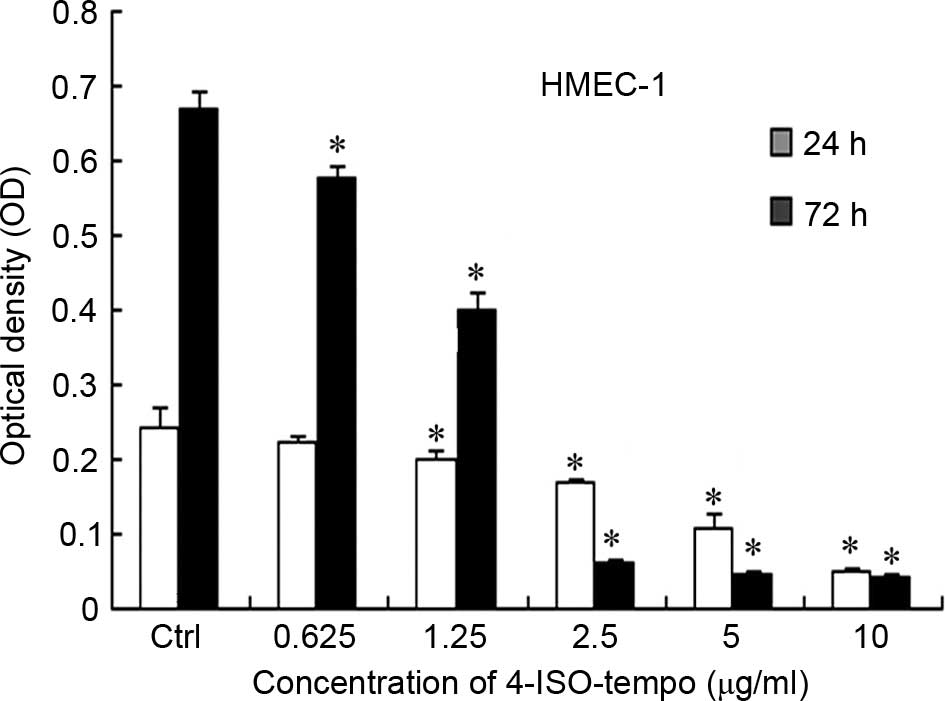
Since the growth of tumor cells is closely
correlated with angiogenesis, we investigated the effect of
4-ISO-Tempo on the cell viability of endothelial cells. As shown in
Fig. 2, 4-ISO-Tempo treatment for 24
or 72 h inhibited the cell viability of HMEC-1 cells, with
IC50 values of 4.668 and 1.386 µg/ml, respectively.
Although 4-ISO-Tempo also inhibited the growth of a variety of
tumor cells, the IC50 values demonstrated that
endothelial cells were more sensitive than tumor cells to
4-ISO-Tempo. Moreover, we concluded that treatment with 4-ISO-Tempo
below the concentration of 5 µg/ml for 24 h did not inhibit cell
viability of HMEC-1 cells. Therefore, the following experiments on
the anti-angiogenic effects on HMEC-1 cells were performed with
4-ISO-Tempo below the concentration of 5 µg/ml for 24 h.
Effect of 4-ISO-Tempo on enzymatic
activity of MMP-2 and MMP-9
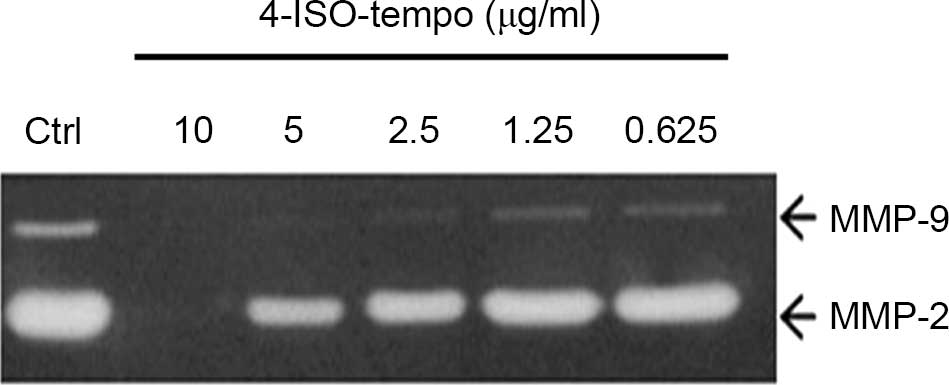
Since the degrading of basement membrane and
extracellular matrix by MMP secreted from tumor and endothelial
cells is crucial for angiogenesis, we analyzed the effect of
4-ISO-Tempo on the secretion of MMP-2 and MMP-9 by HMEC-1 cells. As
shown in Fig. 3, 4-ISO-Tempo caused a
marked decrease in the secretion of MMP-2 and MMP-9 in a
dose-dependent manner. Therefore, it was hypothesized that
4-ISO-Tempo may have the ability to suppress angiogenesis by
inhibiting the enzymatic activity of MMP-2 and MMP-9.
