Introduction
Giant cell tumor of bone (GCTB) is a relatively
rare, locally aggressive benign osteolytic tumor that most commonly
affects young adults. GCTB accounts for 4–10% of all
biopsy-analyzed primary bone tumors (1–3).
The majority of lesions (85%) develop at the
epiphyses of long bones, but may also occur in the sacrum,
vertebral body and occasionally in the small bones of the hands and
feet (4). In a small number of cases
(1–4%), pulmonary metastasis has been reported (5,6).
Spontaneous transformation to an overt malignancy occurs in <10%
of cases (2,3).
The most common symptoms of GCTB include pain,
swelling, impaired mobility of the joints, pathological fractures
of involved bones and deformation of the bone (2–4). On
radiography, GCTB most commonly presents as a nonsclerotic,
osteolytic lesion with clearly defined margins. In addition,
pathological fractures located in the metaphysis of long bones that
extend to the epiphysis in the subarticular region are common
(3,7).
Core needle biopsy, open biopsy or intra-operative
frozen section analysis are performed to establish the final
diagnosis prior to or during surgery, due to the aggressive nature
of the tumor and its tendency for malignant transformation
(8–10). Microscopically, GCTB is composed of
neoplastic and reactive cell populations. The cell population is
composed of osteoclast-like multinucleated giant cells, rounded
mononuclear histiocytic cells and round/ovoid mononuclear stromal
cells, which represent the proliferative neoplastic component
(8,11–13). The
stromal cells grow in a syncytium, exhibit ill-defined cell borders
with little eosinophilic cytoplasm and variable degrees of mitotic
activity. Foci of necrosis and vascular invasion may also be
present. Tumors may demonstrate benign fibrous histiocytoma-like
areas, hemosiderin deposits, secondary aneurysmal bone cyst changes
(10–15% of tumors) and reactive bone formation (6,9,11,14).
Regarding the functional molecular biology of GCTB,
receptor activator of nuclear factor-κB ligand (RANKL) is highly
expressed by neoplastic mononuclear mesenchymal stromal cells
(15–17), whereas RANK is expressed on
osteoclast-like cells, which are recruited secondarily in the
tumor, but are responsive to the aggressive osteolytic activity
(2). RANK-RANKL interactions, which
are involved in normal bone formation and function, and macrophage
colony-stimulating factor exhibit important functions in
osteoclastogenesis by stimulating the recruitment of osteoclastic
cells from blood-born mononuclear osteoclast precursor cells that
differentiate into multinucleated osteoclast-like giant cells
(12,18–20). This
is supported by the observation that giant cells in GCTB exhibit an
osteoclast-like phenotype. Thus, these consistent findings confirm
the involvement of imbalanced RANKL and RANK expression and
dysregulation of the RANKL-RANK-osteoprotegerin signaling pathway
in the pathogenesis of GCTB and induction of bone over-resorption
at the tumor site.
A recent study identified the driver H3F3A
gene mutation in 92% of GCTBs, which occurred exclusively in
stromal cells (21).
Primary malignancy in GCTB is observed at initial
diagnosis as an area of morphologically distinct malignant
mesenchymal tumor cells within an otherwise conventional GCTB. In
secondary malignant GCTB, sarcomas arise subsequent to previous
radiation or surgical treatment and the pre-existing GCTB is not
always evident (8,11).
One study hypothesized that the histological
features of GCTB indicate subsequent behavior and thus may predict
prognosis while providing valuable guidance in treatment (22). GCTB is classified into 3 types: Grade
I, tumors exhibit sparse stroma and giant cells predominate; Grade
II (atypical/borderline GCTB, identified using H3F3A
mutation testing), tumors composed of a smaller giant cell
population with atypical cells or single atypical mitoses in the
more pronounced stroma; Grade III, tumors represent overt malignant
sarcoma (occasionally low-grade) (22). This grading system primarily shows
continuum between histologically benign and sarcomatous tumors,
underscoring the presence of borderline lesions, which have
worrisome features at imaging examinations, but provided they have
a positive H3F3A mutation status, still respond well to denosumab
treatment. The majority of GCTB cases are classified as grade I,
however, ≤20% of cases, even in the absence of histological
malignant traits, invade the cortex and directly extend into
adjacent soft tissues. This results in major discrepancies between
histological tumor grade and radiological stage (23). Radiological staging is considered more
important than histological grading for predicting the clinical
behavior of GCTB, including recurrence and metastatic potential
(2,5,7).
It is also difficult to differentiate GCTB from
other mimicking benign bone lesions, such as aneurysmal bone cyst,
giant cell reparative granuloma, brown tumor of
hyperparathyroidism, benign fibrous histiocytoma or
chondroblastoma, as well as malignant lesions, such as giant cell
rich or teleangiectatic osteosarcoma and undifferentiated
pleomorphic sarcoma (24).
The primary treatment for GCTB is surgery, however
local recurrence or metastasis may occur. The type of surgical
treatment selected depends on the feasibility of curettage compared
with resection and the risk of local recurrence. The most common
surgical treatment is local curettage, which exhibits varying rates
of local recurrence depending on the use of local adjuvants such as
phenol, liquid nitrogen and polymethylmethacrylate cement,
described as improved (12–27% of local recurrence) compared with
local controls. If local adjuvants are not utilized, the mean
recurrence rate is higher (21–65%) (2,7).
