Introduction
Pleural effusion is a relatively common complication
that can result from many medical conditions such as congestive
heart failure, pneumonia, cancer, liver cirrhosis and kidney
disease (1–5). In clinic, the etiology is determined by
specimens obtained from a series of thoracentesis (6,7), and
therapy uses appropriate treatment after diagnosis and puncture
drainage therapy (8,9). Over the years, the use of
ultrasound-guided catheterization in the diagnosis and treatment of
pleural effusion has increased, tending to replace the traditional
puncture method (10–12).
From January 2013 to May 2014, 435 cases with
different causes of pleural effusion were collected to treat with
pleural puncture drawing fluid and ultrasound-guided thoracentesis
catheter drainage and analyzed.
Patients and methods
Patients
In total, 435 patients with pleural effusion from
January 2013 to May 2014 were included in the present study. The
control group (group A) included 37 cases of pleural effusion that
were treated using standard care pleural puncture to draw fluid.
The intervention group (group B) included 398 cases of
ultrasound-guided thoracentesis catheter drainage; 230 males and
205 females, with an average age of 60 years (range, 16–93 years).
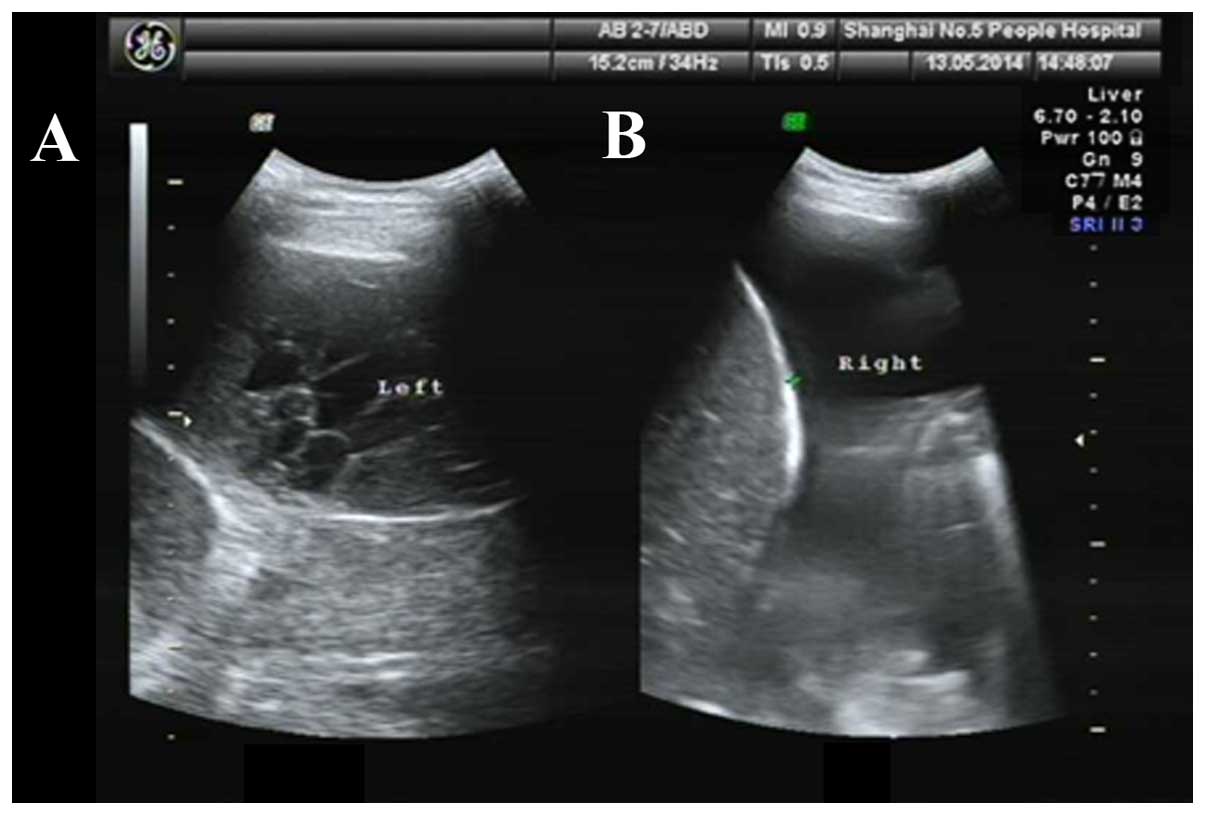
In the intervention group B, there were 140 separated pleural
effusion cases (Fig. 1A) and 258
non-separated pleural effusion cases (Fig. 1B).