Effect of 4-ISO-Tempo on migration of
endothelial cells
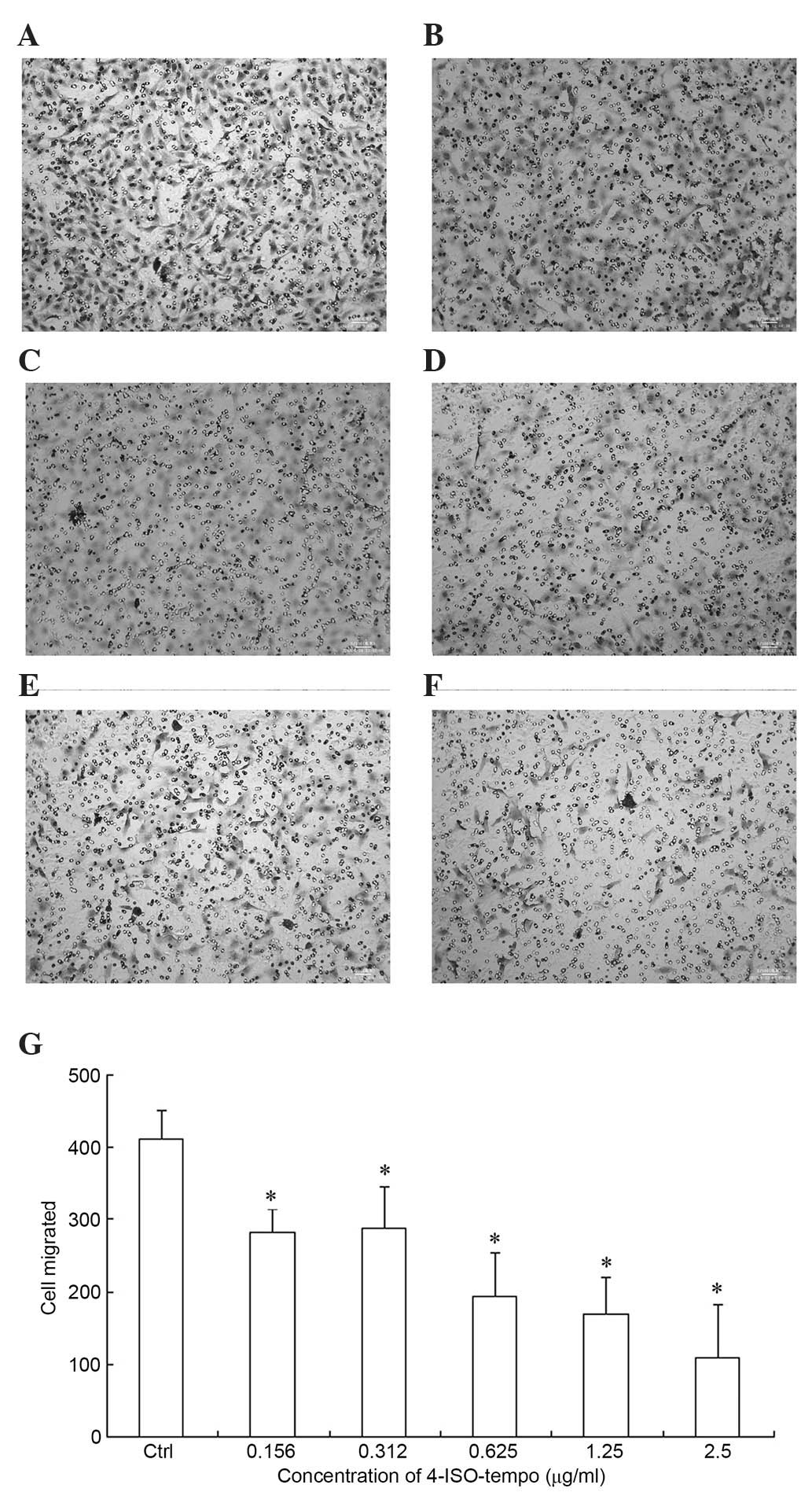
Since the migration of endothelial cells is involved
in angiogenesis and plays a crucial role in the formation of new
blood vessels, we investigated the effect of 4-ISO-Tempo on
endothelial cell migration. As shown in Fig. 4, a large amount of HMEC-1 cells
migrated to the lower side of the filter through the Transwell
membrane. However, the amount decreased significantly when
4-ISO-Tempo was added to the upper chamber, with inhibition rates
of 31.3, 29.9, 52.8, 58.7 and 73.6% at concentrations of 0.156,
0.312, 1.625, 1.25 and 2.5 µg/ml.
