Introduction
Primitive neuroectodermal tumors (PNETs) are rare,
undifferentiated sarcomas deriving from cells that originate from
the neural crest. PNETs often arise in the soft-tissues and bones
of adolescents and young adults (aged <35 years), with a slight
male preponderance (1). Primary
peripheral PNET (pPNET) of the lung without chest wall or pleural
involvement is extremely rare. Due to the similarities of PNET and
Ewin's tumor, it is difficult to estimate the exact incidence of
PNET. The most common symptoms are a cough, fever, dyspnea,
hemoptysis and chest pain; however, none of the clinical
manifestations are specific to PNET (2). The diagnosis of PNET is generally made
by a histopathological analysis, and the treatment of PNET involves
various combinations of surgical resection, chemotherapy and
radiotherapy (3). Similar to Ewing's
sarcoma, PNET is a highly malignant tumor with a poor prognosis:
the 5-year survival rate is <25% (4).
The present study reports the rare case of a patient
with pPNET localized to the lung, who was successfully treated by a
combination of surgical resection, radiotherapy (RT), chemotherapy
and traditional Chinese medicine, including Kanglaite and Shenqi
Fuzheng injections.
Case report
In March 2013, a 37-year-old female patient
presented at the Department of Thoracic Surgery of Xuzhou Central
Hospital (Xuzhou, China), with a history of a dry cough, mild
dyspnea and slight pain in the left chest for 3 months. A computed
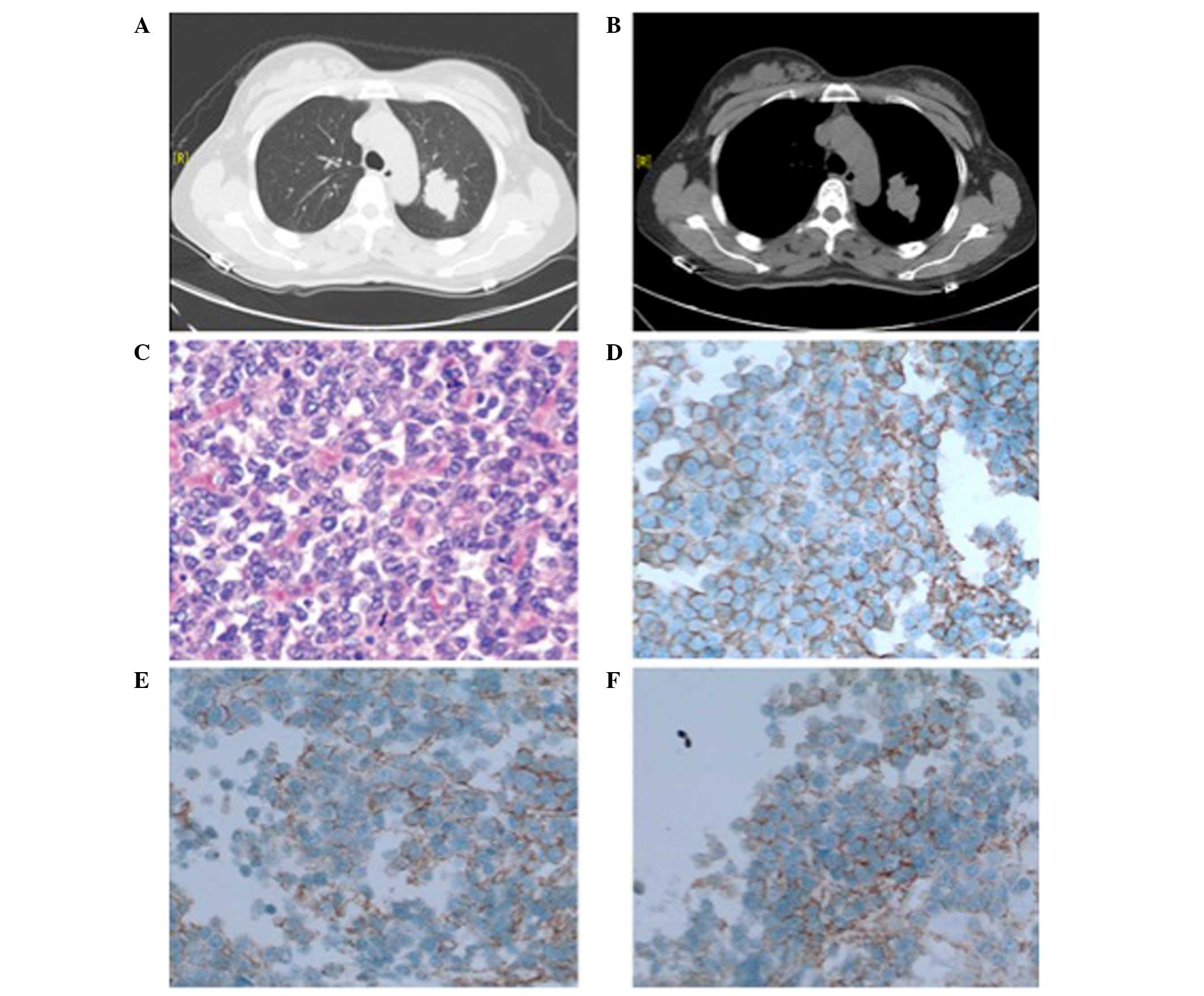
tomography (CT) scan of the chest revealed a mass with lobulated
margins (Fig. 1A). The mass was not
connected to the chest walls, pleurae or any other adjacent organs
(Fig. 1B). Bronchoscopy did not
reveal any abnormal results. Standard staging procedures, including
bone emission CT, brain magnetic resonance imaging and abdominal
ultrasonography, identified no distant metastasis. The values of
the serum tumor markers neuron-specific enolase (NSE),
carcinoembryonic antigen, squamous cell carcinoma and cytokeratin
19 fragments were not elevated.