After approval of the Ethics Committee of Huashan
Hospital of Fudan University and informed consent of patients or
relatives were obtained, the cases were randomly divided into the
control and intervention groups.
Methods
Instruments
V730 GE ultrasonic diagnostic apparatus,
thoracentesis package, disposable central venous catheter package
(containing 16 G drainage tube; Shanghai Puyi Medical Instruments
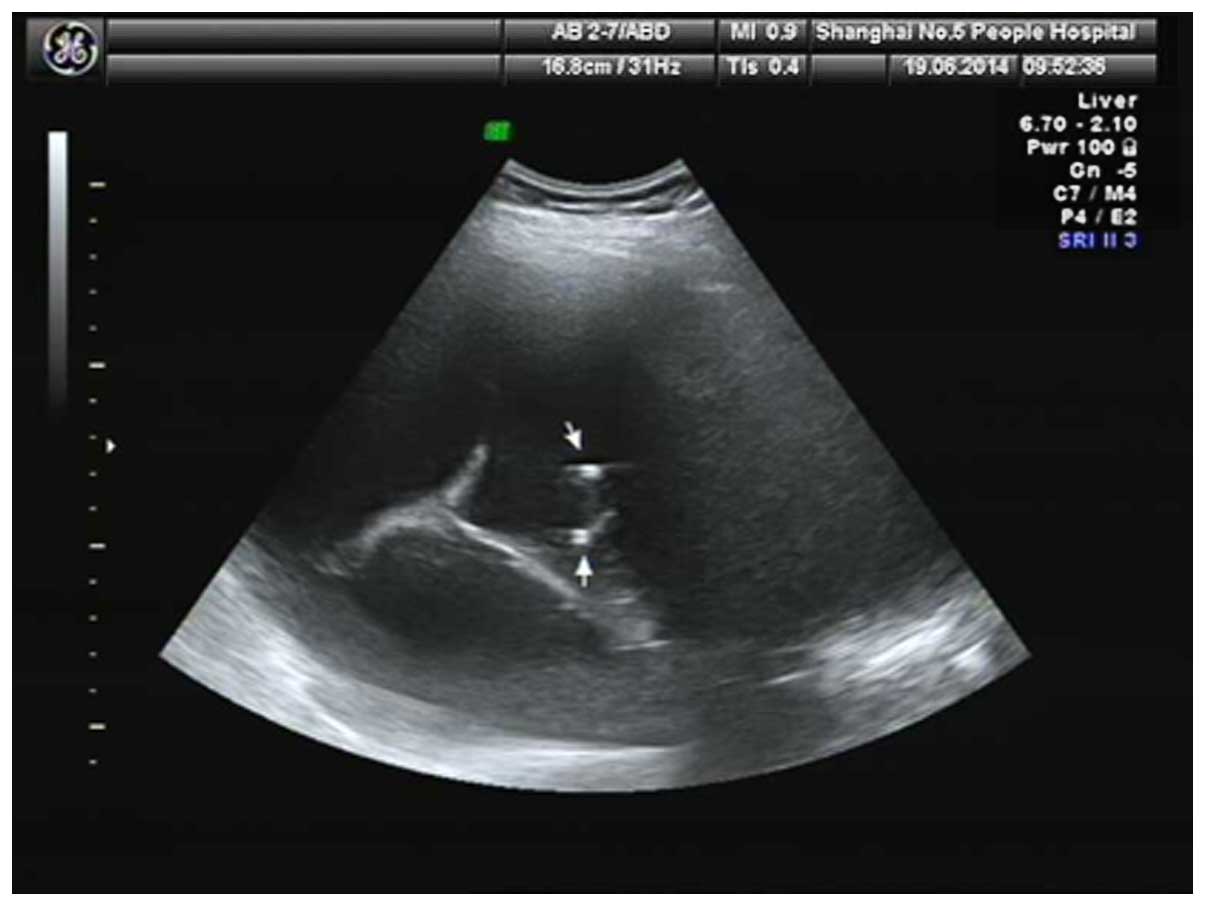
Co., Ltd., Shanghai, China), and SKATER drainage tube (Fig. 2) (including 6 and 8 F; Anjie Tai
Medical Technology Co., Ltd.).