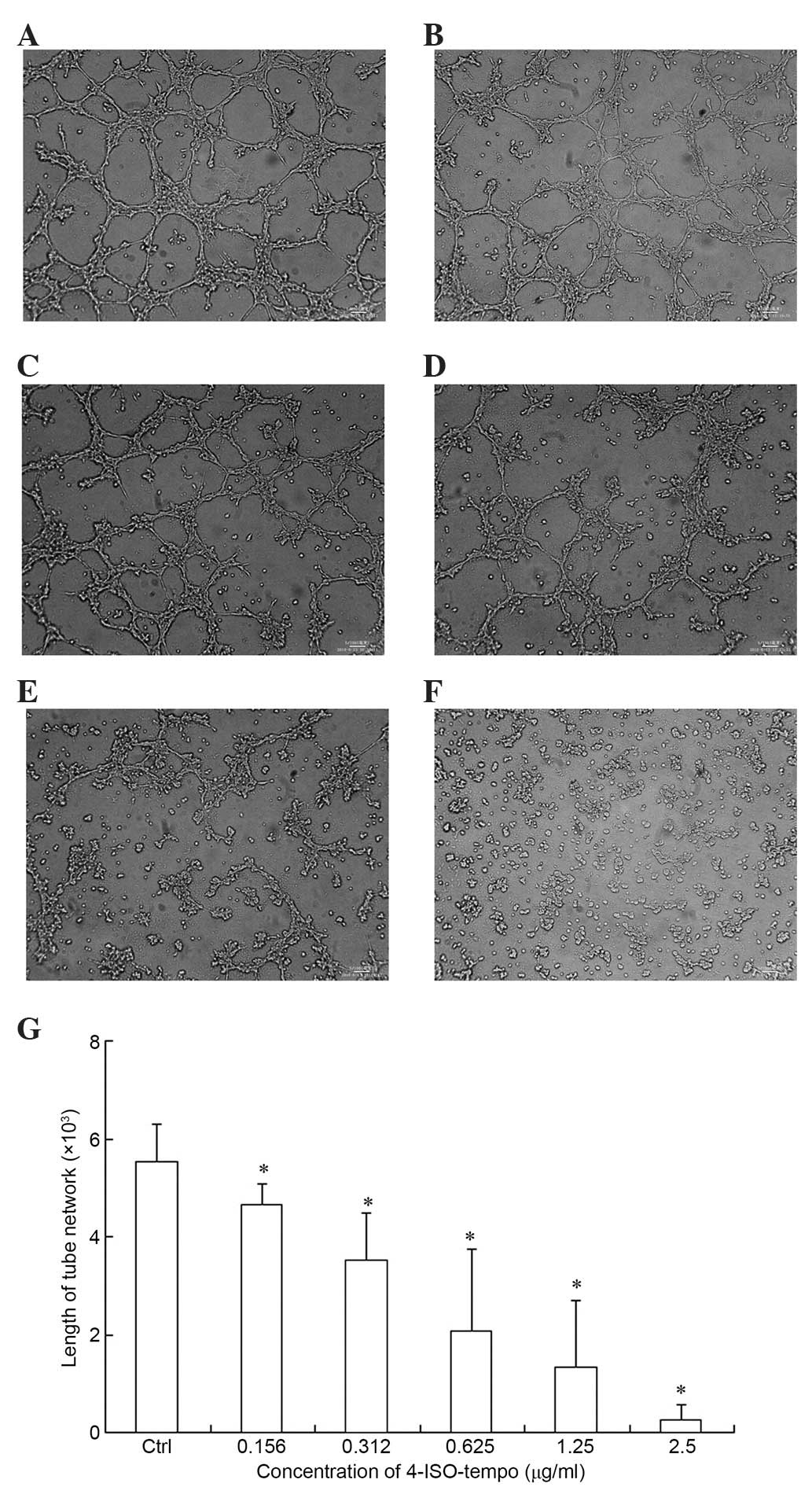
Effect of 4-ISO-Tempo on tube
formation of endothelial cells
Since the differentiation of endothelial cells is
required for angiogenesis, we tested the effect of 4-ISO-Tempo on
the capillary-like structure formation of endothelial cells. The
capillary-like structure formation was inhibited by 4-ISO-Tempo
with inhibition rates of 15.9, 36.3, 62.3 and 75.9% at
concentrations of 0.156, 0.312, 0.625 and 1.25 µg/ml. There were no
completely formed networks in the 2.5 µg/ml treatment group
(Fig. 5).
