Introduction
The emergence, development and drug resistance of a
tumor are the results of certain characteristics of cancer stem
cells. The use of targeted therapy to tumor stem cells can achieve
treatment of a tumor from its origin. All types of enrichment
methods for tumor stem cells (including those based on stem cell
surface markers, intracellular enzyme activity, concentration of
reactive oxygen species, mitochondrial membrane potential,
promoter-driven fluorescent protein expression, autofluorescence,
suspension/adherent culture, cell division, identification of side
population cells, resistance to cytotoxic compounds or hypoxia,
invasiveness/adhesion, immunoselection and physical properties)
have contributed to significant progress since Al-Hajj et al
(1) isolated breast cancer stem cells
successfully in 2003, thus facilitating further research on breast
cancer stem cells (1). Breast cancer
stem cells originate from normal mammary stem cells or
differentiated breast cancer cells, which are characterized by the
capability of self-renewal, differentiation and tumorigenicity
(2). Breast cancer stem cells have
been observed to be highly resistant to radiotherapy, chemotherapy
and hormone therapy (3). Traditional
radiotherapy and chemotherapy cannot easily kill breast cancer stem
cells, leading to recurrence or metastasis in breast cancer. Only
targeted elimination or permanent killing of breast cancer stem
cells is the point at which breast cancer may be cured completely.
Therefore, based on phenotypic and biological characteristics of
breast cancer stem cells, research regarding technological strategy
for breast cancer stem cells may benefit breast cancer treatment.
The Phage Peptide Library is an efficient and simple tool that has
been widely used in the development of antitumor drugs and tumor
diagnostic markers (4). In the
current study, breast cancer stem cells were isolated from
MDA-MB-231 cells using the serum-free culture method and phage
display technology was subsequently employed to screen the phages
that are able to specifically bind to breast cancer stem cells.
Materials and methods
Materials
Human breast hs578bst, MDA-MB-231 and MCF-7 cell
lines were obtained from the State Key Laboratory of Oncology in
Southern China (Sun Yat-sen University, Guangzhou, China). Ph.D-12
Phage Display Peptide Library and its host cell (Escherichia
coli; ER2738) were purchased from New England Biolabs Ltd.,
Hitchin, UK). Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and trypsin were purchased from HyClone; GE
Healthcare Life Sciences (Logan, UT, USA). Isopropyl
β-D-1-thiogalactopyranoside was purchased from Roche Diagnostics
(Basel, Switzerland) and X-gal from Sigma-Aldrich; Merck Millipore
(Darmstadt, Germany). Horseradish-peroxidase (HRP)-conjugated
anti-M13 phage antibody (27942101) was purchased from GE Healthcare
Life Sciences, and ELISA staining reagents were obtained from
Beyotime Institute of Biotechnology (Haimen, China). An M13 Phage
Plasmid Isolation kit was purchased from Biomiga, Inc. (San Diego,
CA, USA). The National Center for Biotechnology Information
database (https://www.ncbi.nlm.nih.gov/) was used for
bioinformatics sequence analysis.
Cell culture and isolation of breast
cancer stem cells
Normal cell culture was mixed with DMEM containing
10% FBS and penicillin-streptomycin solution under 37°C and 5%
CO2. Medium was replaced every half day, and cytokines
were added as appropriate. Epidermal growth factor, basic
fibroblast growth factor and B27 were placed in serum-free medium
to isolate the MDA-MB-231 breast cancer stem cells. Microsphere
formation was viewed under a light microscope. Samples were
centrifuged at 201 × g for 1 min at room temperature, and
the medium was replaced every 2 or 3 days. The cells were cultured
in DMEM with 10% FBS following each generation of serum-free
culture. Dead cells were removed as appropriate.
Phage screening for breast cancer stem
cells
The hs578bst, MDA-MB-231 and breast cancer stem
cells were isolated and cultured in poly-L-lysine-coated plates
with 105/dish density and adherent culture. Serum-free
DMEM was added for 2 h and the cells were sealed with 0.5% bovine
serum albumin (BSA) (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) for 1 h. The Phage Display Peptide Library
was added to the dish coated with the hs578bst cells with
1011 pfu/titer, and the cells were cultured at 37°C.