Furthermore, the risk of local recurrence is markedly increased by
soft tissue extension (20–25% of all GCTBs) (7,25). More
aggressive forms of surgical treatment, such as en bloc wide
resection, may potentially decrease the risk of local recurrence
(3), however, this procedure may lead
to reconstruction problems and impaired functional anatomy.
Prosthesis may be used for local treatment, which results in a good
quality of life, however, the risk of local recurrence following
this procedure is unclear, and possible complications, particularly
in relatively young patients affected by GCTB, must be considered
when planning therapy (26,27). En bloc resection should be considered
in cases of multiple recurrent GCTB, impossible joint salvage,
extensive cortex destruction (insufficient cortex left to curette)
and extensive soft tissue involvement (2,7).
Moderate-dose radiotherapy (40–55 Gy) has previously
been demonstrated as an effective primary treatment in unresectable
GCTB and in cases of residual or recurrent disease whereby surgery
would result in unacceptable morbidity, however, with the
introduction of RANKL inhibitors this must be redefined and limited
to individualized cases. In addition, the risk of malignant
transformation after radiotherapy is 0–5% (3).
Bisphosphonates and interferon-α have also been used
in GCTB treatment. Bisphosphonates inhibit osteoclast activity and
promote their apoptosis, which prevents bone resorption. A previous
case study reported that biphosphonate treatment reduced the local
recurrence rate of GCTB from 30% in the control group to 4.2% in
the bisphosphonates-treated group (28).
Recently, the RANKL inhibitor, denosumab, has been
investigated as a treatment for advanced GCTB (1,29).
Denosumab is a human monoclonal antibody that binds to RANKL and
prevents RANKL activation, thereby inhibiting the maturation of
osteoclasts (29,30). The high efficiency of GCTB denosumab
treatment has been confirmed in two phase II studies. An open-label
phase II study reported an objective response to denosumab therapy
in 86% of patients, whereby an objective response was defined as
>90% elimination of giant cells on histopathological evaluation
or no radiographical progression of the lesion (31). A second, larger study revealed that
96% of surgically unsalvageable GCTB patients exhibited no disease
progression during treatment (median follow-up time, 13 months) and
acceptable drug toxicity (31–33).
Denosumab treatment should be continued until radical resection of
the tumor is possible or progression or unacceptable toxicity has
occurred.
The present study is the largest single center
analysis of denosumab treatment in GCTB patients used in routine
practice to date.
Materials and methods
Patients and procedures
A total of 35 patients with histologically confirmed
GCTB treated with denosumab in a referral center (Maria
Skłodowska-Curie Memorial Cancer Center and Institute of Oncology)
without participation in clinical trials between May 2013 and
September 2015 were included in the study. All pathological
diagnoses were reviewed by a reference pathologist in our center.
Seven cases could not be diagnosed pathologically and thus,
mutational status of the H3F3A gene was tested, which
revealed that all patients were positive for the H3F3A gene
mutation.
The lesions were located in the lower limbs in 17
(49%) patients (49%), in the upper limbs of 11 patients (31%) and
the axial skeleton in 7 patients (20%). All cases were evaluated by
a multidisciplinary team (MDT) prior to the start of therapy with
denosumab. A total of 24 (68%) patients exhibited primary tumors
after diagnostic biopsy, while 11 (31%) patients exhibited
recurrent tumors after undergoing previous surgical procedures. A
total of 10 (29%) cases, which were predominantly located in axial
locations, were defined as unresectable and 24 patients exhibited
locally advanced tumors with soft tissue involvement [grade III,
according to radiographic staging systems by Campanacci et
al (6)], and the majority of
these cases exhibited penetration of the joint, were not suitable
for limb-sparing surgery or exhibited an extremely high risk of
tumor recurrence.
Patient demographics and clinicopathological
characteristics are shown in Table I.
A slight female predominance (60%) was observed. The mean age of
the patient cohort was 32 years-old (range, 19–74 years-old).
 | Table I.Demographic and clinicopathological
characteristics of 35 GCTB denosumab-treated patients included in
the present study. |
Table I.
Demographic and clinicopathological
characteristics of 35 GCTB denosumab-treated patients included in
the present study.
| Parameter | Value |
|---|
| Gender, n (%) |
|
|
Female | 21 (60) |
| Male | 14 (40) |
| Median age, years
(range) | 32 (19–74) |
| GCTB disease type, n
(%) |
|
|
Unresectable primary
tumor | 9
(26) |
|
Resectable high-risk primary
tumor | 26 (74) |
| Prior GCTB
therapies, n (%) |
|
| Biopsy
only | 24 (68) |
| RT | 4
(11) |
|
Subtotal resection | 3 (9) |
| Radical
resection | 3 (9) |
| Surgery
+ adjuvant RT | 1 (3) |
| Tumor localization,
n (%) |
|
| Lower
limb | 17 (49) |
|
Tibia | 10 (29) |
|
Femur | 6
(17) |
|
Fibula | 1 (3) |
| Upper
limb | 11 (31) |
|
Humerus | 4
(11) |
|
Radius | 5
(14) |
|
Ulna | 1 (3) |
|
Metacarpal
bone | 1 (3) |
|
Axial | 7
(20) |
|
Sacrum | 5
(14) |
|
Ilium | 1 (3) |
|
Ischium | 1 (3) |
A total of 4 patients had received radiotherapy
prior to denosumab treatment. Furthermore, 3 patients had undergone
total resection and 3 patients had undergone subtotal resection
prior to denosumab treatment. One patient had been treated with
surgery and radiotherapy.