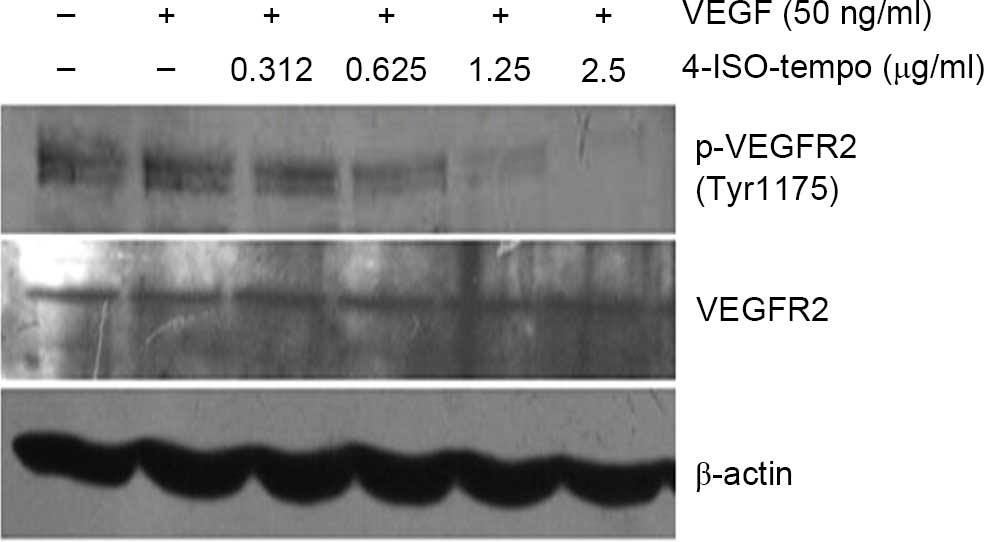
Effect of 4-ISO-Tempo on VEGFR2
phosphorylation in HMEC-1
As a specific mitogen of endothelial cells, VEGF
plays a vital role in endothelial cell activation, proliferation,
migration and survival by activating VEGF/VEGFR2 signaling cascades
(24). To evaluate the molecular
mechanism by which 4-ISO-Tempo inhibited angiogenesis, we examined
its effect on phosphorylation of VEGFR2 stimulated by VEGF. We
observed that 4-ISO-Tempo was unable to affect the basal expression
of VEGFR2, while it strongly blocked phosphorylation of VEGFR2 at
Tyr 1175 in a dose-dependent manner after stimulating VEGF165 (50
ng/ml) for 10 min (Fig. 6). Our
results demonstrated that 4-ISO-Tempo may suppress angiogenesis by
interrupting VEGF-induced VEGFR2 phosphorylation.
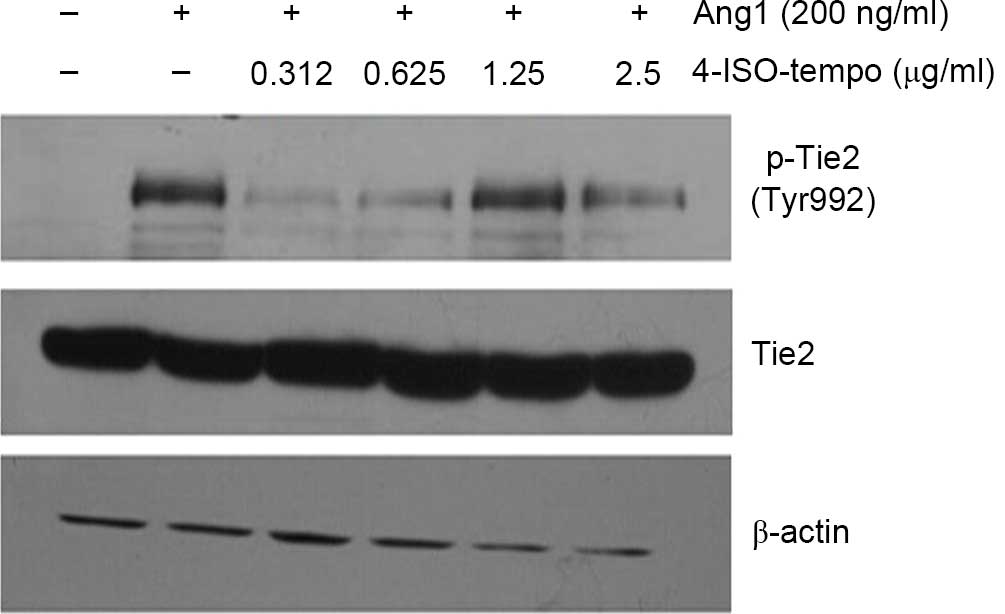
Effect of 4-ISO-Tempo on Tie2
phosphorylation in HMEC-1
Ang1 mediates vessel maturation and integrity by
favoring the recruitment of pericytes and smooth muscle cells
(25). During angiogenesis, Ang1 is
able to induce the phosphorylation of Tie2, which is subsequently
able to transduce a biological signal to endothelial cells
(25). As shown in Fig. 7, Ang1 (200 ng/ml) treatment induced
phosphorylation of Tie2 at Tyr 992 significantly, while 4-ISO-Tempo
blocked the phosphorylation of Tie2 at all concentrations. Our
results demonstrated that 4-ISO-Tempo may inhibit angiogenesis by
blocking Ang1-induced Tie2 phosphorylation.
