Introduction
Laryngeal carcinoma is the most common malignancy
among head and neck tumors, accounting for 1–2.5% of all
malignancies throughout the body. Although the clinical outcome of
laryngeal carcinoma has gradually improved, the prognosis of this
tumor remains poor, with a 5-year overall survival rate of ~60%
(1). Local invasion, lymph node
metastasis and distant metastasis of laryngeal carcinoma are
primarily responsible for this unsatisfactory prognosis (2). The symptoms of laryngeal carcinoma are
frequently non-specific and consequently, the diagnosis and
appropriate therapy are usually delayed. In one study, only 27.8%
of patients with laryngeal carcinoma were noted to have undergone
surgery due to a late diagnosis (3).
Therefore, a better understanding of the molecular mechanisms
driving laryngeal carcinogenesis may aid in the identification of
novel predictive and prognostic biomarkers, and in the development
of novel treatment strategies for this cancer.
The marker for cellular proliferation, Ki-67, has
been generally used in judging clinical progress and estimating the
prognosis of malignant tumors. It has been demonstrated that Ki-67
acts as a positive regulator of cancer progression in gliomas,
breast cancer, salivary gland tumors and squamous cell carcinoma
(4–7).
However, Ki-67 expression in laryngeal carcinoma and its
significance in laryngeal carcinoma progression remain largely
unknown. The present study investigated the expression of Ki-67 in
human laryngeal squamous carcinoma tissues and adjacent
non-cancerous tissues from 50 laryngeal squamous carcinoma patients
using immunohistochemistry and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). Moreover, the effects of Ki-67
gene silencing by small interfering (si)RNA on the proliferation of
HEp2 human laryngocarcinoma cells were observed in
vitro.
Materials and methods
Cell culture and tissues samples
This study was approved by the Institutional Review
Board and Human Ethics Committee of the First Affiliated Hospital
of Xi'an Jiaotong University School of Medicine (Xi'an, Shaanxi,
China). All experiments were performed in accordance with the
principles of the Declaration of Helsinki and all participants
provided their written informed consent to participate in the
study. The human laryngeal carcinoma HEp2 cell line was obtained
from the Center of Biomedical Research in Xi'an Jiaotong University
School of Medicine and routinely cultured in RPMI 1640 medium
(Wuhan Boster Biological Technology, Ltd., Wuhan, China) with 10%
fetal bovine serum (Wuhan Boster Biological Technology, Ltd.) at
37°C. In total, 100 paraffin embedded tissues, consisting of 50
laryngeal squamous carcinoma and 50 adjacent normal tissues, were
obtained from patients treated with surgical resection between
January 2013 and February 2015 in the First Affiliated Hospital of
Xi'an Jiaotong University School of Medicine. None of these
patients received pre-operative radiotherapy and chemotherapy
regimens. The histological diagnosis of the carcinoma was
determined according to World Health Organization criteria
(8).
Immunohistochemical staining
Immunohistochemical staining was performed by the
standard streptavidin-peroxidase technique with the ready-to-use
immunohistochemical detection MaxVision kit (Fuzhou Maixin Biotech.
Co., Ltd., Fuzhou, China). Rabbit monoclonal antibody against human
Ki-67 was obtained from Neomarkers (catalog no., PA5-16446; 1:100
dilution; Fremont, CA, USA). The primary antibody was replaced with
phosphate-buffered saline for the negative control group. For each
tissue section, 10 representative high-power fields (×400
magnification) were selected for histological evaluation. The
expression of the protein was evaluated based on the positive rate
(the percentage of positively-stained cells: ≤10% scored as 0,
11–30% scored as 1, 31–60% scored as 2 and >60% scored as 3) and
staining intensity (0, absence of staining; 1, weakly-positive; 2,
moderately-positive and strongly-positive). The sum of the scores
provide the final score for Ki-67 expression in each sample, in
which a final score of <4 was defined as low/negative expression
and a final score of ≥4 was defined as high expression.
RT-qPCR
Total RNA was isolated from paraffin-embedded
tissues using the RecoverAll™ Total Nucleic Acid Isolation kit
(Ambion, TX, USA) according to the manufacturer's instructions. For
cultured cells, total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocols. RT-qPCR was performed to
evaluate Ki-67 expression using the PrimeScript RT reagent kit
(Takara Bio, Inc., Shiga, Japan) according to the manufacturer's
instructions. Each reaction was performed in a total volume of 25
µl, containing 12.5 µl of 2X Tli RNaseH Plus, 2 µl of primers, 2 µl
of template cDNA, and 8.5 µl of dH2O. The thermal
profile for the qPCR was 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec, 60°C for 30 sec and 72°C for 5 sec on a Bio-Rad
CFX96 RT-qPCR system (Bio-Rad, Hercules, CA, USA). The primers for
Ki-67 gene were as follows: Forward, 5′-AATTCAGACTCCATGTGCCTGAG-3′
and reverse, 5′-CTTGACACACACACATTGTCCTCAGC-3′. The β-actin gene
(forward, 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and
5′-ATGGAGCCACCGATCCACA-3′) was used as an internal control. Melting
curve analysis was then performed. The RT-qPCR procedures were
performed in triplicate and the data were analyzed using the
comparative Cq method (9).
siRNA transfection
For the downregulation of endogenous Ki-67
expression, the following siRNA duplex (Aoke Biological Technology
Co., Ltd., Shanghai, China) was used: 5′-GGUAUGAAAAUGAAAGUCUTT-3′.