All patients received subcutaneous injections of
denosumab (120 mg) every 28 days, with additional injections on
days 8 and 15 of the first month, in addition to calcium (1,000
mg/day) and vitamin D (400 IU/day) supplements. Treatment was
continued until complete tumor resection was feasible (as assessed
by the MDT) or tumor progression or unacceptable toxicity had
occurred. Adverse events were recorded and graded according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events (version 4.0) (34). Tumor
status was assessed every 3 months by computed tomography or
magnetic resonance imaging. In addition, X-rays were performed
every 2–3 months.
Written informed consent was obtained from all
patients for the publication of this study, and study approval was
obtained from the Institutional Review Board of Maria
Sklodowska-Curie Memorial Cancer Center and Institute of Oncology
(Warsaw, Poland).
Results
Denosumab treatment
The mean denosumab treatment duration was 7.4 months
(median, 7 months) (Table II).
 | Table II.Duration of treatment with denosumab
in the present study. |
Table II.
Duration of treatment with denosumab
in the present study.
| Group | Patients, n | Mean treatment
time, months (range) |
|---|
| Total | 35 | 7.4 (2–16) |
| Surgery | 17 | 7.2 (5–12) |
| No surgery | 18 | 7.8 (2–16) |
The median treatment time in the patients who
underwent surgery after neoadjuvant therapy, but had not received
denosumab postoperatively, was 7.2 months (5–12 months). In
patients who continued monthly denosumab treatment who had not yet
undergone surgery (or those who were considered as definitively
unresectable), the median treatment time was 7.8 months (2–16
months) (Table II). A total of 17
(49%) patients at the data cut-off date (study end date of
September 2015) had undergone surgery. A total of 15 patients
continue to receive treatment with denosumab (two additionally as a
salvage therapy following tumor recurrence after surgery). Of the
18 patients who did not undergo surgery, 2 patients developed