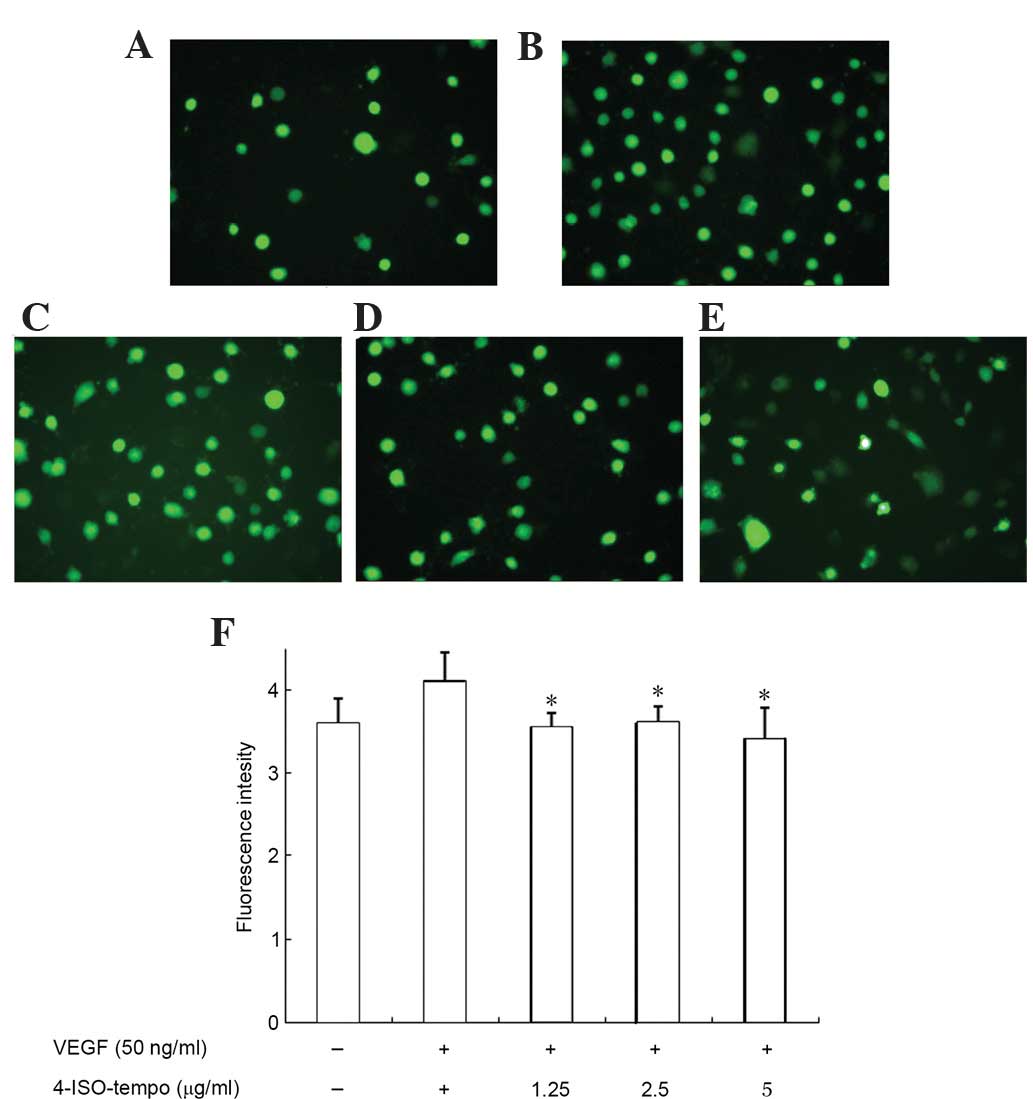
Effect of 4-ISO-Tempo on ROS
generation of endothelial cells
We subsequently investigated the effect of
4-ISO-Tempo on ROS generation in HMEC-1 cells. As shown in Fig. 8, treatment with VEGF increased
intracellular ROS production, while 4-ISO-Tempo decreased this
VEGF-induced effect. Therefore, it was hypothesized that
4-ISO-Tempo inhibited endothelial cell growth, migration and
tube-like formation by reducing ROS generation.