Pleural puncture drawing fluid
For ultrasound preoperative localization or
percussion localization, the site was disinfected prior to
conventional puncture site and draped locally. After lidocaine was
used as the local anesthetic, thoracic puncture needle was slowly
inserted into the skin from the puncture site along the rib edge.
When there was a break, the syringe pump was used to pump pleural
effusion (the first time <600 ml and ≤1,000 ml each time
thereafter).
Pleural puncture catheter drainage
Ultrasonic localization and conventional
disinfection drape was used. After lidocaine was used as the local
anesthetic, thoracic puncture needle was inserted into pleural
effusion under ultrasound guidance. A guide wire was used with the
puncture needle to insert the drainage tube (central venous
catheter or SKATER drainage tube) (Fig.
2), and the guide wire was then removed. The drainage bag was
connected and the tube fixed on the body surface. Pleural effusion
was drawn out periodically.
Pleural effusion control criteria
Complete remission (CR): Pleural effusion
disappearing maintained at least 4 weeks. Partial remission (PR):
Pleural effusion reduction was more than 50% and maintained 4
weeks. Stable remission (SR): Pleural effusion reduction was
<50% without a decrease or increased trend. Progressive disease
(PD): No reduction or a decrease or increased trend of pleural
effusion. Overall effectiveness was calculated as, CR + PR.
Statistical analysis
SPSS 19.0 statistical software (Chicago, IL, USA)
was used for statistical analysis. Measurement data were analyzed
using a Student's t-test. Counting data are expressed as a
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
As shown in Table I,
37 patients in group A were treated with pleural puncture and fluid
was drawn a total of 57 times and the disposable puncture success
rate was 84% (48/57). CR was seen in 4 cases, and PR in 15 cases
(overall effectiveness: CR + PR = 19 cases total). Furthermore,
there were 11 stable patients, and progression was observed in 7
patients. Complications were observed in 4 cases (2 cases of
pneumothorax and 2 cases of pleural reaction).
 | Table I.Effectiveness and complication rates
for standard care (group A) vs. ultrasound-guided thoracentesis
(group B). |
Table I.
Effectiveness and complication rates
for standard care (group A) vs. ultrasound-guided thoracentesis
(group B).
|
|
|
| Complications |
|---|
|
|
|
|
|
|---|
| Groups | N | Overall
effectivenessa | Pneumothorax | Pleural reaction |
|---|
| A | 37 | 19 | 2 | 2 |
| B | 398 | 309 | 4 | 1 |
| P-value |
| 0.001 | 0.038 | 0.020 |
In group B, 398 patients were treated with
ultrasound-guided thoracentesis catheter drainage (419 total
procedures) with a 100% success rate. CR was observed in 135 cases,
PR in 174 cases, stable in 56 cases and progression in 33 cases
(Table I). There were 5 complications
reported (4 cases of pneumothorax and 1 case of pleural reaction);
the drainage tube fell off in 8 cases and was obstructed in 11
cases.
In 258 cases of non-separated pleural effusion,
central venous catheter was identified in 103 cases, 117 used
6F-SKATER drainage tube and 38 the 8F-SKATER (Table II). In 140 cases of separated pleural
effusion, 18 were with central venous catheter, 50 using 6F-SKATER
drainage tube, and 77 with 8F-SKATER.
 | Table II.Influence of drainage tube on pleural
effusion treatment success. |
Table II.
Influence of drainage tube on pleural
effusion treatment success.
|
| 16 G central venous
catheter | 6 F-SKATER drainage
tube | 8 F-SKATER drainage
tube |
|
|---|
|
|
|
|
|
|
|---|
| Case | N | ORb | N | OR | N | OR | P-value |
|---|
| Non-separation | 103 | 89 | 117 | 100 | 38 | 34 | 0.822 |
| Separation | 13 | 5 | 50 | 26 | 77 | 55 | 0.018
(0.037a) |
| P-value | 0.000 | 0.000 | 0.034 |
|
|---|
Discussion
Numerous factors cause excess body fluid to collect
in the pleural cavity to produce pleural effusion (13–17): An
increase in the venous pressure of pleural capillaries, an increase
of pleural permeability, and a decrease of colloid osmotic pressure
of the pleural capillaries, lymphatic drainage barrier and
damage.