Supernatant was subsequently added to the dishes with the
MDA-MB-231 cells. Supernatant was added to the dishes with breast
cancer stem cells following the same process. The supernatant was
discarded after 1 h of incubation at 37°C. The dishes were washed
with 0.05% TBS and Tween 20 (TBST) (v/v) three times, for 1 min
each time. The enriched phages were eluted with glycine elution
buffer (pH, 2.2) and neutralized with Tris-HCl (pH, 9.1). These
phages were gathered from the first round of screening. A small
amount of the eluate was obtained for amplification and used to
infect its host bacteria for the next round of screening. The
concentration of Tween-20 in TBST was increased to 0.1% (v/v) and
the incubation time was increased in the following experiments. Up
to 10 µl of the eluate was collected to measure the phage titer and
compare the enrichment ratio prior to and following each screening.
A total of 10 blue plaques were randomly selected at the final
round of phage tittering. These phages may be used for
identification and DNA sequencing following amplification.
ELISA identification of concentrated
phages
The concentrated breast cancer stem cells were
inoculated at a density of 104/hole in 96-well plates
and handled with serum-free DMEM for approximately 2 h following
adherence. The cells were subsequently collected and fixed in 4%
paraformaldehyde for ~15 min, washed with PBS and disposed with
0.1% TritonX-100 for 10 min, 0.05% PBST thrice and 2% PBS-BSA for 1
h. The phages were added subsequent to amplification and washed
with 0.05% PBST for 2 h. HRP-conjugated anti-M13 antibody (diluted
in 2% PBS-BSA at 1:5,000) was added after 1 h and washed three
times with 0.05% PBST. HCl was used to terminate the reaction after
3,3′,5,5′-tetramethylbenzidine color development. The absorbance
was measured by an ELISA microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 450 nm. The phages were randomly
selected from the original library as a control, and if the phage
optical density (OD) / control OD was >2, this was defined as
positive.
3,3′-Diaminobenzidine (DAB)
identification of concentrated phages
hs578bst, MDA-MB-231 and concentrated breast cancer
stem cells were placed in 24-well plates at a density of
104/hole, following the steps described earlier in the
‘Phage screening for breast cancer stem cells’ paragraph. The
differences between the two procedures were detected using one DAB
chromogenic reagent kit (Beijing Dingguo Changsheng Biotechnology
Co., Ltd., Beijing, China) for color development. The color
reaction was inhibited with distilled water following 10 min.
Mayer's hematoxylin was used for subsequent staining and the
results were observed under a light microscope.
Extraction of phage DNA and
sequencing
The positive phage clone in described earlier in the
‘Phage screening for breast cancer stem cells’ paragraph was
amplified. DNA was extracted using an M13 Phage Plasmid Isolation
kit and was sent to Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for DNA sequencing.
Results
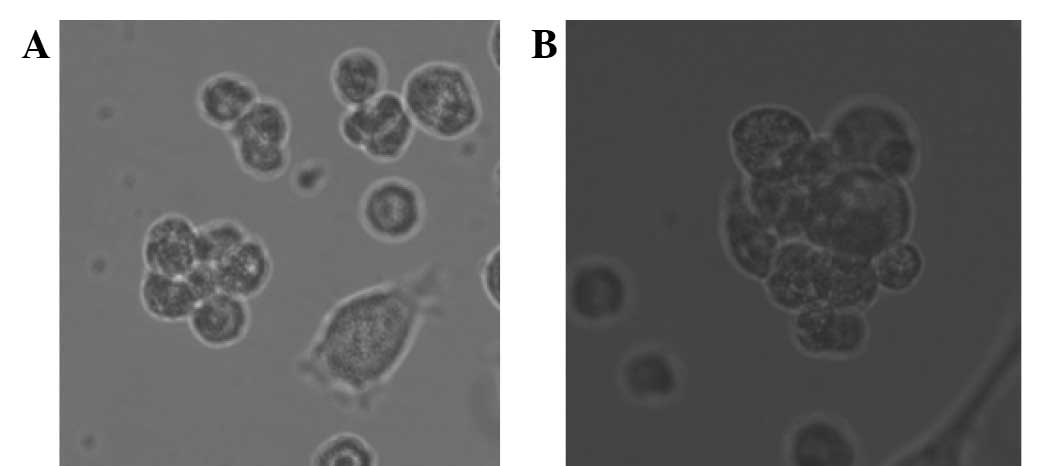
Suspension cultivation of breast
cancer stem cell microspheres
Following the serum-free culture of breast cancer
cells and three rounds of ‘serum-free with serum’, numerous breast
cancer stem cell microspheres were suspended in the cell culture
medium. The microspheres increased in volume with increased
incubation time and growth adhering to the wall when represented in
the serum medium (Fig. 1).
