Introduction
Gallbladder carcinoma (GBC) is rarely observed in
Europe and the United States; however, the incidence and mortality
rates in Asian countries, including China, Japan and Korea, are
much higher (5.2/100,000, 4/100,000 and 5.6/100,000 individuals,
respectively) (1). The majority of
patients with GBCs are diagnosed at an advanced stage, which is
due, in part, to the aggressive nature of the tumor and its rapid
progression. Once the cancer has reached an advanced stage,
curative surgical resection can no longer be performed (2), leaving any GBC patients with a poor
prognosis; the median overall survival time has been reported at
only 8.2 months and the 1-year survival rate for patients with
stage IV disease is estimated at 1% (3,4).
The current diagnostic methods for GBC include
medical imaging and bile cytological analysis. Combination of the
two modalities has been shown to facilitate improved rates of
diagnosis (5), but the accuracy and
utility of each of these strategies are limited by their reliance
on a physician's subjective evaluation and how the sampled tissue
is handled prior to testing. Thus, accurate and early diagnosis
remains a significant clinical challenge. Furthermore, GBC usually
presents with an occult onset, therefore malignancies are commonly
detected incidentally during cholecystectomy for benign diseases
(6). Neglecting to further pursue
these incidental findings may lead to an undiagnosed GBC, thus
allowing the cancer to become more advanced and
life-threatening.
The treatment options for late-stage GBC treatment
include enrolling in a clinical trial, gemcitabine- or
fluoropyrimidine-based chemotherapy, and/or supportive care, all of
which provide only palliative relief for GBC patients (7). The specific dose, length of treatment or
combination regimens of chemotherapy are not included in the
National Comprehensive Cancer Network (NCCN) guidelines (7). Furthermore, the use of chemoradiation,
targeted therapies, and immunotherapy remain controversial. The
optimal comprehensive treatment for GBC patients has yet to be
determined. The current study presents a case of GBC that should
have been detected at an early stage at a different institution 2
years beforehand. The advanced GBC was then managed by a
multidisciplinary collaboration, whereby the survival time of the
patient was extended to 26 months.
Case report
A 62-year-old male with a 1.5-year history of
cholelithiasis and cholecystectomy was admitted to the Cancer
Centre of Nanjing Drum Tower Hospital (Nanjing, Jiangsu, China) in
February 2011, due to a dull pain in the right upper quadrant of
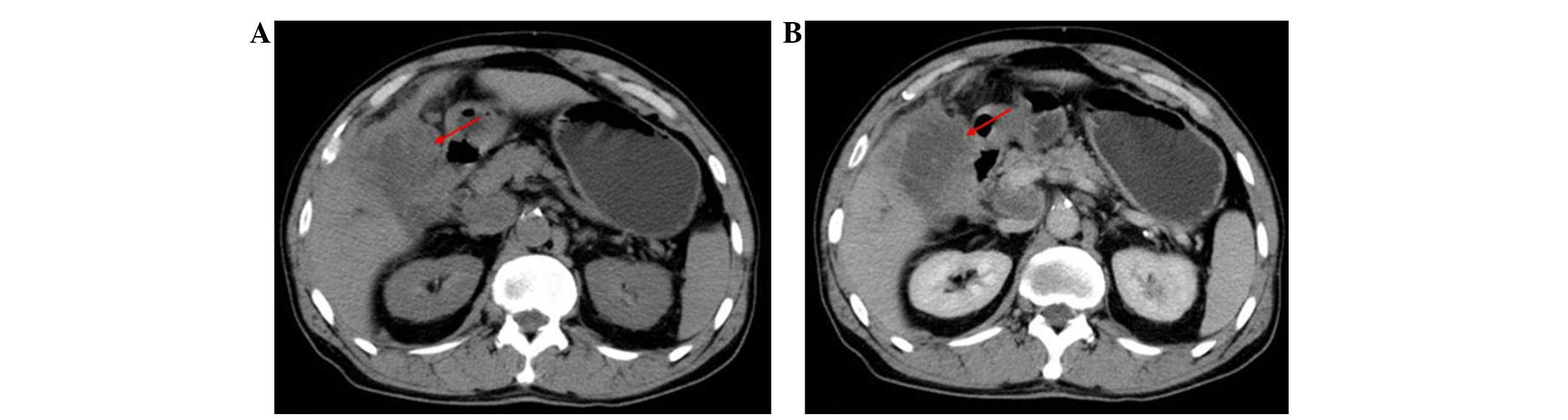
the abdomen for the previous 30 days. Computed tomography (CT) scan
results (Fig. 1A and B) demonstrated
a mass located on the gallbladder and cancer invasion of the
surrounding tissues. Multiple abdominal and retroperitoneal lymph
node metastases, implantation metastases, and dilation of the
intrahepatic and extrahepatic bile ducts were also detected.