Pleural effusion can be divided into transudate and
exudate (18–21). The former mainly treats primary
disease without repeated drainage, but draining of the pleural
cavity is needed when the etiology is unknown, and drainage
treatment should be done regardless of tuberculosis, tumor or
purulence of exudate.
When excessive pleural effusion leads to dyspnea,
both transudate and exudate need to be drained to improve breathing
(22). Traditional puncture drainage
operation requires is repeated, bringing suffering to patients many
times, and increases the reaction of hemothorax, pneumothorax and
pleura and the risk of infection (23). Traditional puncture drainage operation
require repeated conduction and increases the chance of hemothorax,
pneumothorax and risk of infection.
A pleural puncture tube can be drained repeatedly in
one operation, reducing the risk of complications. In particular,
the application of central venous catheters in pleural effusion has
proven to be an effective, safe and economical method (24,25). The
first and crucial step of thoracentesis is choosing a puncture
point.
There are three clinical methods for choosing a
puncture point to treat pleural effusion: Blind puncture,
ultrasonic locating of puncture and ultrasound-guided puncture
(26,27). Blind puncture is used by clinicians to
locate the puncture according to imaging data, experience and
percussion; ultrasound puncture locates the puncture point via
ultrasound, then clinicians continue to puncture the locating point
back on the ward, and ultrasound-guided puncture is performed under
real-time monitoring.
Ultrasound location often has associated problems,
such as failure to puncture the exact ultrasound localization, or
the pleural effusion drainage is not satisfactory (28). To ensure the safety of the puncture,
clinicians mainly choose the effusion surface of largest area as a
puncture point. However, after drainage, with the reduction of
pleural effusion, lung recruitment or diaphragm elevation, the
drainage tube area has no effusion, and the closeness to the pleura
causes poor drainage and drainage is incomplete. The
ultrasound-guided catheterization cannot only avoid the above
factors, but can also guide and correct the placement of the
drainage tube. Compared to the traditional thoracentesis, it has a
lower incidence of pneumothorax and pleural reaction.
Pleural effusion caused by congestive heart failure
and hypoalbuminemia is mostly transudate, which is low in protein
content, with a few cells and good fluidity (29,30). While
the exudate of tuberculous pleurisy and the malignant pleural
effusion produced by malignancy invasion in pleura are high in
protein content, with many cells, tending to form fibrous bands,
separation and even honeycomb. The existence of separation reduces
the fluidity of pleural effusion. Fibrous bands block the drainage,
and interfere or limit the diffusion of drug injected in the
thoracic cavity. Early drainage of separated pleural effusion is
important, especially for tuberculous pleural effusion, to reduce
adhesions between pleural and lung tissue or decrease pleural
thickening (31,32). To achieve early recovery of lung
function, the key is a clean drainage of pleural effusion on the
basis of reasonable and standard anti-tuberculosis therapy.
Currently, thoracentesis catheter drainage is mainly
via central venous catheter (24,25). It
has the traits of flexibility and good tissue compatibility but is
easily blocked. Some researchers believe that small-diameter
drainage tubes (diameter, ≤14 F) have no effect on drainage
(33), but the present study shows
this is not always true. For a non-separated pleural effusion
drainage tube, diameter has no effect on the drainage efficacy,
while for a separated pleural effusion drainage tube, diameter does
affect drainage efficacy. Two kinds of drainage tubes were used: a
central venous catheter and the SKATER drainage tube (34). The side aperture of central venous
catheter is less than its diameter, and the drainage effect depends
on the size of side holes. Central venous catheters of different
diameter have small side holes, which are easily blocked by fibrous
bands within separated pleural effusion, thus lowering efficacy.
The SKATER drainage tube has an elongated side hole, whose diameter
is greater than the drainage pipe, so its efficacy depends on the
diameter of the drainage tube. Therefore, for a small-diameter
drainage tube, in fibrous bands or separation within pleural
effusion, the efficacy is related to the diameter. For a separated
or potentially separated effusion like tuberculous pleural
effusion, a drainage tube with a big side hole like SKATER should
be used while a lower cost central venous catheter can be used for
non-separated pleural effusions.