Specificity phage screened for bonding
to breast cancer stem cells
The phages were enriched nearly 500 times following
three rounds of screening using the hs578bst and MDA-MB-231 cells
as the control screen group and breast cancer stem cells as the
experimental group. Screening phages decreased in degree prior to
and following each round of determination (Table I).
 | Table I.Screening phage drop degree prior to
and following each round. |
Table I.
Screening phage drop degree prior to
and following each round.
| Round | Phages in place,
cpu | Phage washed out,
cpu | Enrichment ratio |
|---|
| First round | 1.0×1011 | 4.9×103 | 4.9×10-8 |
| Second round | 1.5×1011 | 6.5×104 | 4.3×10-7 |
| Third round | 1.5×1010 | 3.2×105 | 2.1×10-5 |
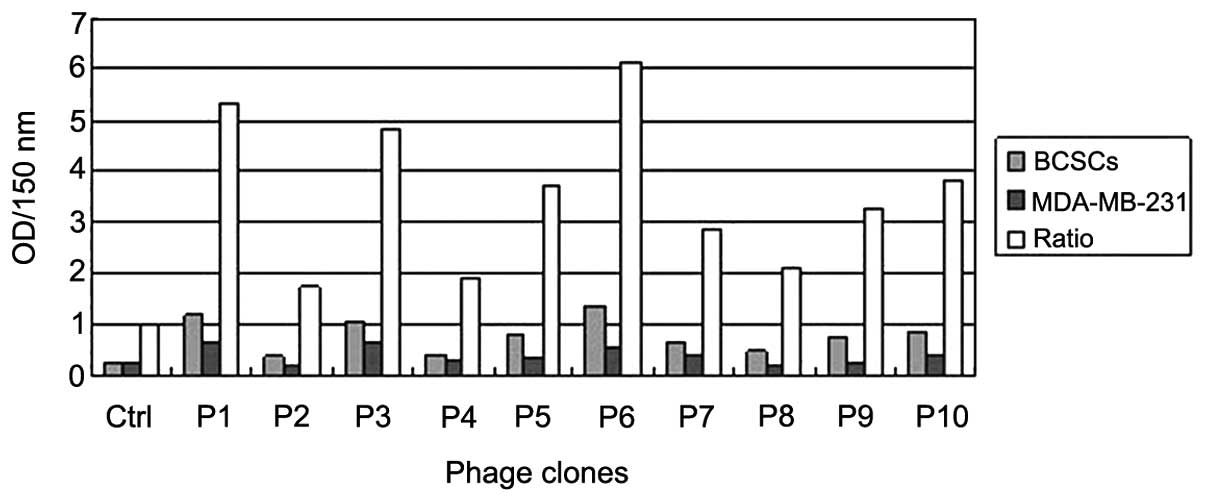
Identification of phage specificity to
breast cancer stem cells by ELISA
A total of 10 independent phagocytic loci coerulei
were selected randomly for amplification from the last round of
phage titration tablet. Following three rounds of screening, the
affinity of each phage to breast cancer stem cells was identified
by ELISA and compared with the original library phage that was
randomly selected (Fig. 2). Among the
phages, no. 6 exhibited the highest affinity to target cells and
was labeled KL-6.