Laboratory testing indicated normal liver function; however, the
blood levels of carcinoembryonic antigen (CEA; 40.9 ng/ml; normal
range, 0–10 mg/ml) and cancer antigen 19–9 (CA19-9; 378 U/ml;
normal range, 0–30 U/ml) were elevated. An exploratory laparotomy
was performed at 6 days after the CT scan, and a suspicious mass
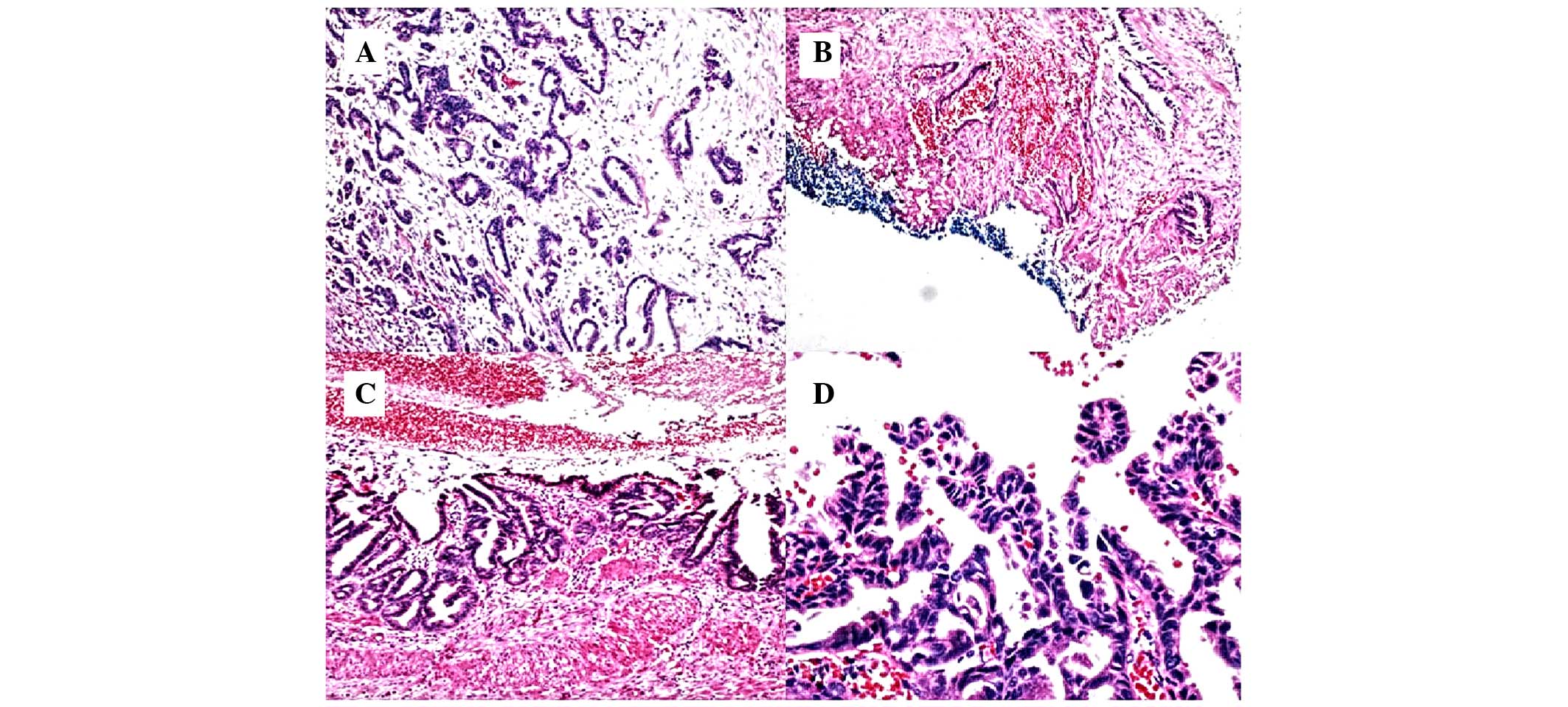
was resected from the gastrocolic ligament. Hematoxylin and eosin
(HE) staining of excised connective tissue from the gastrocolic
ligament showed cells that were poorly differentiated, with a loss
of polarity, an increased nucleoplasm ratio and nuclear fission and
an incomplete glandular cavity with mucus, all of which indicated
metastatic invasive adenocarcinoma of the gallbladder (Fig. 2A). The healthcare team decided that
the risk benefit profiles of any potential treatment options were
unacceptable in light of the late stage of the disease and the mild
clinical manifestation. Previously prepared HE-stained
paraffin-embedded sections (thickness, 5 µm) obtained from a
cholecystectomy performed at the Department of General Surgery
(Nanjing Drum Tower Hospital, Nanjing, Jiangsu, China) 2 years
previously were subjected to a histopathological review, and
chronic cholecystitis and extensive intraepithelial neoplasia (IN)
with an invasive growth pattern were observed (Fig. 2B-D).