A left upper lobectomy associated with mediastinal
lymph node dissection was performed in the Department of Thoracic
Surgery in April 2013, and neither the pleurae or the chest wall
were involved. Microscopic analysis indicated that the resected
tissue consisted of small round cells (Fig. 1C). Immunohistochemical analysis of
4-µm formalin-fixed, paraffin-embedded tissue sections stained with
hematoxylin and eosin and visualized using the MaxVision TM
HRP-Polymer anti-Mouse/Rabbit IHC kit (cat. no. KIT-5030; Fuzhou
Maixin Biotech., Co., Ltd., Fuzhou, China) demonstrated that the
tumor cells were positive for cluster of differentiation (CD) 99
(mouse anti-CD99 monoclonal antibody; cat. no. MAB-0059) (Fig. 1D) and CD56 (mouse anti-CD56 monoclonal
antibody; cat. no. MAB-0256) (Fig.
1E), focally positive for vimentin (rabbit anti-vimentin
monoclonal antibody; cat. no. RMA-0547) (Fig. 1F), and negative for thyroid
transcription factor 1 (TTF-1; mouse anti-TTF-1 monoclonal
antibody; cat. no. MAB-0266), S-100 (mouse anti-S-100 monoclonal
antibody; cat. no. MAB-0697), leukocyte common antigen (LCA; mouse
anti-CD45 monoclonal antibody; cat. no. MAB-0037) and chromogranin
A (mouse anti-chromogranin A; cat. no. MAB-0202; all: Fuzhou Maixin
Biotech., Co., Ltd.). These findings were indicative of a pPNET,
and the diagnosis was supported by the presence of the
t(11;22)(q24;q21) translocation in the tumor cells, as detected by
a cytogenetic analysis.
Following the diagnosis of pPNET, the patient
initially received RT. The total RT dose was 50 Gy, which was
administered with a daily standard fractionation schedule of 2 Gy.
Following RT, the patient underwent a combined chemotherapy regime
with cyclophosphamide (750 mg/m2), cisplatin (75
mg/m2) and vincristine (1.4 mg/m2) every 3
weeks for a total of 6 cycles. Following completion of
chemotherapy, a chest CT scan was performed, which showed no
disease progression. Traditional Chinese medicine, including
Kanglaite (200 ml/day for 21 days; Zhejiang Kanglaite
Pharmaceutical Co., Ltd., Zhejiang, China) and Shenqi Fuzheng (250
ml/day for 10 days; Livzon Pharmaceutical Group, Inc., Guangdong,
China) injections, were administered monthly until the present in
order to improve the patient's immune functions and decrease
adverse events associated with chemotherapy and radiotherapy.
Follow-ups were scheduled every 3 months, and the patient is
currently in a good condition. Written informed consent was
obtained from the patient for the publication of this study.
Discussion
pPNETs belong to the family of ‘small round cell
tumors’, which exhibit various degrees of neuroectodermal
differentiation and derive from cells originating from the neural
crest (5). This rare neoplasm is more
frequent in children and adolescents than in adults (1). PNETs involving the thoracopulmonary
region were first reported as ‘malignant small cell tumors of the
thoracopulmonary region in childhood’ by Askin et al in
1979, which resulted in them being termed as Askin's tumors
(6).
Although pPNETs have often been reported in the
literature, the majority are located in the kidneys, chest wall,
urinary bladder, myocardium, retroperitoneum, pancreas and the
female genital tract (7–9). Reports of pPNETs arising in the lung
without chest wall or pleural involvement, such as the one in the
present case, are extremely rare.