The unspecific scrambled siRNA duplex (5′-AAUGGGAAGAAUAUAGUCUTT-3′;
Aoke Biological Technology Co., Ltd.) was used as negative
control.
A total of 1×105 HEp2 cells were seeded
in 6-well plates 24 h prior to transfection. The transfection of
the cells was performed using Oligofectamine (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Specific silencing of targeted genes was confirmed by
at least three independent experiments.
Four groups were included in the present study: i)
The blank control group; ii) the Lipo2000 group (cells treated with
5 µl Lipofectamine 2000); iii) the control siRNA group (cells
treated with 5 µl Lipofectamine 2000 plus 50 pmol negative control
siRNA); and iv) the Ki-67-siRNA group (cells treated with 5 µl
Lipofectamine 2000 plus 50 pmol Ki-67 siRNA).
Cell proliferation assay
Cell proliferation was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay daily over a 3-day time course. The HEp2 cells
were seeded into eight 96-well plates and incubated in RPMI 1640
medium (Wuhan Boster Biological Technology, Ltd.) at a density of
2×103 cells/well at 37°C for 24, 48 and 72 h following
treatment, respectively. Cell culture was added with 20 µl of 5
mg/ml MTT agent (Sigma, Saint Louis, MO, USA) and incubated at 37°C
for 4 h, followed by addition of 200 µl dimethylsulfoxide and a
further 15-min incubation. The absorbance was then determined using
a Multiskan Spectrum microplate reader (Thermo Fisher Scientific
Inc.) at 490 nm. A growth curve was produced according to the
optical density value alterations. The experiment was performed
three times independently.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer. Cellular proteins were collected and subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then
electrotransferred onto Immobilon-P membranes (Invitrogen; Thermo
Fisher Scientific, Inc.). The membranes were then incubated for 2 h
at 37°C with rabbit anti-human EGFR (catalog no., sc-367974;
1:1,000 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or rabbit anti-human E-cadherin (catalog no., sc-7870; 1:1,000
dilution; Santa Cruz Biotechnology, Inc.). This was followed by
incubation with horseradish peroxidase-conjugated donkey
anti-rabbit immunoglobulin G secondary antibody for 1 h at 37°C
(catalog no., NA934; 1:1,000 dilution; Amersham Pharmacia Biotech,
Piscataway, NJ, USA). The rabbit anti-human β-actin antibody
(catalog no., sc-7210; 1:1,000 dilution; Santa Cruz Biotechnology,
Inc.) was used as an internal marker for control purposes.
Statistical analysis
Continuous data were presented as mean ± standard
deviation and categorical data were presented as frequencies.
Statistical differences were calculated by Student's t-test
or chi-square test using SPSS 16.0 statistical software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of Ki-67 protein in paired
human laryngeal squamous carcinoma tissues and corresponding
non-cancerous tissue samples
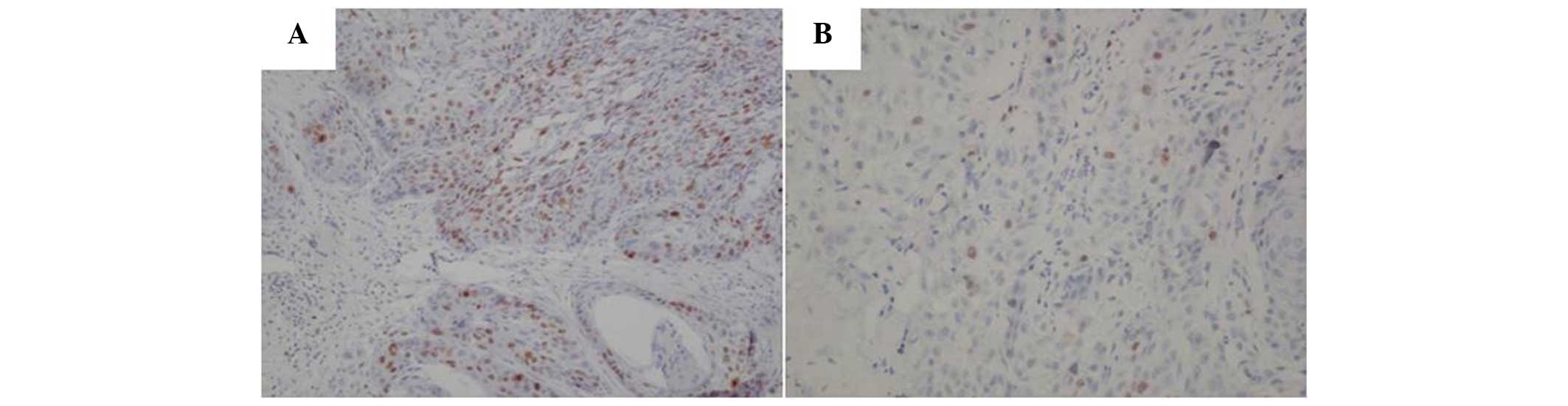
Ki-67 was detected in 34 out of 50 (68%) laryngeal
squamous carcinoma tissues and in only 8 out of 50 (16%)
corresponding non-cancerous tissues (Fig.