In conclusion, ultrasound-guided thoracentesis
catheter drainage is an efficient, safe, minimally invasive
procedure with a high rate of success. Its efficacy is related to
the separation of pleural effusion, drainage tube type and tube
diameter. The choice of the drainage tube should be based on the
nature of pleural effusion, separation of pleural effusion and
cost.
References
|
1
|
Bauwens AM, de Graaff CS and Boersma WG:
Pleural effusion and empyema as complications of pneumonia. Ned
Tijdschr Geneeskd. 146:464–469. 2002.(In Dutch). PubMed/NCBI
|
|
2
|
Han ZJ, Wu XD, Cheng JJ, Zhao SD, Gao MZ,
Huang HY, Gu B, Ma P, Chen Y, Wang JH, et al: Diagnostic accuracy
of natriuretic peptides for heart failure in patients with pleural
effusion: a systematic review and updated meta-analysis. PLoS One.
10:e01343762015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madrid A, Ortega G, Boix M Molina, Salinas
F, Santesteban J, González-Conde A and Carmena R: Pleural effusion
in cancer patients. Review of 118 cases. Med Clin (Barc).
80:823–825. 1983.(In Spanish). PubMed/NCBI
|
|
4
|
Zarogoulidis P, Chatzaki E,
Hohenforst-Schmidt W, Goldberg EP, Galaktidou G, Kontakiotis T,
Karamanos N and Zarogoulidis K: Management of malignant pleural
effusion by suicide gene therapy in advanced stage lung cancer: a
case series and literature review. Cancer Gene Ther. 19:593–600.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assouad J, Barthes FP, Shaker W, Souilamas
R and Riquet M: Recurrent pleural effusion complicating liver
cirrhosis. Ann Thorac Surg. 75:986–989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pignotti MS, Messeri A and Donzelli G:
Thoracentesis in pericardial and pleural effusion caused by central
venous catheterization: a less invasive neonatal approach. Paediatr
Anaesth. 14:349–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhuniya S, Arunabha DC, Choudhury S, Saha
I, Roy TS and Saha M: Role of therapeutic thoracentesis in
tuberculous pleural effusion. Ann Thorac Med. 7:215–219. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lazarev SM, Reshetov AV, Kakysheva OE,
Nikolaev GV, Kirillov IuV and Volgin GN: The assessment of surgical
treatment of patients with malignant pleural effusion. Vestn Khir
Im I I Grek. 172:32–38. 2013.(In Russian). PubMed/NCBI
|
|
9
|
Miraglia R, Maruzzelli L, Piazza M, Gallo
G, D'Amico M, Spada M, Vitulo P and Luca A: Real-time
ultrasound-guided placement of a pigtail catheter in supine
position for draining pleural effusion in pediatric patients who
have undergone liver transplantation. J Clin Ultrasound.
44:284–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marchetti G, Valsecchi A, Indellicati D,
Arondi S, Trigiani M and Pinelli V: Ultrasound-guided medical
thoracoscopy in the absence of pleural effusion. Chest.
147:1008–1012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abusedera M and Alkady O:
Ultrasound-guided pleural effusion drainage with a small catheter
using the single-step trocar or modified seldinger technique. J
Bronchology Interv Pulmonol. 23:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu LN, Xu HX, Lu MD and Xie XY:
Percutaneous ultrasound-guided thermal ablation for liver tumor
with artificial pleural effusion or ascites. Chin J Cancer.
29:830–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolczyński A: Etiology of pleural effusion
based on material from the Hospital of Lung Diseases and
Tuberculosis. Pneumonol Alergol Pol. 69:239–246. 2001.(In Polish).
PubMed/NCBI
|
|
14
|
Ferreiro L, San José E, González-Barcala
FJ, Alvarez-Dobaño JM, Golpe A, Gude F, Anchorena C, Pereyra MF,
Zamarrón C and Valdés L: Eosinophilic pleural effusion: incidence,
etiology and prognostic significance. Arch Bronconeumol.
47:504–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qari FA: Etiology of pleural effusion in
Western Saudi Arabia. Saudi Med J. 23:351–352. 2002.PubMed/NCBI
|
|
16
|
Sahoo RC and Acharya PR: Pleural effusion
of a dual etiology. J Assoc Physicians India. 56:55–56.