progression and started chemotherapy, and 16 remain on denosumab
treatment. All patients remain alive.
Adverse events
In general, treatment was well tolerated and no
grade IV toxicity was observed. However, 2 patients exhibited grade
III toxicity: 1 patient experienced hypophosphatemia after 10
months of denosumab treatment and 1 patient exhibited hypocalcemia
after 7 months of treatment with denosumab. A total of 11 patients
exhibited grade II adverse events (Table III).
 | Table III.Adverse effects exhibited in giant
cell tumor of bone patients following treatment with denosumab. |
Table III.
Adverse effects exhibited in giant
cell tumor of bone patients following treatment with denosumab.
| Adverse effect | Grade II toxicity,
n (%) | Grade III toxicity,
n (%) |
|---|
|
Hypophosphatemia | 8 (23) | 1 (3) |
| Hypocalcemia | 3
(9) | 1 (3) |
Surgery
Of all denosumab-treated patients, 17 (49%) patients
underwent surgery after denosumab treatment. Wide en bloc resection
was performed in 11 patients, with prosthesis implantation in 10/11
cases, while 6 patients underwent intralesional curettage with
high-speed burr and allograft or bone cement (Table IV). Patients that underwent
prosthetic replacement exhibited a longer median preoperative
duration of denosumab therapy when compared with patients
undergoing surgery without prosthetic implantation.
 | Table IV.Treatment types administered to
patients that underwent surgery following denosumab treatment
(n=17). |
Table IV.
Treatment types administered to
patients that underwent surgery following denosumab treatment
(n=17).
| Treatment type | Patients, n
(%) |
|---|
| Prosthesis
replacement | 10 (59) |
| No prosthesis
replacement | 7
(41) |
Pathological changes
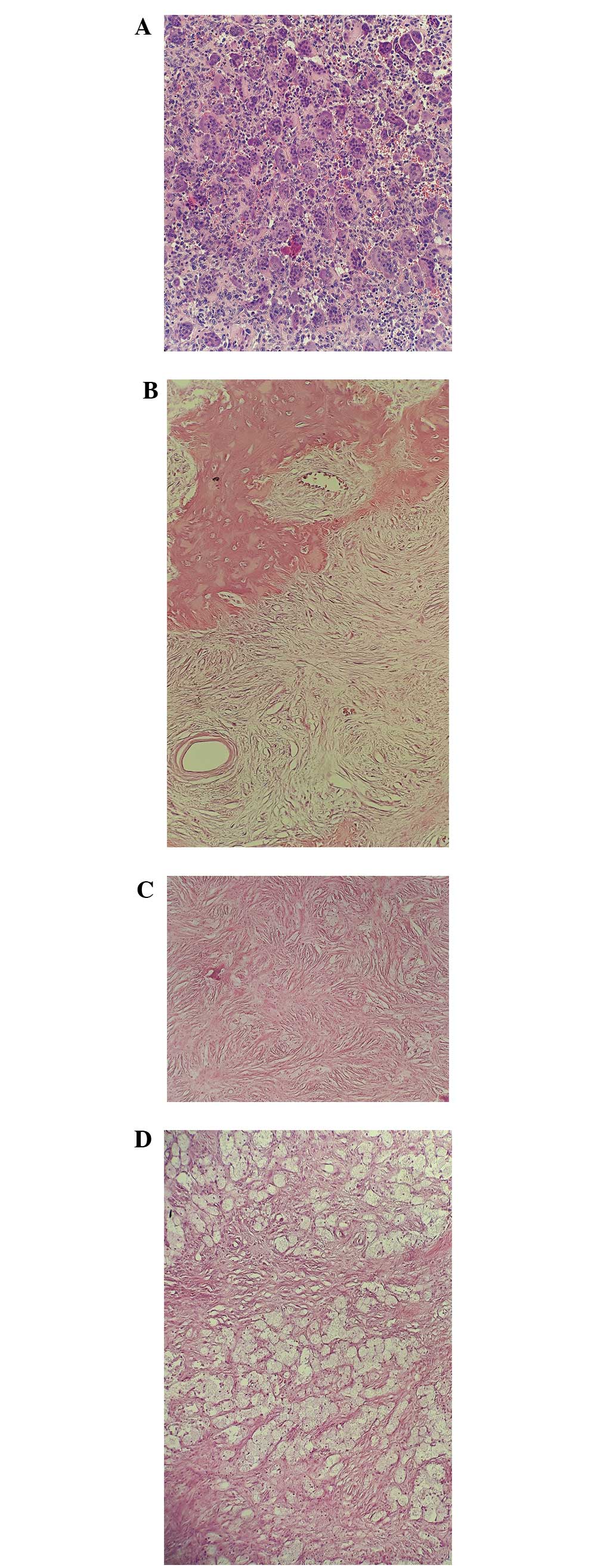
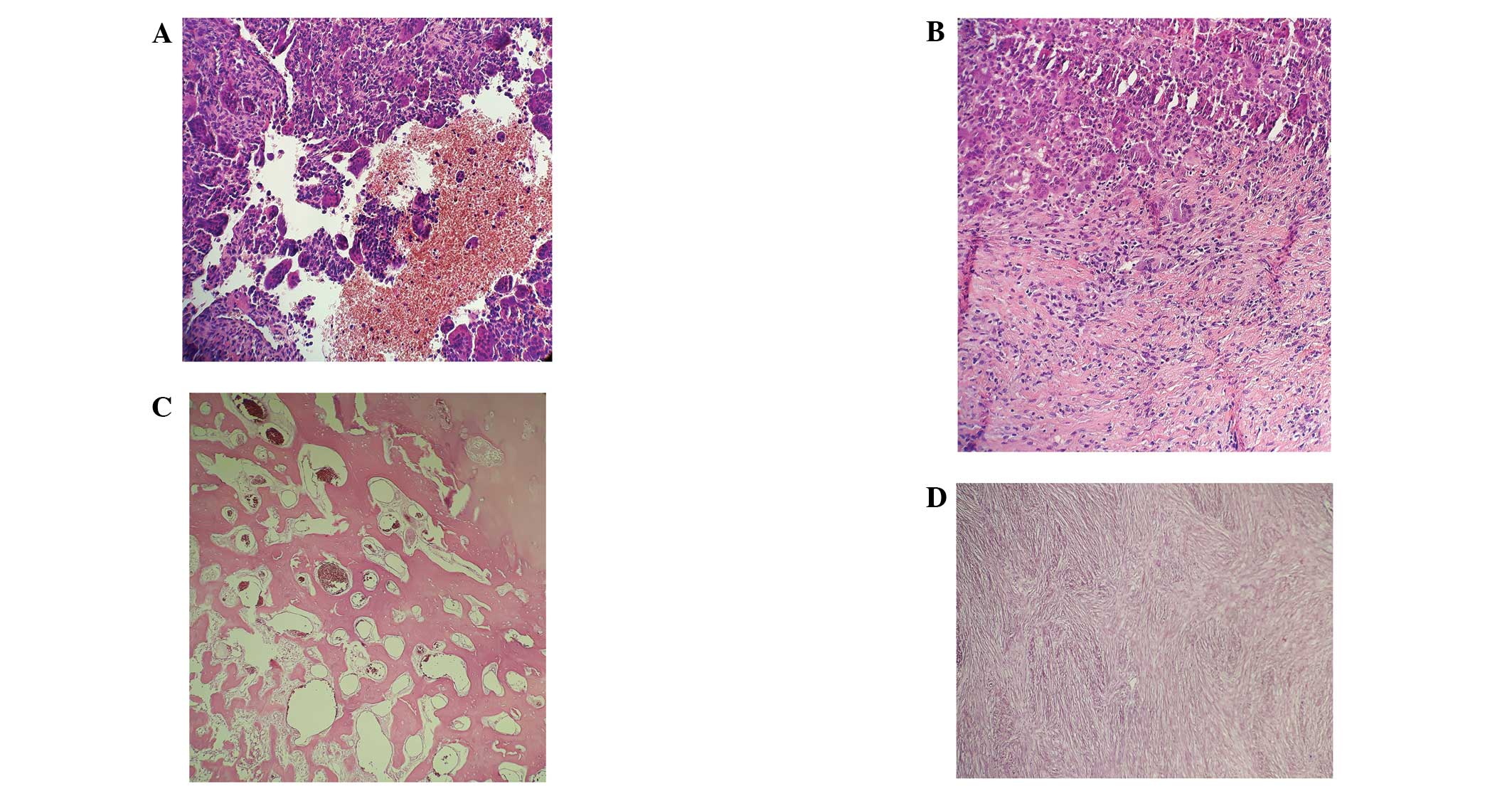
Following therapy with the RANKL inhibitor
denosumab, in GCTB en bloc resection specimens, giant cells
disappeared, the number of mononuclear tumor cells decreased and
bone formation increased (Figs. 1 and
2).