1). The frequency of Ki-67 in the laryngeal squamous carcinoma
tissues was significantly higher than that in the non-cancerous
tissues (P<0.001).
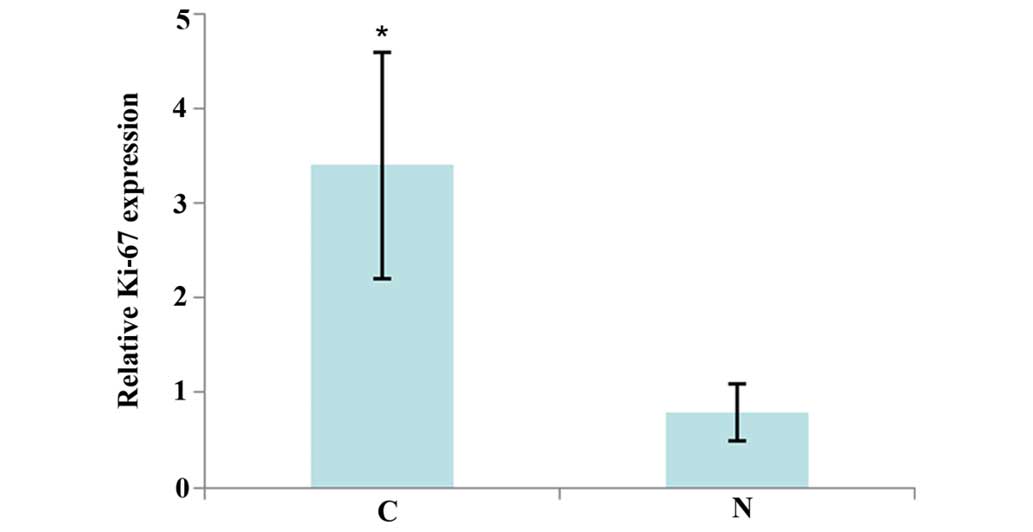
The study further analyzed the expression level of
Ki-67 in the aforementioned tissue samples by RT-qPCR. As shown in
Fig. 2, Ki-67 expression was
significantly increased in the laryngeal squamous carcinoma tissues
compared with the adjacent normal tissues. These observations
suggest that Ki-67 may be a tumor promoter in this cancer.
Increase in Ki-67 expression
correlates with cervical lymph node metastasis and clinical
outcome
All 50 patients were followed up regularly after
surgery. The median follow-up period was 32 months (range, 5–50
months). The expression of Ki-67 in the patients was compared with
regard to gender, age, type of cancer, cervical lymph node
metastasis, histological grade, tumor-node-metastasis stage
(10), recurrence and clinical
outcome. The levels of Ki-67 expression significantly correlated
with cervical lymph node metastasis and clinical outcome. During
the follow-up of 34 patients with high Ki-67 expression, 24
succumbed. This percentage was significantly higher than that in
the patients with low Ki-67 expression (70.6 vs. 12.5%; P<0.001;
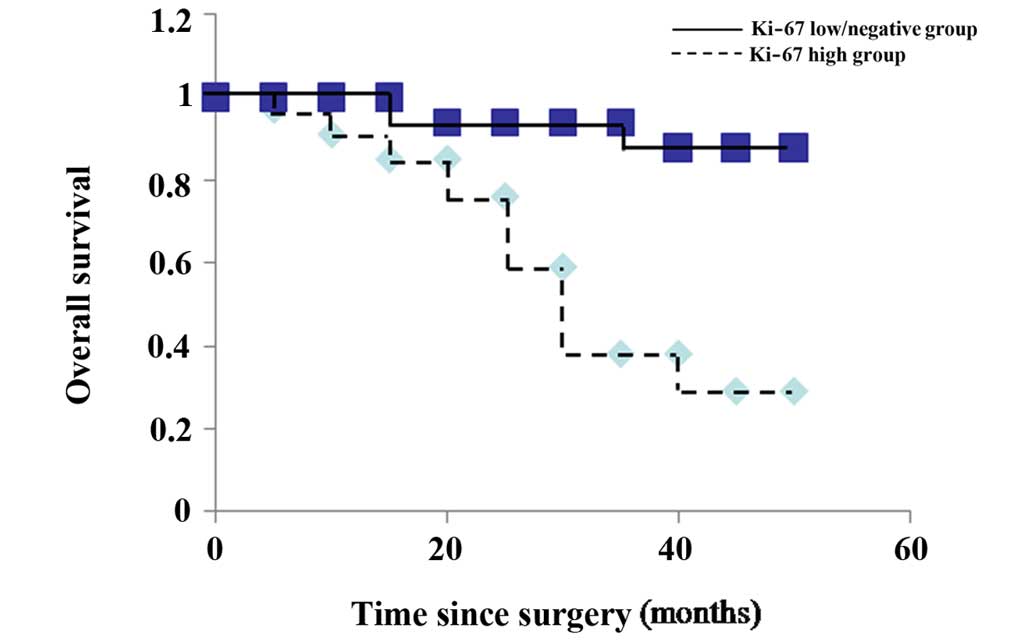
Table I). Using the Kaplan-Meier
method, it was observed that the overall survival time was
significantly shorter in the high Ki-67 expression group than that
in the low/negative Ki-67 expression group (P<0.001) (Fig. 3). These results imply that the high
expression of Ki-67 may be a valuable predictor for the prognosis
of laryngeal squamous carcinoma.