2008.PubMed/NCBI
|
|
17
|
Neragi-Miandoab S: Surgical and other
invasive approaches to recurrent pleural effusion with malignant
etiology. Support Care Cancer. 16:1323–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vázquez F, Michelángelo H, Trevisani H,
González F and de Quiros B: Differential diagnosis between exudate
and transudate in pleural effusion. Medicina (B Aires). 56:223–230.
1996.(In Spanish). PubMed/NCBI
|
|
19
|
Gümüş A, Çınarka H, Karataş M, Kırbaş A,
Kayhan S and Şahin Ü: Elevated pleural copeptin levels can
distinguish to exudate from transudates. Tuberk Toraks. 62:267–272.
2014.(In Turkish). View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia Pachon E and Padilla Navas I:
Pleural effusion: criteria for distinguishing between transudates
and exudates. An Med Interna. 13:91–94. 1996.PubMed/NCBI
|
|
21
|
Uchiyama T: Pleural effusion (transudates
and exudates). Ryoikibetsu Shokogun Shirizu. 774–777. 1994.(In
Japanese). PubMed/NCBI
|
|
22
|
Oi K, Haraguchi N, Machida S, Beppu T,
Ogawa A, Yeh YF and Sasaki T: Dyspnea resulting from accumulation
of pleural effusion after radical neck dissection. A case report.
Oral Surg Oral Med Oral Pathol. 67:258–261. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krumhaar D: Dangers and errors in pleural
puncture and placing suction drainage. Langenbecks Arch Chir Suppl
II Verh Dtsch Ges Chir. 191–194. 1989.(In German). PubMed/NCBI
|
|
24
|
Madhavi P, Jameson R and Robinson MJ:
Unilateral pleural effusion complicating central venous
catheterisation. Arch Dis Child Fetal Neonatal Ed. 82:F248–F249.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu S and Zhang M: Central venous catheter
for coal workers pneumoconiosis complicated with pleural effusion
and pneumothorax efficacy analysis. Zhonghua Lao Dong Wei Sheng Zhi
Ye Bing Za Zhi. 33:51–53. 2015.(In Chinese). PubMed/NCBI
|
|
26
|
Loeza-Irigoyen JA, Muñoz-Guzmán Y,
Pérez-Guzmán C and Gutiérrez-Mendoza I: Thoracocentesis in patients
with pleural effusion and chronic alcoholic liver disease. Rev Med
Inst Mex Seguro Soc. 46:453–458. 2008.(In Spanish). PubMed/NCBI
|
|
27
|
Kuo YL and Chan TF: Treatment of
unilateral fetal pleural effusion by intrauterine thoracocentesis.
Taiwan J Obstet Gynecol. 51:303–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cavanna L, Mordenti P, Bertè R, Palladino
MA, Biasini C, Anselmi E, Seghini P, Vecchia S, Civardi G and Di
Nunzio C: Ultrasound guidance reduces pneumothorax rate and
improves safety of thoracentesis in malignant pleural effusion:
report on 445 consecutive patients with advanced cancer. World J
Surg Oncol. 12:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woodring JH: Distribution of pleural
effusion in congestive heart failure: what is atypical? South Med
J. 98:518–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pais D, Kuzmenko E, Amir J and Harel L:
Association of hypoalbuminemia with the presence and size of
pleural effusion in children with pneumonia. Pediatrics.
121:e533–e538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferreiro L, San José ME and Valdés L:
Management of parapneumonic pleural effusion in adults. Arch
Bronconeumol. 51:637–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Didilescu C, Ibraim E and Iordan CM:
Management of parapneumonic pleural effusion. Pneumologia.
50:196–198. 2001.(In Romanian). PubMed/NCBI
|
|
33
|
Antunes G, Neville E, Duffy J and Ali N:
Pleural Diseases Group, Standards of Care Committee, British
Thoracic Society: BTS guidelines for the management of malignant
pleural effusions. Thorax. 58:(Suppl 2). ii29–ii38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Medford AR and Maskell N: Pleural
effusion. Postgrad Med J. 81:702–710. 2005. View Article : Google Scholar : PubMed/NCBI
|