Discussion
Tumor angiogenesis is essential for the growth of
solid tumors, since tumors remain in the dormant phase for a long
time in the absence of the initiation of blood vessel formation.
Usually, the tumor mass diameter does not exceed 1–2 mm without
involving angiogenesis, and this is the size limit for simple
diffusion of nutrients and oxygen (1). Therefore, the angiogenesis process could
be an significant target in suppressing tumor growth and
metastasis. There are numerous complex steps involved in
angiogenesis, including basement membrane degradation, migration,
proliferation and formation of capillary tubes of endothelial
cells. Endothelial cells are the main players in the angiogenesis
process and could be a particular target for anti-angiogenic
therapy. Our results revealed that 4-ISO-Tempo significantly
inhibited A549 and SW620 cell viability. In addition, 4-ISO-Tempo
markedly inhibited the proliferation of HMEC-1 cells in a
concentration-dependent manner, and the effective concentration of
4-ISO-Tempo in HMEC-1 cells is much lower than that in A549 and
SW620 cells. This suggests that endothelial cells are more
sensitive to 4-ISO-Tempo than tumor cells.
Endothelial cells release MMPs, a family of
proteinases, to degrade the extracellular matrix for their
migration during the sprouting process in vivo (26,27). The
breakdown of the tissue matrix by MMPs to facilitate the movement
of newly formed endothelial cells for vessel formation is a
critical step in angiogenesis (28).
MMP-2 and MMP-9 are overexpressed in invasive prostate cancer, and
facilitate the invasion of tumor and endothelial cells (27,29).
4-ISO-Tempo notably reduced the secretion of MMP-2 and MMP-9. In
addition, it significantly inhibited the migration and
capillary-like structure formation of endothelial cells in a
concentration-dependent manner in vitro. This indicated that
4-ISO-Tempo suppressed neovascularization through a multi-step
process in vitro.
During initial angiogenesis and vasculogenesis, a
variety of growth factors and cytokines are upregulated, and exert
their functions through autocrine or paracrine actions. Of these,
VEGF is the most significant mitogenic and survival factor for
vascular endothelial cells, since its receptors are selectively
located in endothelial cells (30).
There is now considerable evidence that VEGFR2 is the main mediator
of VEGF-driven responses in endothelial cells, and it is considered
to be a crucial signal transducer in physiological and pathological
angiogenesis. The VEGF binds to VEGFR2, leading to receptor
dimerization and phosphorylation at Tyr 1175, which has been
identified as a major autophosphorylation site and is critical in
mediating migratory responses (31,32). Our
results demonstrated that 4-ISO-Tempo had no effect on the
expression of VEGFR2, whereas it strongly inhibited phosphorylation
of VEGFR2 at Tyr 1175 after stimulating VEGF for 10 min.
The activation of Tie2 promotes endothelial cell
migration and survival as well as the maturation of new blood
vessels. Blockade of the Ang1/Tie2 and VEGF/VEGFR2 signaling
pathways strengthened the anti-VEGF therapy efficacy on
angiogenesis (33,34). In our study, we observed that
4-ISO-Tempo blocked the phosphorylation of Tie2 at Tyr 992 after
stimulating Ang1 (200 ng/ml) for 30 min. These results demonstrated
that 4-ISO-Tempo not only inhibited the phosphorylation of VEGFR2
but also the phosphorylation of Tie2, indicating that 4-ISO-Tempo
is an anti-angiogenic agent with bifunctional inhibition of
sprouting angiogenesis and maturation of blood vessels.