Treatment outcomes
Tumor progression after surgical treatment was
observed in 2 patients (9 and 11 months after surgery) that
underwent intralesional curettage without prosthesis implantation.
Both patients were subsequently administered salvage denosumab
treatment (Table V).
 | Table V.Patient outcomes following surgery
with or without prosthesis replacement (n=17). |
Table V.
Patient outcomes following surgery
with or without prosthesis replacement (n=17).
| Outcome | Patients with
prosthesis replacement, n (%) | Patients without
prosthesis replacement, n (%) |
|---|
| Disease
progression | 0 (0) | 5
(71)a |
| No progression | 10 (100) | 2 (29) |
In addition, tumor progression was observed in 2
patients during denosumab treatment. One patient exhibited
progression to osteosarcoma 3 months after initiation of denosumab
treatment and thus, chemotherapy with doxorubicin and cisplatin was
initiated. The second patient exhibited tumor progression 7 months
after the initiation of denosumab therapy, which was confirmed by
magnetic resonance imaging and subsequently, pathologically
malignant GCTB was diagnosed. This patient underwent amputation and
chemotherapy was administered. Both patients had undergone
radiotherapy prior to denosumab treatment.
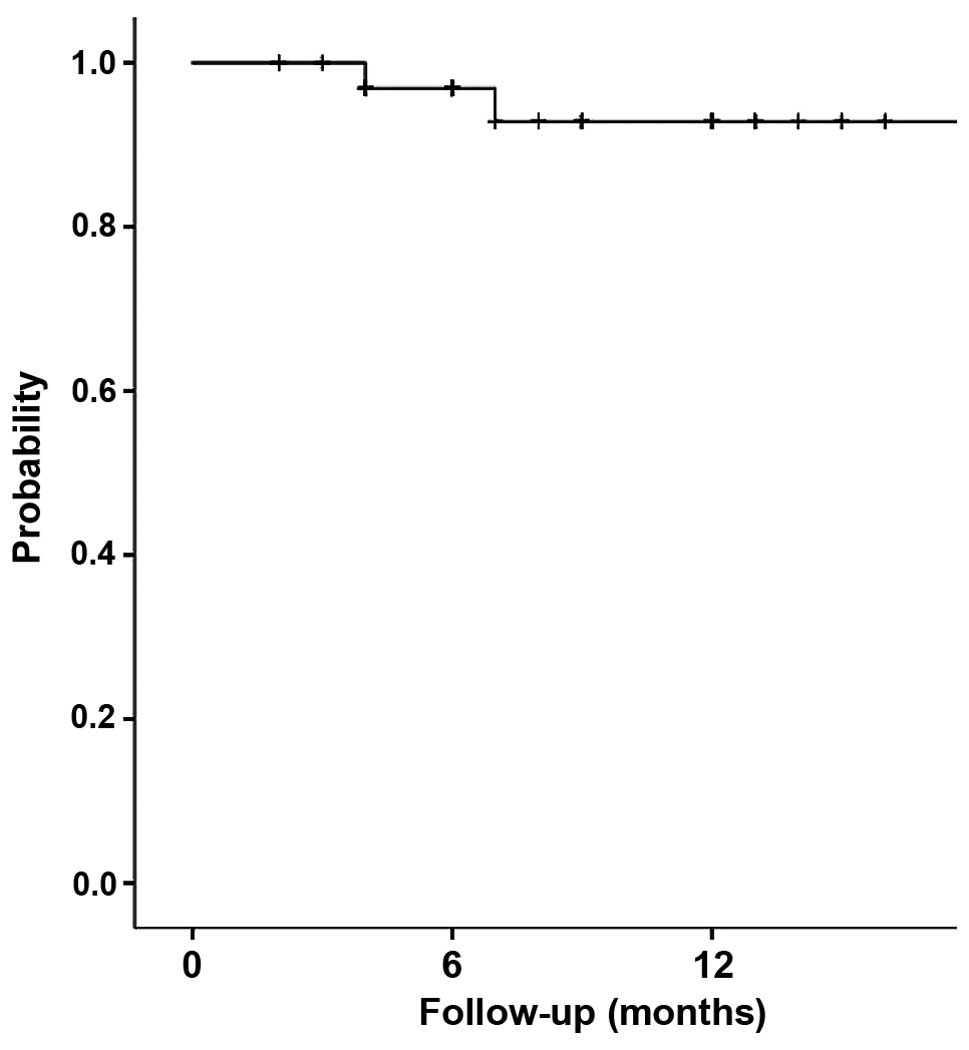
The overall 1-year progression-free survival rate
was 92.8% (95% confidence interval, 83.2–100) (Fig. 3).