 | Table I.Association between
clinicopathological variables and Ki-67. |
Table I.
Association between
clinicopathological variables and Ki-67.
|
|
| Expression of Ki-67,
n |
|
|---|
|
|
|
|
|
|---|
| Features | Cases, n | + | − | P-value |
|---|
| Gender |
|
|
| 0.938 |
| Male | 34 | 23 | 11 |
|
|
Female | 16 | 11 | 5 |
|
| Age, years |
|
|
| 0.895 |
|
>50 | 35 | 24 | 11 |
|
| ≤50 | 15 | 10 | 5 |
|
| Type of cancer |
|
|
| 0.960 |
|
Supraglottic | 31 | 21 | 10 |
|
|
Glottic | 19 | 13 | 6 |
|
| Cervical lymph node
metastasis |
|
|
| 0.021a |
| Yes | 37 | 29 | 8 |
|
| No | 13 | 5 | 8 |
|
| Histological
grade |
|
|
| 0.884 |
| I | 15 | 10 | 5 |
|
| II | 17 | 11 | 6 |
|
| III | 18 | 13 | 5 |
|
| TNM stage |
|
|
| 0.909 |
| I | 7 | 4 | 3 |
|
| II | 15 | 10 | 5 |
|
| III | 18 | 13 | 5 |
|
| IV | 10 | 7 | 3 |
|
| Recurrence |
|
|
| 0.054 |
|
Yes | 19 | 16 | 3 |
|
| No | 31 | 18 | 13 |
|
| Clinical
outcome |
|
|
| 0.000a |
|
Alive | 24 | 10 | 14 |
|
|
Succumbed | 26 | 24 | 2 |
|
Ki-67-knockdown in HEp2 cells