The diagnosis of pPNETs is based on light microscopy
following identification of a small round cell tumor (10). Immunohistochemically, pPNETs are
positive for CD99, NSE, CD56 and vimentin, and negative for LCA,
cytokeratin, epithelial membrane antigen and desmin (10). In order to diagnose a tumor as a
pPNET, it should be positive for at least two of the aforementioned
neural markers. In addition, reciprocal translocation
(11;22)(q24;q12) is considered to be characteristic of this tumor
family (11,12). Any tumor suspected to be a pPNET
should undergo biopsy, either by needle or a complete and wide
surgical excision in order to obtain tissue from the lesion for all
aforementioned tests. Hence, the diagnosis of pPNETs is based on
histopathological, immunohistochemical, and, when possible, genetic
analyses.
Due to the different therapeutic schedules and
prognostic characteristics for distinct tumor types, differential
diagnosis is essential for PNETs. Usually, PNETs share a similar
histological appearance with small round blue cell tumor (except
for the presence of rosettes), and CD99 expression and cytogenetic
translocation t(11;22)(q24;q12) with Ewing's sarcoma. Neural
differentiation indicates the presence of PNET rather than Ewing's
sarcoma (13). The differential
diagnosis of PNET also includes small-cell carcinoma,
neuroblastoma, lymphoma and rhabdomyosarcoma, which are all
indistinguishable by conventional light microscopy (5). Positive immunohistochemical staining for
CD99, CD56, vimentin, NSE and synaptophysin are favorable in the
differential diagnosis of PNET (6).
Neuroblastomas are also positive for NSE and
synaptophysin, but negative for CD99, and the presence of
Homer-Wright rosettes is a characteristic of these lesions
(14). LCA positivity supports the
diagnosis of lymphoma, but T cell lymphoblastic lymphoma may be
positive for CD99 and CD3, and negative for LCA. Small-cell
carcinoma is almost always positive for cytokeratin, while
rhabdomyosarcoma is positive for actin, desmin and myoglobin
(15–18); therefore, the immunohistochemical
results observed in the present case (positivity for CD99, vimentin
and CD56, and negativity for CD3, desmin, and LCA) highly support
the diagnosis of a pulmonary PNET.
Treatment for pPNETs includes surgical resection,
chemotherapy and radiotherapy. It has been reported that complete
surgical excision with wide (2–3 cm) margins may improve long-term
survival for patients with PNETs (19). The most commonly recommended
chemotherapy regimens include several cycles with agents such as
cyclophosphamide, vincristine, doxorubicin, etoposide and
ifosfamide (13,20). A number of studies have reported poor
long-term survival rates in PNETs despite multimodal treatment
(21,22). The patient in the present case was
treated by a multimodal treatment strategy that included surgery,
radiotherapy and 6 cycles of chemotherapy with cyclophosphamide,
cisplatin and vincristine. Furthermore, traditional Chinese
medicine, including Kanglaite and Shenqi Fuzheng injections, were
used for the treatment. Kanglaite injection is an antitumor agent
that has been shown to significantly decrease the occurrence of
cancer cachexy and improve the quality of life of cancer patients
(23). In addition, it may ameliorate
the development of multiple drug resistance in cancers when
combined with radiotherapy and chemotherapy, as well as
strengthening the overall response rate and reducing the side
effects of nausea and vomiting (24).
Shenqi Fuzheng injection is commonly used to improve immune
function against cancer, and was reported to reduce the toxicity of
radiotherapy and chemotherapy (25).
At the time of writing, the patient had been alive without any
signs of recurrence or metastasis for 19 months, which demonstrated
that the treatment had been adequate.
In conclusion, despite the rarity of arising from
the lung without chest wall or pleural involvement in adult
patients, PNETs should be considered in the differential diagnosis
of all parenchymal lung nodules. In addition, multimodal treatment,
including surgical excision, radiotherapy, feasible chemotherapy
regimens and traditional Chinese medicine, has been shown to be
beneficial.
Acknowledgements
The present study was supported by the project Six
Talent Peaks Project of Jiangsu Province (grant no. WSN-065).
References
|
1
|
de Alava E and Gerald WL: Molecular
biology of the Ewing's sarcoma/primitive neuroectodermal tumor
family. J Clin Oncol. 18:204–213. 2000.PubMed/NCBI
|
|
2
|
Dong M, Liu J, Song Z, Li X, Shi T, Wang
D, Ren D and Chen J: Primary Multiple Pulmonary Primitive
Neuroectodermal Tumor: Case Report and Literature Review. Medicine
(Baltimore). 94:e11362015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andrei M, Cramer SF, Kramer ZB, Zeidan A
and Faltas B: Adult primary pulmonary primitive neuroectodermal
tumor: Molecular features and translational opportunities. Cancer
Biol Ther. 14:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Subbiah V, Anderson P, Lazar AJ, Burdett
E, Raymond K and Ludwig JA: Ewing's sarcoma: Standard and
experimental treatment options. Curr Treat Options Oncol.