Discussion
The present study summarized the results of
denosumab treatment in 35 locally advanced GCTB patients without
participation in clinical trials. The results revealed that
denosumab exhibits high efficacy with long term responses. This
study comprises the largest study of locally advanced GCTB patients
without metastasis to receive denosumab in routine practice, and in
≥50% of patients denosumab was administered as a preoperative
modality combined with radical local surgery. No patients received
denosumab as adjuvant therapy. The primary treatment for GCTB is
surgery and the most important challenge in surgical management is
the relatively high recurrence rate after curettage (21–65%)
(2,7).
Two patients exhibited disease recurrence after curettage, which
raises significant concerns regarding curettage after denosumab
treatment as the recurrence rate was high (6.33%). This indicates
that if intralesional surgery is planned after neoadjuvant
denosumab, denosumab therapy should be administered for a
relatively short period of time (~3 months) as the calcified rim of
the tumor may be too thick following 3 months of denosumab
treatment, which would prevent radical curettage of the tumor
cells. However, a different situation arises in cases whereby wide
radical surgery is planned after denosumab therapy. In the present
study, prosthetic replacement was performed in 10/17 cases that
underwent surgery as the patients exhibited locally advanced
tumors, penetration to the joint or pathological fractures. In such
situations calcification of tumors that initially penetrate the
soft tissues after denosumab therapy may facilitate or enable
radical tumor resection with a low risk of tumor recurrence.
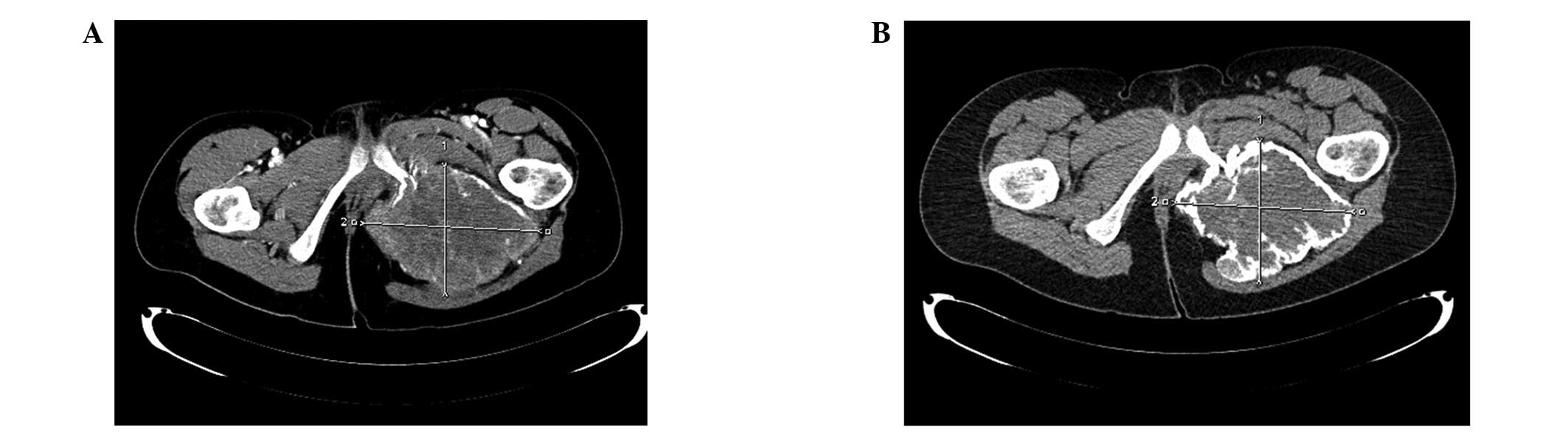
Therefore, we postulate that in cases where en bloc resection is
planned neoadjuvant therapy should be administered for a longer
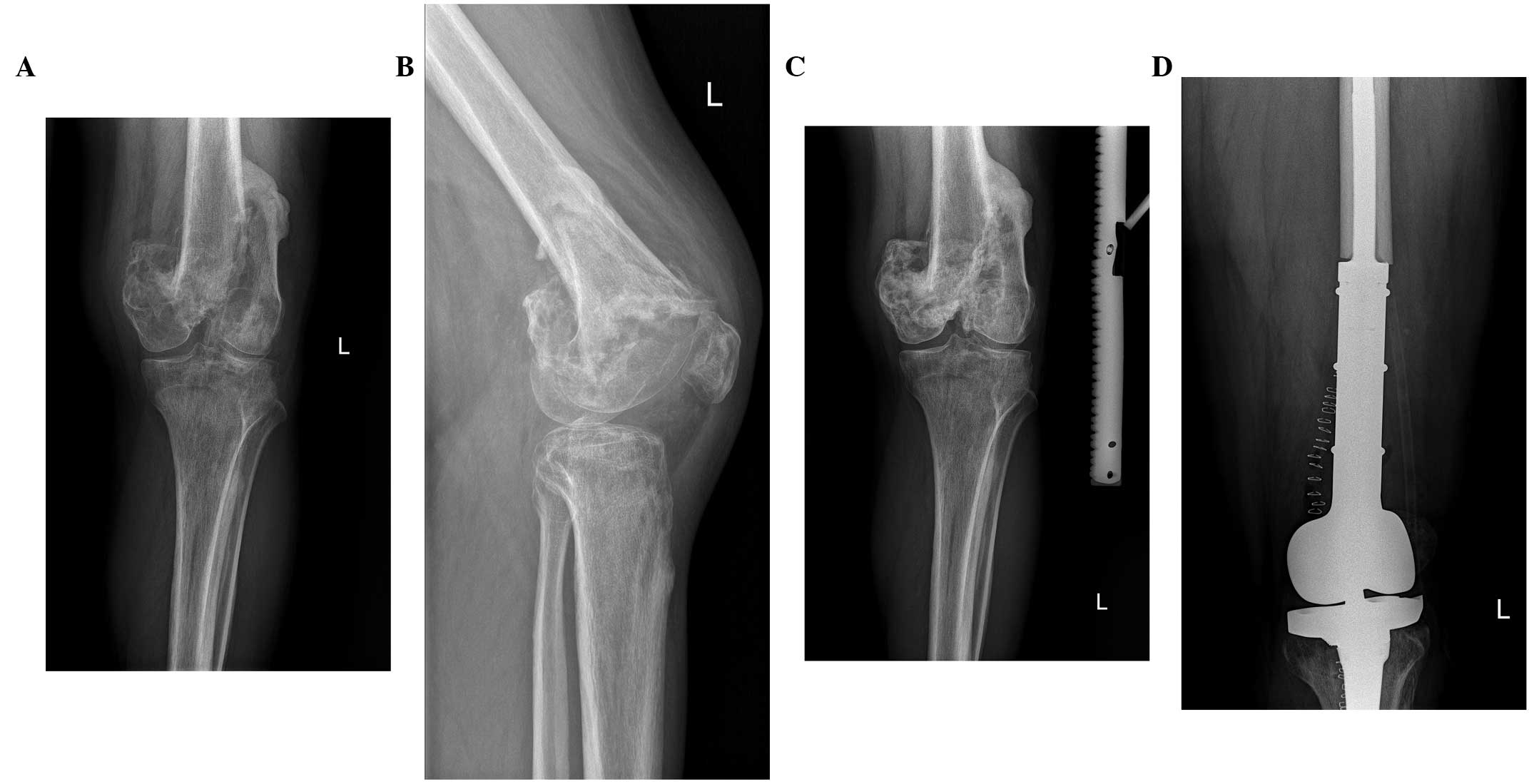
duration to allow maximal calcification of the tumor (Figs. 4 and 5)
and response plateau observed on control imaging. Whether denosumab
maintenance is required after radical surgery remains unclear,
however, in the present study, no disease recurrence was
observed.
A phase II clinical trial assessed 222 patients for
possible downstaging with denosumab for planned surgery (35). The majority of patients received
adjuvant denosumab for 6 months after surgery. Of the 116 patients
who underwent surgery (median postsurgical follow-up time, 13
months), local recurrence occurred in 17 (15%) patients, however,
by contrast to the present study, the majority of these patients
underwent intralesional curettage.
Furthermore, a number of patients present with
inoperable GCTB and thus require life-long denosumab treatment.
Although good tolerance of treatment has been reported previously
(33,31), data regarding long-term use of
denosumab for metastatic/inoperable GCTB is limited. It may be