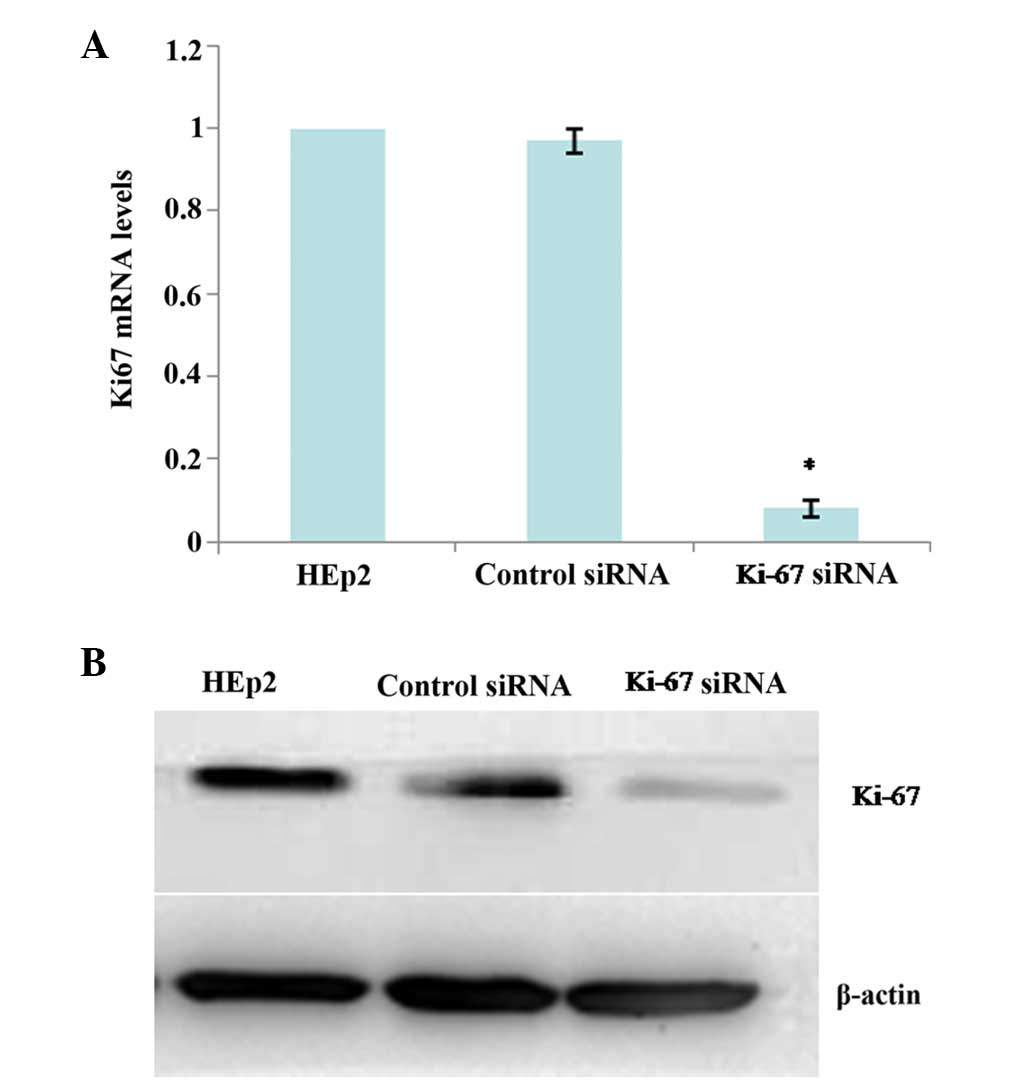
RT-qPCR and western blot analysis were applied to
validate the silencing efficiency of the target gene after RNA
interference. Stable Ki-67 siRNA-transfected HEp2 cells
(Ki-67-siRNA) and a mock-transfected control cell line (control
siRNA) were established as aforementioned. Compared with the
parental HEp2 cells and control siRNA cells, the mRNA and protein
expressions of Ki-67 was significantly reduced in the Ki-67-siRNA
cells at 24 h after siRNA transfection (all P<0.001; Fig. 4A and B), which persisted for at least
72 h (data not shown).
Gene silencing of Ki-67 reduces cell
proliferation in HEp2 cells
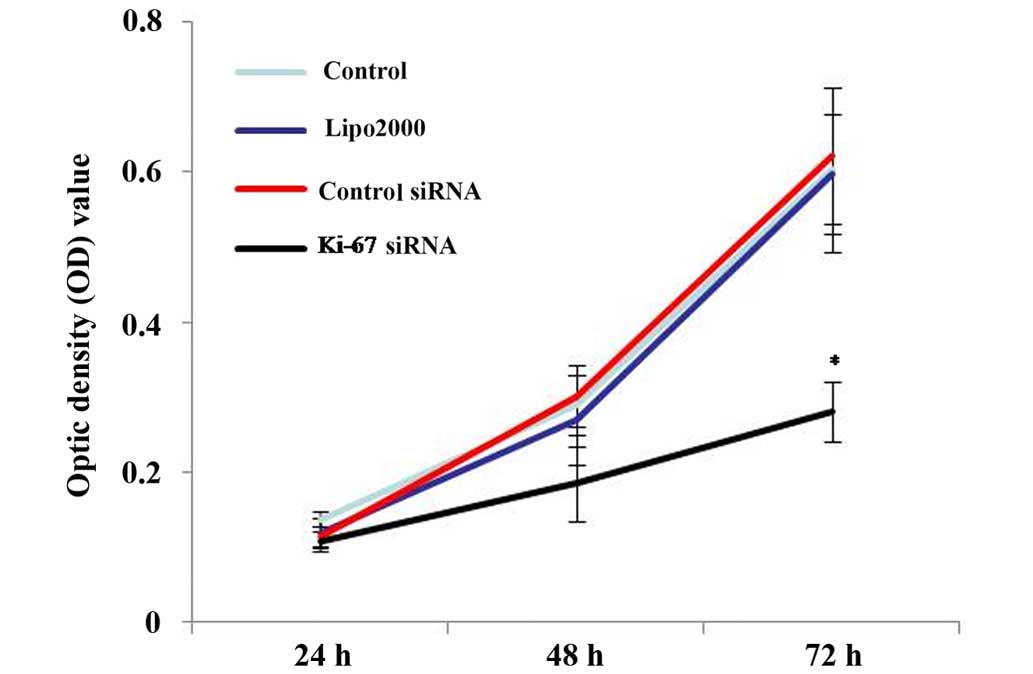
To test the effect of the knockdown of Ki-67 on HEp2
cell growth, a MTT cell proliferation assay was performed. As
demonstrated by MTT assays, Ki-67-siRNA group cells showed
decreased cell proliferation compared with the blank control,
Lipo2000 and control siRNA group cells, supporting the role of
Ki-67 in cell growth in HEp2 cells (P<0.001; Fig. 5).