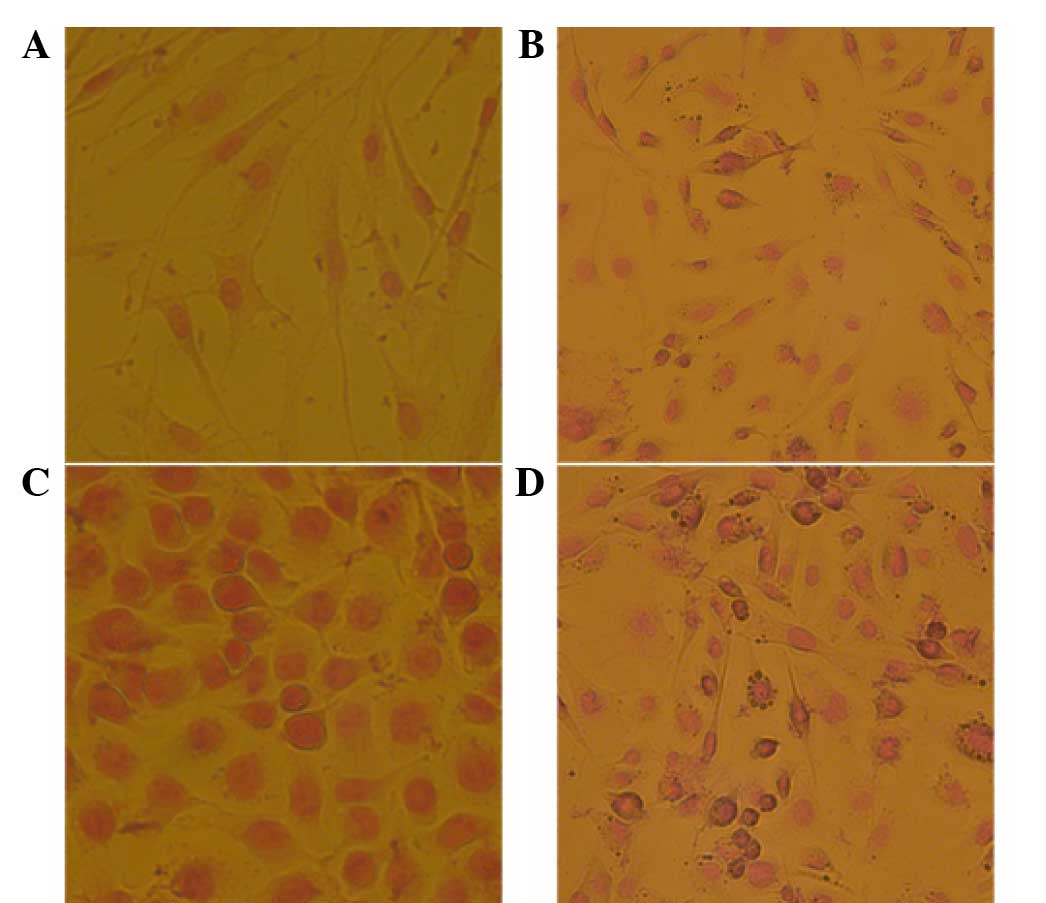
DAB identification of phage bonding
and specificity to breast cancer stem cells
DAB staining was used to identify the specificity of
the positive phage clone KL-6 to breast cancer stem cells (Fig. 3). KL-6 exhibited the highest affinity
and specificity to the breast cancer stem cells, which were clearly
stained brown. KL-6 demonstrated small affinity and specificity to
the MDA-MB-231 breast cancer cells, with a few partially stained
cells, and exhibited no affinity or specificity to the MCF-7 breast
cancer cells and breast cells, in which no stained cells were
identified.