At a follow-up examination performed 3 months after
the laparotomy, the disease was found to have progressed. The
patient presented with a variety of disease-related symptoms,
including icteric sclera, xanthochromia, yellowish discoloration of
the urine, light-colored excrement and generalized pruritus.
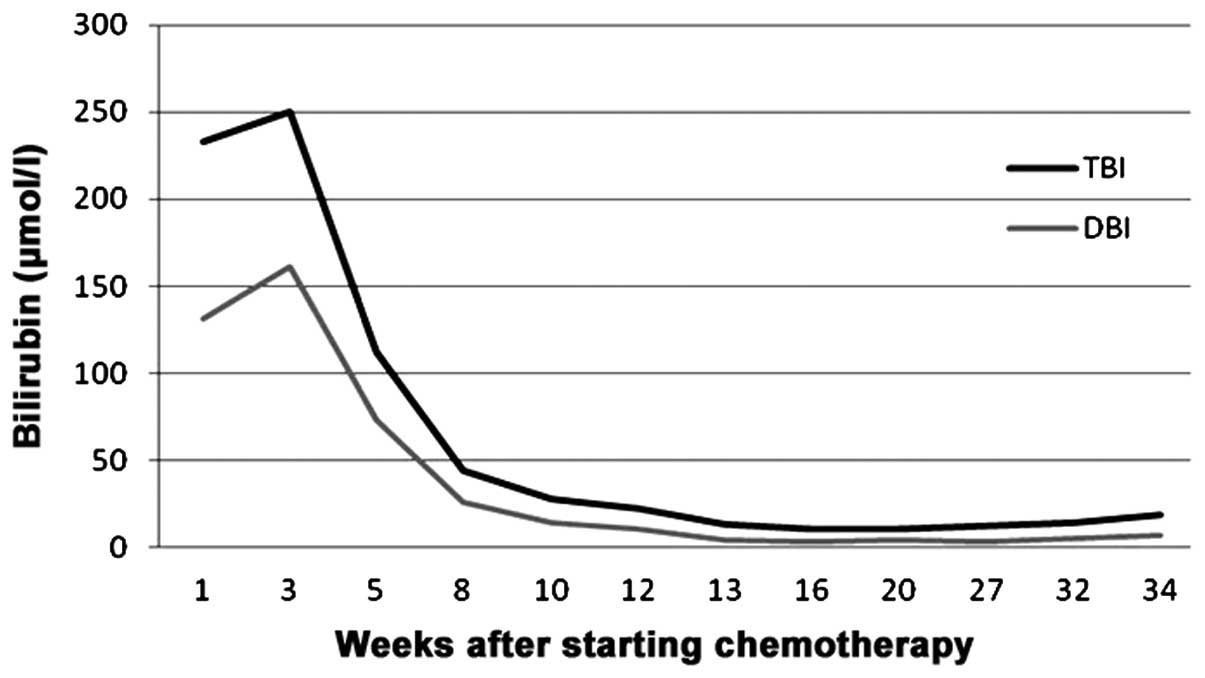
Laboratory tests revealed markedly elevated levels of total
bilirubin (TBI; 255.8 µmol/l; normal range, 5–20.5 µmol/l) and
direct bilirubin (DBI; 161.3 µmol/l; 1.7–6.8 µmol/l). Moreover, the
blood levels of tumor markers CEA (87.5 ng/ml) and CA19-9 (933.3
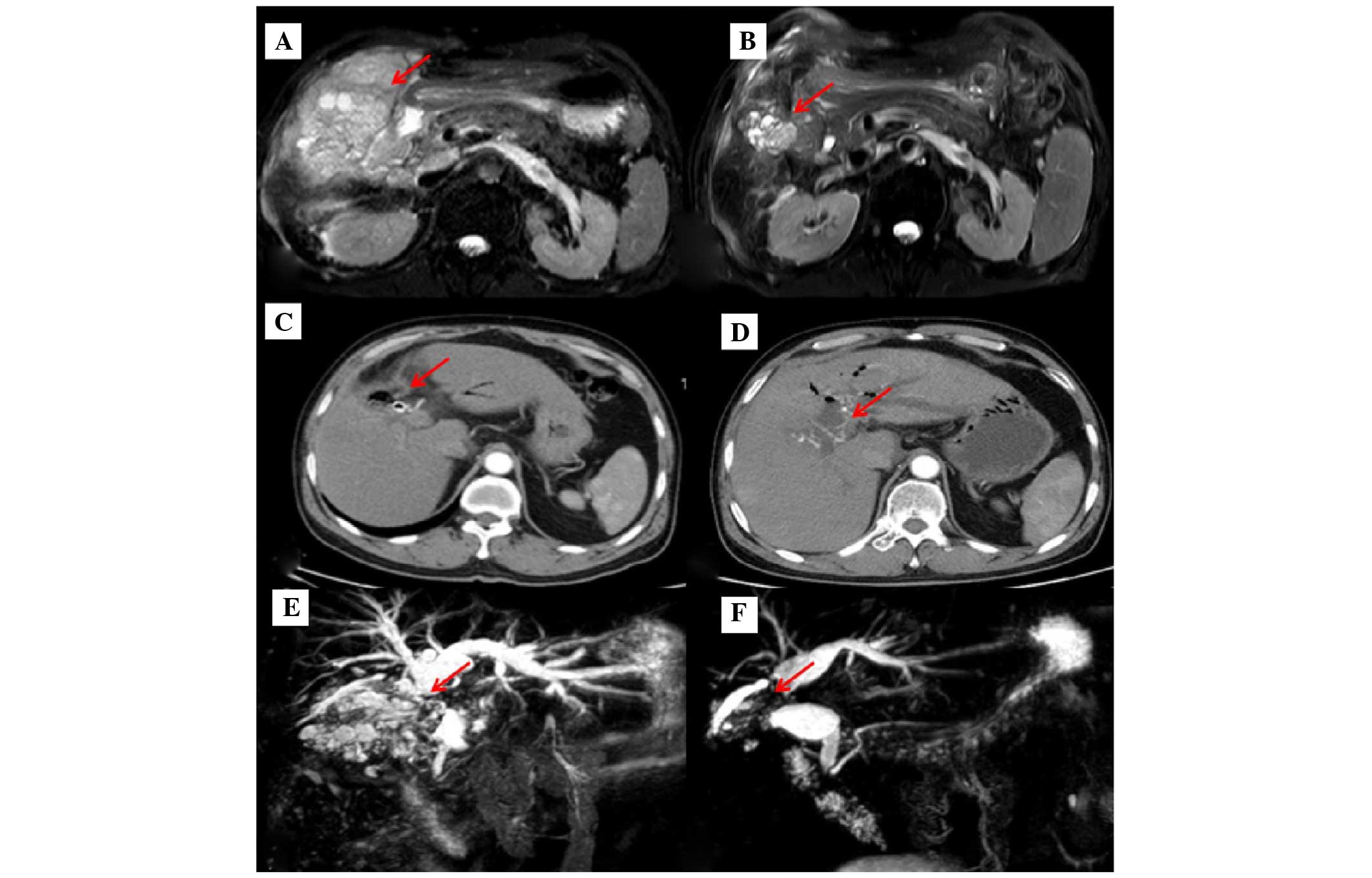
U/ml) were also increased. Examination by magnetic resonance
cholangiopancreatography indicated the recurrence of GBC with
invasion of the liver, its surrounding peritoneum and the hilar
area, as well as retroperitoneal lymph node metastasis (Fig. 3A and E). The intrahepatic bile duct
was also observed to be dilated. Gene mutation analysis was
performed on the tumor cells to investigate the KRAS
proto-oncogene, GTPase (KRAS) gene, which is frequently mutated in
cholangiocarcinoma, and indicated that the gene was the wild-type.
Tumor-node-metastasis staging of the tumor, according to the
American Joint Committee on Cancer (AJCC) grading system for
gallbladder cancer (7th edition, 2010) (8), provided a classification of T4N2M1.
The patient underwent multiple treatments of various
modalities, including percutaneous transhepatic cholangial drainage
(PTCD), chemotherapy, chemoradiation, targeted therapy and
immunotherapy. The strategy consisted of first delivering 12 cycles
of chemotherapy, each using a 2-week schedule of gemcitabine (1 g
via a peripherally inserted central catheter (PICC) on day 1),
oxaliplatin (50 mg via PICC, on days 1 and 2), nimotuzumab (200 mg
via PICC on day 1), recombinant human interleukin-2 (IL-2; 500,000
units via subcutaneous injection, twice a day on days 5–12) and
granulocyte-macrophage colony-stimulating factor (GM-CSF; 75 µg via