10:126–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jo VY and Fletcher CD: WHO classification
of soft tissue tumours: An update based on the 2013 (4th) edition.
Pathology. 46:95–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Askin FB, Rosai J, Sibley RK, Dehner LP
and McAlister WH: Malignant small cell tumor of the
thoracopulmonary region in childhood: A distinctive
clinicopathologic entity of uncertain histogenesis. Cancer.
43:2438–2451. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khosla D, Rai B, Patel FD, Sreedharanunni
S, Dey P and Sharma SC: Primitive neuroectodermal tumor of the
uterine cervix diagnosed during pregnancy: A rare case with review
of literature. J Obstet Gynaecol Res. 40:878–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakkar S, Gupta D, Kaur G and Rana V:
Primary primitive neuroectodermal tumor of kidney: A rare case
report with diagnostic challenge. Indian J Pathol Microbiol.
57:298–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banerjee SS, Eyden BP, McVey RJ, Bryden AA
and Clarke NW: Primary peripheral primitive neuroectodermal tumor
of the urinary bladder. Histopathology. 30:486–490. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jimenez RE, Folpe AL, Lapham RL, Ro JY,
O'Shea PA, Weiss SW and Amin MB: Primary Ewing's sarcoma/primitive
neuroectodermal tumor of the kidney: A clinicopathologic and
immunohistochemical analysis of 11 cases. Am J Surg Pathol.
26:320–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YY, Kim do H, Lee JH, Choi JS, In KH,
Oh YW, Cho KH and Roh YK: Primary pulmonary Ewing's
sarcoma/primitive neuroectodermal tumor in a 67-year-old man. J
Korean Med Sci. 22(Suppl): S159–S163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda M, Urano M, Abe M, Mizoguchi Y,
Horibe Y, Murakami M, Tashiro K and Kasahara M: Primary primitive
neuroectodermal tumor of the kidney. Pathol Int. 50:967–972. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carvajal R and Meyers P: Ewing's sarcoma
and primitive neuroectodermal family of tumors. Hematol Oncol Clin
North Am. 19:501–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Small AG, Thwe le M, Byrne JA, Lau L, Chan
A, Craig ME, Cowell CT and Garnett SP: Neuroblastoma, body mass
index, and survival: A retrospective analysis. Medicine
(Baltimore). 94:e7132015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mentzel T, Flaschka J, Mentzel HJ,
Eschholz G and Katenkamp D: Primary primitive neuroectodermal tumor
of the urinary bladder: Clinicopathologic case report and
differential small cell tumor diagnosis of this site. Pathologe.
19:154–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi L, Guo Z and Wu X: Primary pulmonary
primitive neuroectodermal tumor metastasis to the pancreas: A rare
case with seven-year follow-up. Diagn Pathol. 8:512013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cetiner H, Kir G, Gelmann EP and Ozdemirli
M: Primary vulvar Ewing sarcoma/primitive neuroectodermal tumor: A
report of 2 cases and review of the literature. Int J Gynecol
Cancer. 19:1131–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colecchia M, Dagrada G, Poliani PL,
Messina A and Pilotti S: Primary primitive peripheral
neuroectodermal tumor of the prostate: Immunophenotypic and
molecular study of a case. Arch Pathol Lab Med. 127:e190–e193.
2003.PubMed/NCBI
|
|
19
|
Craver RD, Lipscomb JT, Suskind D and
Velez MC: Malignant teratoma of the thyroid with primitive
neuroepithelial and mesenchymal sarcomatous components. Ann Diagn
Pathol. 5:285–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grier HE, Krailo MD, Tarbell NJ, Link MP,
Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers
PA, et al: Addition of ifosfamide and etoposide to standard
chemotherapy for Ewing's sarcoma and primitive neuroectodermal
tumor of bone. N Engl J Med. 348:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mulsow J, Jeffers M, McDermott R, Geraghty
J and Rothwell J: Complete clinical response to neoadjuvant
chemotherapy in a 54-year old male with Askin tumor. Thorac
Cardiovasc Surg. 58:306–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gunluoglu MZ, Kara HV, Demir A and Dincer
SI: Results of multimodal treatment of two patients with thoracic
primitive neuroectodermal tumor. Is surgery really helpful for
survival? Thorac Cardiovasc Surg. 55:460–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang JC, Tian JH, Ge L, Gan YH and Yang
KH: Which is the best Chinese herb injection based on the FOLFOX
regimen for gastric cancer? A network meta-analysis of randomized
controlled trials. Asian Pac J Cancer Prev. 15:4795–4800. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Wang JC, Ma B, Gao W, Chen P, Sun R
and Yang KH: Shenqi Fuzheng Injection for advanced gastric cancer:
A systematic review of randomized controlled trials. Chin J Integr
Med. 21:71–79. 2015. View Article : Google Scholar : PubMed/NCBI
|