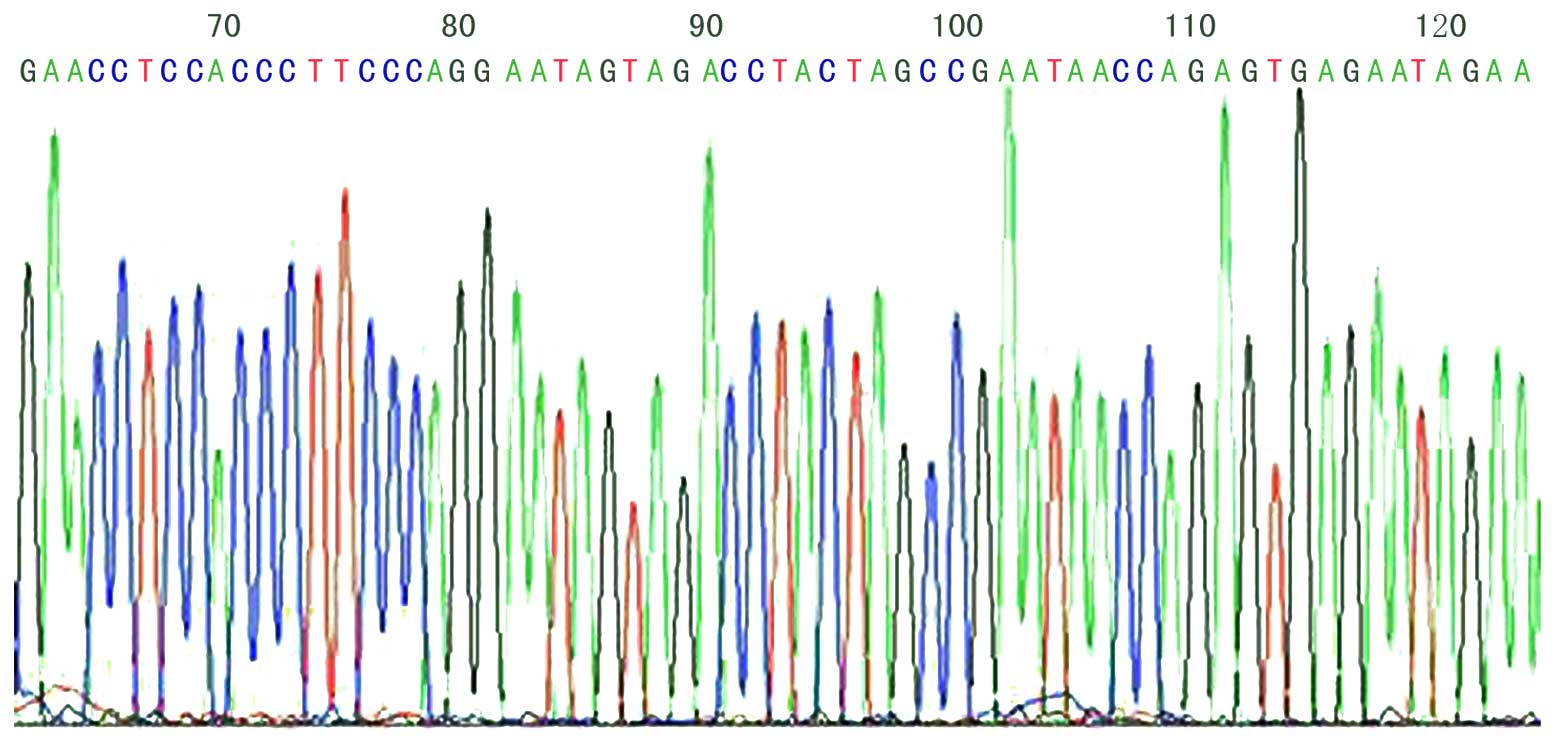
DNA sequencing for positive
phages
Following ELISA identification and amplification, 8
positive phages were collected by M13 Phage Plasmid Isolation kit.
Approximately 5 µg DNA was obtained from 3 ml phage incubation
buffer. The amino acids translated by DNA sequences are presented
in Table II, in which a sequence
(GYSASRSTIPGK) appeared thrice; this sequence is unknown when
compared with the Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/Blast). The DNA
sequencing of KL-6 phage 12 peptide fragments is illustrated in
Fig. 4.
 | Table II.Amino acids translated by DNA
sequences for positive phages. |
Table II.