ROS have been suggested as significant mediators of
angiogenesis (35). VEGF promotes
proliferation and migration of endothelial cells via VEGFR2. ROS
are involved in VEGFR2 autophosphorylation and angiogenic-related
responses in endothelial cells. A previous study revealed that
diphenyleneiodonium, a nicotinamide adenine dinucleotide
phosphate-oxidase inhibitor, blocked the phosphorylation of AKT and
p44/42 MAPK via inhibition of ROS generation induced by Ang1
(36). In the present study,
stimulation with VEGF also induced massive ROS production by
endothelial cells. This result is consistent with that of the
aforementioned study by Chen et al (36). However, pretreatment with 4-ISO-Tempo
significantly inhibited VEGF-induced ROS generation, which
indicated that the inhibitory effect of 4-ISO-Tempo on VEGFR2 and
Tie2 phosphorylation was attributed to decreasing ROS
generation.
4-ISO-Tempo exhibits anti-neoplastic activity in
vitro. In addition, 4-ISO-Tempo also inhibits proliferation,
migration and tube formation of HMEC-1 cells. These results may be
partly attributed to reducing ROS generation and further inhibiting
phosphorylation of VEGFR2 and Tie2. The most promising feature of
4-ISO-Tempo is its ability to inhibit VEGFR2 and Tie2
phosphorylation in the VEGF/VEGFR2 and Ang1/Tie2 pathway
simultaneously to minimize the possibility of resistance to
anti-VEGF therapy in angiogenesis. Although the exact mechanism of
anti-angiogenesis of 4-ISO-Tempo remains elusive, we have
demonstrated that this agent is a potent inhibitor of angiogenesis,
suggesting that 4-ISO-Tempo could be investigated for its
usefulness in anti-angiogenesis therapies.
Acknowledgements
This study was supported by the Medical Foundation
of Lanzhou University (LZUYX200617) and research funds obtained
from Key Lab of Preclinical Study for New Drugs of Gansu Province
(GSKFKT-0702), and partly supported by National Natural Science
Foundation of China (30700142).
References
|
1
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent. J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macchiarini P, Fontanini G, Hardin MJ,
Squartini F and Angeletti CA: Relation of neovascularisation to
metastasis of non-small-cell lung cancer. Lancet. 340:145–146.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
SmithMcCune KK and Weidner N:
Demonstration and characterization of the angiogenic properties of
cervical dysplasia. Cancer Res. 54:800–804. 1994.PubMed/NCBI
|
|
5
|
Ferrara N and Bunting S: Vascular
endothelial growth factor, a specific regulator of angiogenesis.
Curr Opin Nephrol Hypertens. 5:35–44. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones N, Iljin K, Dumont DJ and Alitalo K:
Tie receptors: new modulators of angiogenic and lymphangiogenic
responses. Nat Rev Mol Cell Biol. 2:257–267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koch AE, Polverini PJ, Kunkel SL, Harlow
LA, DiPietro LA, Elner VM, Elner SG and Strieter RM: Interleukin-8
as a macrophage-derived mediator of angiogenesis. Science.
258:1798–1801. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu N, Gao Y, Ling Y, Chen Y, Yang Y, Gu
HY, Qi Q, Liu W, Wang XT, You QD and Guo QL: Wogonin suppresses
tumor growth in vivo and VEGF-induced angiogenesis through
inhibiting tyrosine phosphorylation of VEGFR2. Life Sci.
82:956–963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zachary I and Gliki G: Signaling
transduction mechanisms mediating biological actions of the
vascular endothelial growth factor family. Cardiovasc Res.
49:568–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kowanetz M and Ferrara N: Vascular
endothelial growth factor signaling pathways: therapeutic
perspective. Clin Cancer Res. 12:5018–5022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madhusudan S and Ganesan TS: Tyrosine
kinase inhibitors in cancer therapy. Clin Biochem. 37:618–635.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, GarciaCarbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): a randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh R, Kim WJ, Kim PH and Hong HJ:
Combined blockade of HER2 and VEGF exerts greater growth inhibition
of HER2-overexpressing gastric cancer xenografts than individual
blockade. Exp Mol Med. 45:e522013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sivonová M, Tatarková Z, Duracková Z,
Dobrota D, Lehotský J, Matáková T and Kaplán P: Relationship
between antioxidant potential and oxidative damage to lipids,
proteins and DNA in aged rats. Physiol Res. 56:757–764.
2007.PubMed/NCBI
|
|
15
|
Peshavariya HM, Liu GS, Chang CW, Jiang F,
Chan EC and Dusting GJ: Prostacyclin signaling boosts NADPH oxidase
4 in the endothelium promoting cytoprotection and angiogenesis.