subcutaneous injection, twice a day on days 5–12). The patient's
levels of TBI, DBI and tumor markers were found to have
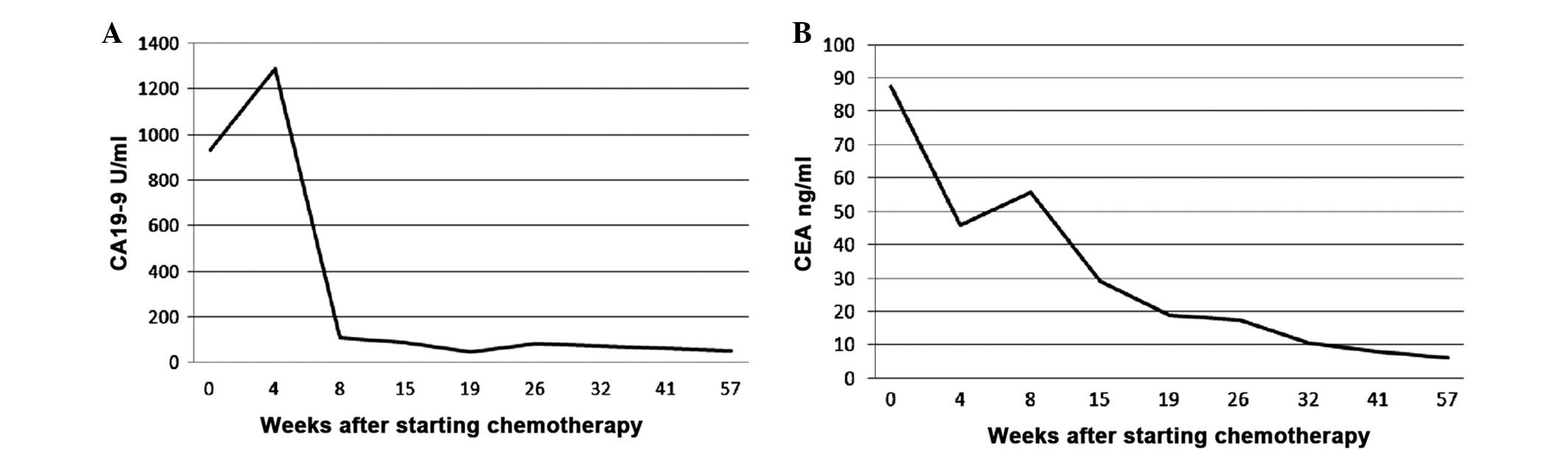
significantly decreased after 4 cycles of treatment (Figs. 4 and 5).
A partial response (PR) was achieved at treatment weeks 8, 64 and
84 (Fig. 3B-F). Next, a 5-week course
of radiation therapy was given to the hepatic portal vein, which
consisted of delivering a total dose of 50 Gy in 25 fractions. This
radiotherapy was administered concurrently with another
chemotherapy strategy consisting of 5-fluorouracil (1.25 g via
continuous infusion over 120 h every week) and nimotuzumab (200 mg
every 2 weeks). The patient attained a complete response (CR) after
completion of the chemoradiation regimen, when surgery was
performed to restore the biliary duct drainage by inserting a
biliary stent. A sustaining therapy regimen of compound tegafur
tablets (324 mg, twice a day, on days 1–14, every 21 days) was
established with the aim of assisting the patient in attaining
longer-term maintenance of the CR status. The patient tolerated all
the treatments well, with only mild liver dysfunction and grade 2
myelosuppression experienced.
At 3 months after being discharged, the patient was
readmitted to the hospital with recurrent icteric sclera, yellowish
discoloration of the urine and a fever. Laboratory tests showed
elevated levels of TBI (125.9 µmol/l), DBI (91.6 µmol/l) and CA19-9
(1,349.0 U/ml); however, CEA levels were observed to be within the
normal range. CT imaging revealed gas aggregation in the
intrahepatic bile duct and fluid accumulation around the liver.
Blood culture indicated E. coli infection, which may have
been associated with the biliary stent implantation, and the
patient was subsequently treated with PTCD and imipenem (0.5 g,
intravenous drop, every 8 hours). However, the patient did not
survive the infection and succumbed 26 months after the initial
diagnosis of GBC. The patient's family consented to the publication
of patient data in the present case report.
Discussion