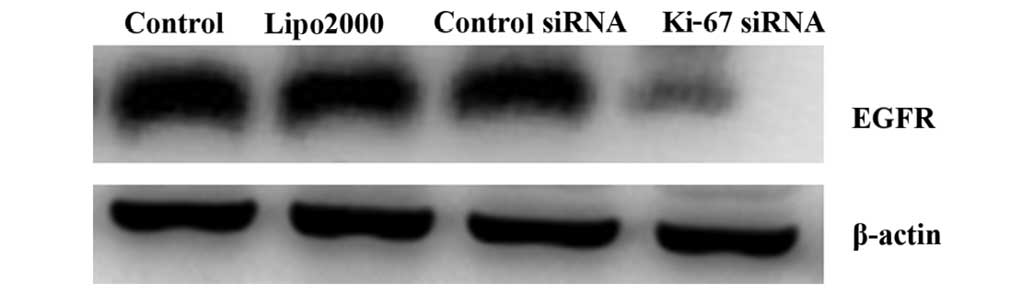
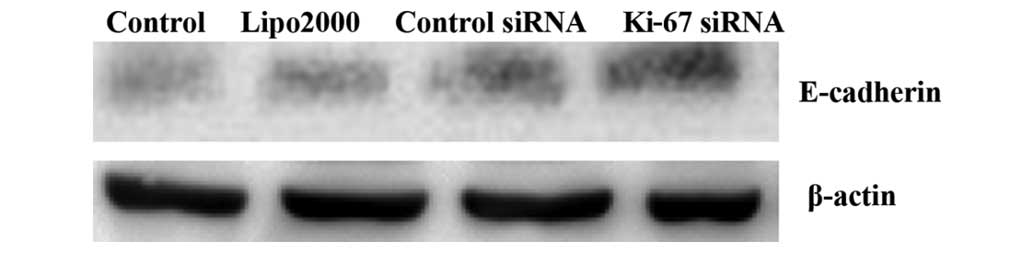
Silencing of Ki-67 by siRNA stimulates
the activation of E-cadherin and suppresses the activation of EGFR
in HEp2 cells
In this study, changes in the protein levels of EGFR
and E-cadherin after transfection were detected by western blot
analysis. The results demonstrated that at 72 h after Ki-67-siRNA
transfection, the protein levels of EGFR and E-cadherin were
significantly decreased and increased, respectively, in the treated
HEp2 cells, compared with those in the cells in the other groups
(all P<0.001; Figs. 6 and 7).
Discussion
Ki-67 is a type of DNA binding protein that is
mainly located in the cell nucleus and is closely associated with
cell proliferation. Ki-67 expression begins to appear in the G1
phase of the cell cycle, then increases gradually, reaches the peak
in the M phase and declines quickly in the late stage of cell
division (11). Ki-67 is involved in
maintaining the structure of the stable DNA in mitosis and has been
used as an indicator of the proliferation rate in a number of human
malignancies (12). Various studies
have shown that the level of Ki-67 is closely associated with the
development and progression of a variety of cancer types, including
lung adenocarcinoma, breast cancer, adrenocortical carcinoma and
gastric cancer (13–16). In laryngeal carcinoma, however, there
is no consensus on the role of Ki-67. Leopardi et al found a
higher degree of Ki-67 positivity in the neoplastic tissue of
laryngeal epithelial lesions compared with in the pre-cancerous
tissues or in benign lesions (17).
Liu et al reported that Ki-67 expression was significantly
higher in primary squamous cell carcinoma of the larynx and
hypopharynx with lymph node metastasis compared with the expression
in those without lymph node metastasis, and that it was correlated
with pathological T-stage and tumor differentiation. In univariate
analysis, the high expression of Ki-67 was inversely correlated
with overall and disease-free survival (18). However, in another study, Teppo et
al reported that Ki-67 did not significantly affect the
prognosis of laryngeal cancer when using the Cox regression model
(19). In the present study, it was
found that the mRNA and protein levels of Ki-67 in the laryngeal
squamous carcinoma tissues were significantly higher compared with
those in the adjacent non-tumor tissues. Based on that, further
clinical pathological analysis found that the high expression of
Ki-67 in cancer was significantly correlated with cervical lymph
node metastasis and clinical outcome. These results suggested that
Ki-67 may play a promotive role in the development and progression
of laryngeal squamous carcinoma, and that it could be used as a
predictor of poor prognosis for laryngeal squamous carcinoma
patients.
To provide evidence for this assumption, the effect
of Ki-67 silencing on the proliferation of human laryngocarcinoma
HEp2 cells in vitro was investigated. The expression of
Ki-67 in the cells was specifically knocked down using RNA
interference. The results demonstrated that the downregulation of
Ki-67 resulted in a significant suppression of HEp2 cell
proliferation, which strongly indicated that Ki-67 was involved in
the canceration processes of laryngeal carcinoma. These data are in
line with previous studies on the expression and functional roles
of Ki-67 (13–16).