possible to reduce the dose frequency (e.g., to once every 3
months) in patients who have achieved long-term stable disease on
monthly denosumab (36).
In conclusion, denosumab therapy in GCTB is
associated with a high rate of tumor response with a good toxicity
profile. The results of the present study confirmed that denosumab
exhibits excellent efficacy and short-term tolerability. For
patients with advanced, unresectable, progressive, or symptomatic
heavily pretreated GCTB, denosumab provides a therapeutic option
not previously available and thus, has become the standard therapy
for multidisciplinary management of advanced/high-risk or
unresectable GCTB. Furthermore, the results of the study data
suggest that neoadjuvant therapy with denosumab may present a
therapeutic option for patients with locally advanced, high-risk
tumors to facilitate complete surgical resection or avoid damaging
surgery, however, the risk of recurrence after curettage of GCTB
following denosumab therapy raises questions regarding the optimal
preoperative duration of treatment. This study confirmed the
efficacy of denosumab in 35 patients with locally advanced GCTB
treated without participation in clinical trials. Complete tumor
resection was feasible in 50% of patients. Therefore, denosumab
became the standard therapy for the multidisciplinary management of
GCTB.
References
|
1
|
Amanatullah DF, Clark TR, Lopez MJ, Borys
D and Tamurian RM: Giant cell tumor of bone. Orthopedics.
37:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jagiello-Wieczorek E, Pieńkowski A and
Rutkowski P: Denosumab for treating giant cell tumor of bone.
Expert Opinion on Orphan Drugs. 3:1219–1229. 2015. View Article : Google Scholar
|
|
3
|
Thomas DM and Skubitz KM: Giant cell tumor
of bone. Curr Opin Oncol. 21:338–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skubitz KM: Giant cell tumor of bone:
Current treatment options. Curr Treat Options Oncol. 15:507–518.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dominkus M, Ruggieri P, Bertoni F,
Briccoli A, Picci P, Rocca M and Mercuri M: Histologically verified
lung metastases in benign giant cell tumours - 14 cases from a
single institution. Int Orthop. 30:499–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Heijden L, Dijkstra PD, van de
Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovée JV, Hogendoorn
PC and Gelderblom H: The clinical approach toward giant cell tumor
of bone. Oncologist. 19:550–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher DM: WHO Classification of Tumours
of Soft Tissue and Bone. 4th. IARC Press; Lyon: 2013
|
|
9
|
Greenspan A, Jundt G and Remagen W:
Differential Diagnosis in Orthopaedic Oncology. 2nd. Lippincott
Williams & Wilkins; Philadelphia, PA: 2007, PubMed/NCBI
|
|
10
|
Biermann JS: Updates in the treatment of
bone cancer. J Natl Compr Canc Netw. 11:(Suppl 5). S681–S683.