GBC is commonly diagnosed at a late stage, not only
due to the aggressiveness of the disease, but also as the clinical
presentation of GBC mimics that of cholelithiasis or chronic
cholecystitis, both of which progress gradually over a long period
of time (9). Hence, there is a
possibility of missing the cancer diagnosis, an example of which is
presented in the current study. Extensive IN was found during a
pathological review that was conducted 2 years after the first
resection sample was taken from the patient. In a retrospective
review of 435 cases performed by researchers at the Memorial
Sloan-Kettering Cancer Center, the incidental diagnosis of GBC
during laparoscopic cholecystectomy occurred in 47% of the cases
examined (6). Considering the
prevalence of incidental GBC and the fact that cholelithiasis
accompanied by chronic inflammation is the most well-established
risk factor for GBC (10), careful
histopathological review should be performed following surgery for
cholelithiasis. Additionally, physicians should be aware of occult
GBC in patients with other high-risk factors, including
calcification of the gallbladder, anomalous pancreaticobiliary duct
junctions and gallbladder polyps.
The 2000 International Agency of Research on Cancer
(11) yielded a consensus on the
naming of gastrointestinal precancerous lesions; the precancerous
and early-stage cancers, which had been previously described as
‘high-grade dysplasia’, ‘carcinoma in situ’, ‘intramucosal
carcinoma’ and ‘local canceration’, were recommended to be renamed
using the more encompassing descriptor, ‘intraepithelial
neoplasia’. However, the understanding of IN is not yet uniform
among scholars in Japan and Western countries. The former argue
that IN belongs to the entity of carcinoma (according to its
cytohistological features), while the latter argue that cancer
should be diagnosed only when metastasis is confirmed. Considering
the case presented here, we support the former, and are concerned
with the possibility of missing the diagnosis of GBC.