EGFR is a member of the ErbB family of receptors and
activation of EGFR signaling may trigger a series of intracellular
signals that ultimately promote cell proliferation and cell growth
(20). Overexpression of EGFR has
been found in numerous cancer types, including hepatocellular
carcinoma, lung cancer, bladder cancer and laryngeal carcinoma
(21–24). Li et al demonstrated that EGFR
expression was significantly correlated with separate Ki-67
expression in gliomas (25). Yang
et al reported that in primary and relapse nasopharyngeal
cancer, a strong significant correlation between EGFR and Ki-67
molecules expression was obtained (26). In the present study, the expression of
EGFR was significantly downregulated in the Ki-67 siRNA-treated
cells compared with that in the control group. This indicated a
positive correlation between Ki-67 and EGFR, and suggested that
Ki-67 may regulate the proliferation of HEp2 cells via modulating
the EGFR signaling pathway.
E-cadherin participates in calcium-dependent somatic
cell adhesion and loss of E-cadherin is believed to enable
metastasis by disrupting intercellular contacts (27). Nakagawa et al found that in T1
esophageal squamous cell carcinoma, reduced E-cadherin expression
was observed in 58.1% of cases and that E-cadherin expression were
closely correlated with nodal metastasis. The study concluded that
after endoscopic treatment, additional therapy may be required if
reduced E-cadherin expression is observed in the primary tumor
specimen (28). Rodrigo et al
found that E-cadherin was an independent predictor of nodal
metastases in supraglottic squamous cell carcinomas (29). In another study, Simionescu et
al found that in low-grade squamous intraepithelial lesions,
E-cadherin had a membranous pattern and the Ki-67 proliferation
index was low, whereas in high-grade lesions, E-cadherin expression
became aberrant and the Ki-67 proliferation index was high
(30). Consistent with this finding,
the present study demonstrated that the expression of E-cadherin
was significantly upregulated in Ki-67 siRNA-treated cells compared
with that in the control group, which indicated a negative
correlation between Ki-67 and E-cadherin, and suggested that Ki-67
may promote HEp2 cell proliferation through the modulation of cell
adhesion and contact.
In summary, the present study data revealed that the
Ki-67 expression in the examined laryngeal squamous carcinoma
tissues was significantly higher than that in the adjacent
non-tumor tissues. High Ki-67 expression was significantly
correlated with cervical lymph node metastasis and may be a
valuable predictor for the prognosis of laryngeal squamous
carcinoma. The cellular proliferation of Ki-67 siRNA-transfected
HEp2 cells was significantly decreased, indicating that Ki-67 may
be involved in the canceration processes of laryngeal carcinoma.
The upregulation of EGFR and the downregulation of E-cadherin may
at least partly contribute to the mechanisms of action of Ki-67.
Further studies are necessary to elucidate the detailed molecular
mechanisms underlying the involvement of Ki-67 in the tumorigenesis
and progression of human laryngeal squamous carcinoma.
References
|
1
|
Markou K, Christoforidou A, Karasmanis I,
Tsiropoulos G, Triaridis S, Constantinidis I, Vital V and Nikolaou
A: Laryngeal cancer: Epidemiological data from Νorthern Greece and
review of the literature. Hippokratia. 17:313–318. 2013.PubMed/NCBI
|
|
2
|
Iovănescu GH, Poenaru M, Doroş C and
Borugă O: Histopathological prognostic and risk factors in patients
with laryngeal neoplasms. Rom J Morphol Embryol. 54:1087–1092.
2013.PubMed/NCBI
|
|
3
|
Traoré CB, Kamaté B, Kéita M, Tchoupa MM,
Timbo SK, Ag MA and Bayo S: Laryngo-pharyngeal cancer at a health
service of last resort in Mali: Anatomo-clinical and therapeutic
aspects. Mali Med. 23:51–54. 2008.(In French).
|
|
4
|
Arshad H, Ahmad Z and Hasan SH: Gliomas:
Correlation of histologic grade, Ki67 and p53 expression with
patient survival. Asian Pac J Cancer Prev. 11:1637–1640.
2010.PubMed/NCBI
|
|
5
|
Klintman M, Bendahl PO, Graban D, Lövgren
K, Malmström P and Fernö M: South Sweden Breast Cancer Group: The
prognostic value of Ki67 is dependent on estrogen receptor status
and histological grade in premenopausal patients with node-negative
breast cancer. Mod Pathol. 23:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tadbir AA, Pardis S, Ashkavandi ZJ,
Najvani AD, Ashraf MJ, Taheri A, Zadeh MA and Sardari Y: Expression
of Ki67 and CD105 as proliferation and angiogenesis markers in
salivary gland tumors. Asian Pac J Cancer Prev. 13:5155–5159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naderi N Jalayer, Tirgari F, Kharazi-Fard
MJ and Parsa F Farahani: A study on the relationship between
clinical features with Ki67 expression and eosinophil cells
infiltration in oral squamous cell carcinoma. Med J Islam Repub
Iran. 28:1152014.PubMed/NCBI
|
|
8
|
Thompson L: World Health Organization
classification of tumours: pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
9
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Jiang HG, Lu N, Lu BH and Chen ZH:
Expression of ki67 in papillary thyroid microcarcinoma and its
clinical significance. Asian Pac J Cancer Prev. 16:1605–1608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015.PubMed/NCBI
|
|
13
|
Liu HB, Gao XX, Zhang Q, Liu J, Cui Y, Zhu
Y and Liu YF: Expression and prognostic implications of FOXO3a and
Ki67 in lung adenocarcinomas. Asian Pac J Cancer Prev.