2013.
|
|
11
|
Athanassou NA, Bensal M, Forsyth R, et al:
Giant cell tumor of boneWHO Classification of Tumours of Soft
Tissue and Bone. Fletcher CDM, Bridge JA, Hogendoorn PCW and
Mertens F: IARC Press; Lyon: pp. 321–324. 2013
|
|
12
|
Hemingway F, Taylor R, Knowles HJ and
Athanasou NA: RANKL-independent human osteoclast formation with
APRIL, BAFF, NGF, IGF I and IGF II. Bone. 48:938–944. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu PF, Tang JY and Li KH: RANK pathway in
giant cell tumor of bone: Pathogenesis and therapeutic aspects.
Tumour Biol. 36:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakarun CJ, Forrester DM, Gottsegen CJ,
Patel DB, White EA and Matcuk GR Jr: Giant cell tumor of bone:
Review, mimics, and new developments in treatment. Radiographics.
33:197–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas DM: RANKL, denosumab, and giant
cell tumor of bone. Curr Opin Oncol. 24:397–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roux S, Amazit L, Meduri G,
Guiochon-Mantel A, Milgrom E and Mariette X: RANK (receptor
activator of nuclear factor kappa B) and RANK ligand are expressed
in giant cell tumors of bone. Am J Clin Pathol. 117:210–216. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan T, Atkins GJ, Trivett MK, Johnson
SA, Kansara M, Schlicht SL, Slavin JL, Simmons P, Dickinson I,
Powell G, et al: Molecular profiling of giant cell tumor of bone
and the osteoclastic localization of ligand for receptor activator
of nuclear factor kappaB. Am J Pathol. 167:117–128. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao TS, Yurgelun MB, Chang SS, Zhang HZ,
Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ and Lee
FY: Recruitment of osteoclast precursors by stromal cell derived
factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res.
23:203–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clézardin P: The role of
RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone
diseases: Physiopathology and clinical implications. Bull Cancer.
98:837–846. 2011.(In French). PubMed/NCBI
|
|
20
|
Forsyth RG, De Boeck G, Baelde JJ,
Taminiau AH, Uyttendaele D, Roels H, Praet MM and Hogendoorn PC:
CD33+ CD14- phenotype is characteristic of multinuclear
osteoclast-like cells in giant cell tumor of bone. J Bone Miner
Res. 24:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behjati S, Tarpey PS, Presneau N, Scheipl
S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, et
al: Distinct H3F3A and H3F3B driver mutations define
chondroblastoma and giant cell tumor of bone. Nat Genet.
45:1479–1482. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huvos AG: Bone TumorsDiagnosis, treatment
and prognosis. 2nd. W.B. Saunders; Philadelphia, PA: 1990
|
|
23
|
Enneking WF: Staging musculoskeletal
tumorsMusculoskeletal Tumor Surgery. Churchill Livingstone; New
York, NY: pp. 87–88. 1983
|
|
24
|
Nielsen GP and Rosenberg AE: Diagnostic
Pathology: Bone. Amirsys Lippincott Williams & Wilkins;
Philadelphia, PA: 2012
|
|
25
|
Dufresne A, Derbel O, Cassier P, Vaz G,
Decouvelaere AV and Blay JY: Giant-cell tumor of bone, anti-RANKL
therapy. Bonekey Rep. 1:1492012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shehadeh A, Noveau J, Malawer M and
Henshaw R: Late complications and survival of endoprosthetic
reconstruction after resection of bone tumors. Clin Orthop Relat
Res. 468:2885–2895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeys LM, Suneja R, Chami G, Grimer RJ,
Carter SR and Tillman RM: Impending fractures in giant cell tumors
of the distal femur: Incidence and outcome. Int Orthop. 30:135–138.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tse LF, Wong KC, Kumta SM, Huang L, Chow
TC and Griffith JF: Bisphosphonates reduce local recurrence in
extremity giant cell tumor of bone: A case-control study. Bone.
42:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Branstetter DG, Nelson SD, Manivel JC,
Blay JY, Chawla S, Thomas DM, Jun S and Jacobs I: Denosumab induces
tumor reduction and bone formation in patients with giant-cell
tumor of bone. Clin Cancer Res. 18:4415–4424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steensma MR, Tyler WK, Shaber AG, Goldring
SR, Ross FP, Williams BO, Healey JH and Purdue PE: Targeting the
giant cell tumor stromal cell: Functional characterization and a
novel therapeutic strategy. PLoS One. 8:e691012013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López-Pousa A, Martín Broto J, Garrido T
and Vázquez J: Giant cell tumour of bone: New treatments in
development. Clin Transl Oncol. 17:419–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Institutes of Health, . Common
Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
|
|
35
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase ii trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bukata SV, Sudan M, Mendanha W, et al:
Considerations for long-term maintenance treatment with denosumab
for stable inoperable Giant Cell Tumor: Making a case for spacing
of doses after initial response. Connective Tissue Oncology
Society: Abstract 047. 2015.
|