Tumor staging is the most critical prognostic factor
for GBC patients (6). In a
retrospective study of 2,574 GBC cases from hospital cancer
registries across the United States, the 5-year survival of stage
0, I and II diseases was reported to be 60, 39 and 15%,
respectively (3). However, survival
rates were found to have markedly decreased in patients diagnosed
with stage III and IV cancers (to 5 and 1%, respectively). The AJCC
(7th edition, 2010) (8) suggest that
T4 tumors (a tumor invading the main portal vein or hepatic artery,
or invading two or more extrahepatic organs or structures) with
lymph node metastasis to the periaortic, pericaval, superior
mesenteric and/or celiac arteries (N2) should be classified as
stage IV disease; this classification implies that curative
surgical resection should not be performed. The patient in the
present study was classified as T4N2M1, and was therefore not a
candidate for complete resection. The overall survival time was
predicted to be ~8 months upon consideration of findings from a
previous meta-analysis (4).
Furthermore, the AJCC strongly recommends a second curative surgery
for incidental GBC (≥T1b), where the malignancy is found during or
after cholecystectomy for benign gallbladder diseases. The extent
of the surgery should be determined according to the stage of the
cancer. The present study patient had lost the opportunity to have
this second procedure performed after cholecystectomy due to the
original missed diagnosis that had occurred years previously at a
different institution. The 5-year survival for incidental GBC has
been reported to be as low as 20% when a second surgery is not
performed. Conversely, the 5-year survival rate has been shown to
increase to 80% when patients undergo this second procedure
(12). We believe that the patient
presented in this case report is an example of diagnostic
negligence. Better clinical outcomes would almost certainly have
been achieved with correct staging and appropriate medical
treatment administered after the first cholecystectomy.
The NCCN recommends biliary drainage, including a
percutaneous approach (PTCD) or using an endoscopic technique such
as endoscopic retrograde cholangiopancreatography, for patients
presenting with jaundice (7). These
procedures can result in pain relief and improved quality of life.
However, it is important to note that the treatment should be
performed according to the patient's condition. Treatment options
for patients diagnosed with advanced-stage GBC include: i)
Enrolling in a clinical trial; ii) gemcitabine- or
fluoropyrimidine-based chemotherapy; and iii) best supportive care
as per the NCCN guidelines (7). These
recommendations were followed in the present study, and
multidisciplinary collaboration was introduced into the management
plan for the patient.
The rapid progression of advanced GBC provides
justification for the use of adjuvant therapy, with the efficacy of
chemotherapy being well established (4,13–17). Single-agent and combination regimens
are applied in current clinical practice. Ueno et al
(15) reported an overall response
rate of 21.1% using the single chemotherapeutic agent oral
fluoropyrimidine derivative S-1. This phase II study also observed
a progression-free survival (PFS) time of 3.7 months and a median
overall survival time of 8.3 months. Furthermore, gemcitabine has
been found to be able to achieve a longer overall survival time
than any of the best supportive care strategies examined (9.1 vs.
2.9 months, respectively), yielding a disease control rate of 69.2%
at the 1-year post-chemotherapy follow-up (14). In a study that used a pooled analysis
of 104 trials, consisting of 2,810 patients with advanced biliary
tract carcinoma, the combination of a gemcitabine- and a
platinum-based regimen demonstrated the highest response and tumor
control rates as compared with other regimens, including
fluoropyrimidines plus platinum compounds, gemcitabine alone and
docetaxel/paclitaxel (4). The results
of a phase II trial assessing the efficacy of gemcitabine combined
with carboplatin in 48 cases of advanced biliary tract carcinomas
(represented by 35 cholangiocarcinoma, 12 gallbladder cancer and 1
ampullary cancer) illustrated that the median PFS time, overall
survival time and 6-month survival rate were 7.8 months, 10.6
months and 85.4%, respectively (17).
Finally, another phase II study demonstrated that gemcitabine and
oxaliplatin were associated with superior response rates (26–50%),
time-to-progression (6.5–10 months) and overall survival (11–14
months) (13,16). Consideration of the aforementioned
results and NCCN recommendations led to the choice of the
combination of gemcitabine and oxaliplatin for treatment of the
present study patient.
Chemoradiation aims to relieve symptoms and prolong
the survival time of patients with advanced GBC. Petera et
al (18) emphasized the
importance of intensity-modulated radiotherapy in a study of
patients with inoperable GBC or cholangiocarcinoma who were treated
with a dose of 50–60 Gy/25 fractions; this treatment achieved a
median overall survival time of 10.4 months. The NCCN guidelines
(7) recommend concurrent
chemoradiation as well, but advise that the therapeutic agents
applied should be limited to either fluorouracil or capecitabine.