16:1443–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen LY, Tsang JY, Ni YB, Chan SK, Chan
KF, Zhang S and Tse GM: Bcl2 and Ki67 refine prognostication in
luminal breast cancers. Breast Cancer Res Treat. 149:631–643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beuschlein F, Weigel J, Saeger W, Kroiss
M, Wild V, Daffara F, Libé R, Ardito A, Al Ghuzlan A, Quinkler M,
et al: Major prognostic role of ki67 in localized adrenocortical
carcinoma after complete resection. J Clin Endocrinol Metab.
100:841–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Deng W, Ma J, Wei B, Guo K, Shen W,
Zhang Y and Luo S: Prognostic evaluation of Nanog, Oct4, Sox2,
PCNA, Ki67 and E-cadherin expression in gastric cancer. Med Oncol.
32:4332015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leopardi G, Serafini G, Simoncelli C,
Ludovini V, Pistola L and Altissimi G: Ki67 and p53 in laryngeal
epithelial lesions: Correlations with risk factors. Acta
Otorhinolaryngeal Ital. 21:243–247. 2001.(In Italian).
|
|
18
|
Liu M, Lawson G, Delos M, Jamart J,
Chatelain B, Remacle M and Marbaix E: Prognostic value of cell
proliferation markers, tumour suppressor proteins and cell adhesion
molecules in primary squamous cell carcinoma of the larynx and
hypopharynx. Eur Arch Otorhinolaryngol. 260:28–34. 2003.PubMed/NCBI
|
|
19
|
Teppo H, Soini Y, Melkko J, Koivunen P and
Alho OP: Prognostic factors in laryngeal carcinoma: The role of
apoptosis, p53, proliferation (Ki-67) and angiogenesis. APMIS.
111:451–457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buckley AF, Burgart LJ, Sahai V and Kakar
S: Epidermal growth factor receptor expression and gene copy number
in conventional hepatocellular carcinoma. Am J Clin Pathol.
129:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carcereny E, Morán T, Capdevila L, Cros S,
Vilà L, de Los Llanos Gil M, Remón J and Rosell R: The epidermal
growth factor receptor (EGRF) in lung cancer. Transl Respir Med.
3:12015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carlsson J, Wester K, De La Torre M,
Malmström PU and Gårdmark T: EGFR-expression in primary urinary
bladder cancer and corresponding metastases and the relation to
HER2-expression. On the possibility to target these receptors with
radionuclides. Radiol Oncol. 49:50–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Kang N, Fu W, Sun X, Gao H and Sun
K: Detection of epidermal growth factor receptor gene amplification
in human laryngeal carcinomas by means of fluorescence in situ
hybridization. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 17:278–280.
2000.(In Chinese). PubMed/NCBI
|
|
25
|
Li M, Wei M, Jiang Z, Wei H, Lu W, Dou W
and Lu S: BRAF overexpression induces rampant glioma proliferation
independent of phospho-EGFR expression. Adv Clin Exp Med.
23:893–899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Xuan J, Yang Z, Han A, Xing L, Yue
J, Hu M and Yu J: The expression of epidermal growth factor
receptor and Ki67 in primary and relapse nasopharyngeal cancer: A
micro-evidence for anti-EGFR targeted maintenance therapy. Med
Oncol. 29:1448–1455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa Y, Ohira M, Kubo N, Yamashita Y,
Sakurai K, Toyokawa T, Tanaka H, Muguruma K, Shibutani M, Yamazoe
S, et al: Tumor budding and E-cadherin expression are useful
predictors of nodal involvement in T1 esophageal squamous cell
carcinoma. Anticancer Res. 33:5023–5029. 2013.PubMed/NCBI
|
|
29
|
Rodrigo JP, Dominguez F, Alvarez C,
Manrique C, Herrero A and Suárez C: Expression of E-cadherin in
squamous cell carcinomas of supraglottic larynx with correlations
to clinicopathological features. Eur J Cancer. 38:1059–1064. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simionescu C, Mărgăritescu C, Stepan A,
Georgescu CV, Niculescu M and Muntean M: The utility of p16,
E-cadherin and Ki67 in cervical squamous intraepithelial lesions
diagnosis. Rom J Morphol Embryol. 51:621–626. 2010.PubMed/NCBI
|