Antioxid Redox Signal. 20:2710–2725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swartz HM: Interactions between cells and
nitroxides and their implications for their uses as biophysical
probes and as metabolically responsive contrast agents for in vivo
NMR. Bull Mag Res. 8:172–175. 1986.
|
|
17
|
Keana JFW, Lex L, Mann JS, et al: Novel
nitroxides for spin-labelling, -trapping and magnetic resonance
imaging applications. Pure Appl Chem. 62:201–205. 1990. View Article : Google Scholar
|
|
18
|
Gariboldi MB, Ravizza R, Petterino C,
Castagnaro M, Finocchiaro G and Monti E: Study of in vitro and in
vivo effects of the piperidinenitroxide Tempol - a potential new
therapeutic agent for gliomas. Eur J Cancer. 39:829–837. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Yang F, Zhang XW, Wang SC, Li MH,
Lin LP and Ding J: Grateloupia longifolia polysaccharide inhibits
angiogenesis by down regulating tissue factor expression in HMEC-1
endothelial cells. Br J Pharmacol. 148:741–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwak HJ, Park MJ, Park CM, Moon SI, Yoo
DH, Lee HC, Lee SH, Kim MS, Lee HW, Shin WS, et al: Emodin inhibits
vascular endothelial growth factor-A-induced angiogenesis by
blocking receptor-2 (KDR/Flk-1) phosphorylation. Int J Cancer.
118:2711–2720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang X, Yi Z, Zhang X, Moon SI, Yoo DH,
Lee HC, Lee SH, Kim MS, Lee HW and Shin WS:
Acetyl-11-keto-beta-boswellic acid inhibits prostate tumor growth
by suppressing vascular endothelial growth factor receptor
2-mediated angiogenesis. Cancer Res. 69:5893–5900. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuang J, Jiang T, Lu D, Luo Y, Zheng C,
Feng J, Yang D, Chen C and Yan X: NADPH oxidase 4 mediates reactive
oxygen species induction of CD146 dimerization in VEGF signal
transduction. Free Radic Biol Med. 49:227–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim CW, Son KN, Choi SY and Kim J: Human
lactoferrinupregulates expression of KDR/Flk-1 and stimulates
VEGF-A-mediated endothelial cell proliferation and migration. FEBS
Lett. 580:4332–4336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grant DS and Kleinman HK: Regulation of
capillary formation by laminin and other components of the
extracellular matrix. EXS. 79:317–333. 1977.
|
|
27
|
Takaha N, Resar LM, Vindivich D and Coffey
DS: High mobility group protein HMGI(Y) enhances tumor cell growth,
invasion, and matrix metalloproteinase-2 expression in prostate
cancer cells. Prostate. 60:160–167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Overall CM and López-Otín C: Strategies
for MMP inhibition in cancer: innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehta PB, Jenkins BL, McCarthy L, Thilak
L, Robson CN, Neal DE and Leung HY: MEK5 overexpression is
associated with metastatic prostate cancer and stimulates
proliferation, MMP-9 expression, and invasion. Oncogene.
22:1381–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veikkola T and Alitalo K: VEGFs, receptors
and angiogenesis. Semin Cancer Biol. 9:211–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SL, Lee ST, Trang KT, Kim SH, Kim IH,
Lee SO, Kim DG and Kim SW: Parthenolide exerts inhibitory effects
on angiogenesis through the downregulation of VEGF/VEGFRs in
colorectal cancer. Int J Mol Med. 33:1261–1267. 2014.PubMed/NCBI
|
|
33
|
Huang S, Yang N, Liu Y, Hu L, Zhao J, Gao
J, Li Y, Li C, Zhang X and Huang T: Grape seed proanthocyanidins
inhibit angiogenesis via the downregulation of both vascular
endothelial growth factor andangiopoietin signaling. Nutr Res.
32:530–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pyriochou A, Tsigkos S, Vassilakopoulos T,
Cottin T, Zhou Z, Gourzoulidou E, Roussos C, Waldmann H, Giannis A
and Papapetropoulos A: Anti-angiogenic properties of a sulindac
analogue. Br J Pharmacol. 152:1207–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paeng SH, Jung WK, Park WS, Lee DS, Kim
GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, et al: Caffeic acid
phenethyl ester reduces the secretion of vascular endothelial
growth factor through the inhibition of the ROS, PI3K and HIF-1α
signaling pathways in human retinal pigment epithelial cells under
hypoxic conditions. Int J Mol Med. 35:1419–1426. 2015.PubMed/NCBI
|
|
36
|
Chen JX, Zeng H, Lawrence ML, Blackwell TS
and Meyrick B: Angiopoietin-1-induced angiogenesis is modulated by
endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol.
291:H1563–H1572. 2006. View Article : Google Scholar : PubMed/NCBI
|