In a retrospective study of 23 patients with non-metastatic bile
duct carcinoma who underwent three-dimensional conformal external
beam radiotherapy (total dose, 50.4 Gy) in combination with a
5-fluorouracil-based chemotherapy after curative resection (4
gallbladder, 7 ampullary and 12 cholangiocarcinomas), the 5-year
local-regional control rate and the overall survival rate were 48.3
and 35.9%, respectively (19). Those
patients who had achieved negative margins after the therapy were
shown to have attained better local control and overall survival
rates than their counterparts with positive/narrow/unknown margins
(67.0 vs. 35.9% and 61.4 vs. 16.7%, respectively). In the present
case, chemoradiation was applied following chemotherapy, which
significantly improved the treatment efficacy and the patient's
quality of life.
Even though various cytotoxic chemotherapeutic
combinations and chemoradiation are utilized to treat advanced GBC,
the PFS and overall survival times remain short. Currently, there
is an increasing interest in improving the knowledge of the
molecular pathways involved in carcinogenesis. Among these
pathways, the epidermal growth factor receptor (EGFR) axis is
believed to be one of the most important pathways involved in
biliary cancers (20). Cetuximab is a
promising EGFR inhibitor and has been demonstrated to be effective
in a phase II trial (21). In this
trial, Cetuximab, in combination with chemotherapy, was employed in
unresectable local-regional or metastatic biliary cancers. In total
63% of the patients achieved an objective response, and 30%
underwent a potentially curative secondary resection after a major
response to therapy. In another study investigating
cetuximab-containing therapy for the treatment of 5 advanced
biliary cancer patients, a CR was achieved in 1 patient, a PR in 3
patients and stable disease (SD) in 1 patient (22). Notably, a CR was observed in the
patient who was wild-type for the KRAS gene. Additionally, the
corresponding K-Ras protein was expressed at normal levels. Thus, a
KRAS gene analysis was performed for the patient reported in the
present to determine whether a mutation was present at this locus.
A genetic mutation in KRAS can lead to abnormal protein function,
which could affect how a patient responds to anti-EGFR therapy.
Since the KRAS gene was wild-type in the patient, nimotuzumab was
applied.
It has also been indicated that chemotherapy is able
to cause ‘inflammatory changes’ in the tumor microenvironment, such
as upregulation of chemokine expression, exposure of tumor
antigens, prevention of immunocyte eradication and inhibition of
vascular structural changes. These changes aid in enhancing the
antineoplastic affect at the tumor site (23). In a previous study combining
gemcitabine, an oxaliplatin, fluorouracil and folinic acid regimen,
and GM-CSF/IL-2, patients with colorectal carcinoma demonstrated a
high tolerance to the treatment and a favorable objective response
rate (68.9%) (24). The results of a
phase II multicenter trial of maintenance biotherapy using
IL-2/GM-CSF/interferon α-2b following induction chemotherapy
revealed superior CR, PR and SD rates (8, 36 and 29% respectively).
The PFS time was extended to 9 months, and overall survival time
was 13.5 months (25). In the present
case, GM-CSF/IL-2 treatment was commenced 48 h after chemotherapy,
as immunotherapy can activate tumor antigen-specific cytotoxic T
lymphocyte aggregation at the tumor site, which may enhance
antitumor efficacy.
In summary, at the time of admission, the patient
presented with local invasion of the liver, peritoneum and lymph
nodes. Cancer staging as cT4N2M1, anatomical stage IVB was clear,
and multidisciplinary treatment, including PTCD, chemotherapy,
chemoradiation, targeted therapy and immunotherapy, were applied.
The therapeutic approach was successful, and the patient tolerated
the treatment well. The overall survival of the patient was
extended to 26 months with a greatly improved quality of life. We
recommend that a multidisciplinary collaborative approach be
integral to the management of GBC, with individual situations taken
into consideration when interpreting consensus guidelines.
References
|
1
|
Randi G, Malvezzi M, Levi F, Ferlay J,
Negri E, Franceschi S and La Vecchia C: Epidemiology of biliary
tract cancers: An update. Ann Oncol. 20:146–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hezel AF and Zhu AX: Systemic therapy for
biliary tract cancers. Oncologist. 13:415–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donohue JH, Stewart AK and Menck HR: The
national cancer data base report on carcinoma of the gallbladder,
1989–1995. Cancer. 83:2618–2628. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eckel F and Schmid RM: Chemotherapy in
advanced biliary tract carcinoma: A pooled analysis of clinical
trials. Br J Cancer. 96:896–902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe Y, Goto H, Hirooka Y, Itoh A,
Taki T, Hayakawa S, Hayakawa T, Naitoh Y, Ohhashi K, Yamao K and
Furukawa T: Transpapillary biopsy in gallbladder disease.
Gastrointest Endosc. 51:76–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duffy A, Capanu M, Abou-Alfa GK, Huitzil
D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH and
O'Reilly EM: Gallbladder cancer (GBC): 10-year experience at
memorial Sloan-Kettering cancer centre (MSKCC). J Surg Oncol.
98:485–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary
Cancers (version 2, 2016). https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdfAccessed.
July 6–2016
|
|
8
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging HandbookFrom the AJCC
Cancer Staging Manual. 7th. Springer; New York: 2010
|
|
9
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, de Ruiz P Alonso, Urista G
Aristi and Nervi F: Epidemiology and molecular pathology of
gallbladder cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheth S, Bedford A and Chopra S: Primary
gallbladder cancer: Recognition of risk factors and the role of
prophylactic cholecystectomy. Am J Gastroenterol. 95:1402–1410.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosman FT, Carneiro F, Hruban RH and
Threise ND: WHO Classification of Tumours of the Digestive System.
4th. IARC Press; Lyon: 2010
|
|
12
|
Shimizu T, Arima Y, Yokomuro S, Yoshida H,
Mamada Y, Nomura T, Taniai N, Aimoto T, Nakamura Y, Mizuguchi Y, et
al: Incidental gallbladder cancer diagnosed during and after
laparoscopic cholecystectomy. J Nippon Med Sch. 73:136–140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebbia N, Verderame F, Di Leo R,
Santangelo D, Cicero G, Valerio MR, Arcara C, Badalamenti G,
Fulfaro F and Carreca I: A phase II study of oxaliplatin (O) and
gemcitabine (G) first line chemotherapy in patients with advanced
biliary tract cancers. J Clin Oncol. 23(suppl): abstract 4132.
2005.
|
|
14
|
Kuriyama H, Kawana K, Taniguchi R, Jono F,
Sakai E, Okubo H, Suzuki H, Kobayashi S, Murata Y, Inamori M, et
al: Single-agent gemcitabine in elderly patients with unresectable
biliary tract cancer. Hepatogastroenterology. 58:26–30.
2011.PubMed/NCBI
|
|
15
|
Ueno H, Okusaka T, Ikeda M, Takezako Y and
Morizane C: Phase II study of S-1 in patients with advanced biliary
tract cancer. Br J Cancer. 91:1769–1774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harder J, Riecken B, Kummer O, Lohrmann C,
Otto F, Usadel H, Geissler M, Opitz O and Henss H: Outpatient
chemotherapy with gemcitabine and oxaliplatin in patients with
biliary tract cancer. Br J Cancer. 95:848–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams KJ, Picus J, Trinkhaus K,
Fournier CC, Suresh R, James JS and Tan BR: Gemcitabine with
carboplatin for advanced biliary tract cancers: A phase II single
institution study. HPB (Oxford). 12:418–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petera J, Kasaová L, Paluska P, Sirák I,
Jansa J, Macingová Z, Dvorák J and Soumarova R: Intensity-modulated
radiotherapy in the treatment of subhepatic carcinomas.
Hepatogastroenterology. 58:331–335. 2011.PubMed/NCBI
|
|
19
|
Beltrán M Bonet, Roth AD, Mentha G and
Allal AS: Adjuvant radio-chemotherapy for extrahepatic biliary
tract cancers. BMC Cancer. 11:2672011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshikawa D, Ojima H, Iwasaki M, Hiraoka
N, Kosuge T, Kasai S, Hirohashi S and Shibata T:
Clinicopathological and prognostic significance of EGFR, VEGF, and
HER2 expression in cholangiocarcinoma. Br J Cancer. 98:418–425.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gruenberger B, Schueller J, Heubrandtner
U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S and
Gruenberger T: Cetuximab, gemcitabine, and oxaliplatin in patients
with unresectable advanced or metastatic biliary tract cancer: A
phase 2 study. Lancet Oncol. 11:1142–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang PY, Cheng MF, Lee HS, Hsieh CB and
Yao NS: Preliminary experience of cetuximab in the treatment of
advanced-stage biliary tract cancer. Onkologie. 33:45–47. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zitvogel L, Apetoh L, Ghiringhelli F,
André F, Tesniere A and Kroemer G: The anticancer immune response:
Indispensable for therapeutic success? J Clin Invest.
118:1991–2001. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Correale P, Cusi MG, Tsang KY, Del Vecchio
MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini
C, et al: Chemo-immunotherapy of metastatic colorectal carcinoma
with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte
macrophage colony-stimulating factor and interleukin-2 induces
strong immunologic and antitumor activity in metastatic colon
cancer patients. J Clin Oncol. 23:8950–8958. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Day SJ, Atkins MB, Boasberg P, Wang HJ,
Thompson JA, Anderson CM, Gonzalez R, Lutzky J, Amatruda T, Hersh
EM and Weber JS: Phase II multicenter trial of maintenance
biotherapy after induction concurrent biochemotherapy for patients
with metastatic melanoma. J Clin Oncol. 27:6207–6212. 2009.
View Article : Google Scholar : PubMed/NCBI
|