Amino acids translated by DNA
sequences for positive phages.
| No. | Amino acids |
|---|
| P1, P7 | LPAEPPKIVKLR |
| P3, P6, P9 | GYSASRSTIPGK |
| P5 | GAIRIRLSEPLS |
| P8 | SIYVDYETNRVV |
| P10 | HSPLSAITNNIM |
Discussion
Reya et al (5)
proposed the ‘theory of tumor stem cells’ in 2001, which suggested
that not all cells in tumor tissues are able to proliferate
aberrantly and eventually become malignant. However, a few cancer
stem cells do control the development, transfer and drug resistance
of tumors, which primarily cause tumorigenesis and recurrence
(6). A number of experiments and
studies have been conducted on cancer stem cells. Based on the
existing stem cell separation technology, the association between
breast cancer stem cells and viruses, expression of estrogen and
radiation treatment have been studied (7–9). In 2012,
three separate teams of researchers used genetic marker technology
to track cell proliferation, particularly cancer cell growth, which
provided strong evidence to confirm the existence of cancer stem
cells (10–12). Surgery, chemotherapy, radiotherapy and
targeted therapies are currently the most common therapeutic
methods for breast cancer. The targeted therapy of stem cells also
exhibits good prospects for development and considerable economic
potentials (13). The development of
targeted peptides for breast cancer stem cells may reduce drug
dosage and side effects of chemotherapy drugs on normal cells.
Breast cancer may also be cured radically in this approach.
Phage display is able to obtain target specificity
phage clones without immunization in a short time (14). The screening procedure is simple and
extremely useful in the field, such as in antigen epitope,
parasites and tumor-specific marker screening (15,16). Gur
et al (17) employed phage
display technology to screen two polypeptides for SUM159 breast
cancer stem cells. These cells may be targeted to identify breast
cancer stem cells. Phage display technology is used in screening
the specificity and polypeptide of tumor stem cells, which has
provided novel potential approaches for breast cancer treatment
(18).
Cluster of differentiation (CD)133 is one of the
numerous markers used in screening targets for cancer stem cells.
However, the roles of CD133 and its natural ligand in breast cancer
stem cells remain unknown. Sun et al (19) employed phage display technology and
obtained a peptide sequence (LQNAPRS) that is able to specifically
bind to CD133 in nude mice. Migration and wound healing experiments
indicated that this peptide may inhibit the migration of breast
cancer cells through concentration dependence (19). In addition, Tian et al
(20) also obtained one phage peptide
using similar experiments and methods, and this phage peptide may
also specifically combine with CD133 and simulate the molecular
ligands and antigen epitope of human colon cancer, which is
expected to develop peptide drugs via antagonism effect.
Furthermore, Yang et al (21)
detected a sequence via Phage Peptide Library screening for NVVRQ
five peptides, and this peptide is able to bind to highly
metastatic tumor cells, including MDA-MB-435 breast cancer cells
specifically in vivo and in vitro.
The current study also designed dual-subtract
biopanning, which is a method that is more rigorous than
conventional screening methods in the process of experimental
screening. This method is able to address the shortcomings of
serum-free culture method, but cannot enrich all the stem cells in
it. The serum-free culture method was employed by the present study
in the steps in enrichment of breast cancer stem cells as this
method causes minimal damage to the stem cells. This method avoided
the disadvantages of the side-group cell separation method,
including surface antigen changes on stem cells (22). The one-sidedness of the separation
method was also resolved using CD44+/CD24− as
a cell-surface marker. This method does not only enrich stem cells
without the change of surface antigens, but also ensures the
diversity of specific antigens on the surface of stem cells. Given
that no method is able to fully select the stem cells, enough
positive phages can be screened by the dual-subtract biopanning
method. The phage-specific evaluation process itself is also the
appraisal process of sorting out the stem cell population. In the
current study, the DNA sequence of the phage was transferred into
an amino acid (GYSASRSTIPGK) following extraction and compared with
the National Center for Biotechnology Information GenBank DNA
database sequence of bioinformatics analysis (https://www.ncbi.nlm.nih.gov/). The results
demonstrated that the phage peptide sequence was unknown, and no
homology with genes and proteins were known. Thus, it may be
speculated that the present study screened one novel surface
antigen-related ligand for breast cancer stem cells using phage
display technology and facilitated further research on stem cell
biology and new breast cancer-targeted therapy.
Cancer stem cells have elicited considerable
attention in the field of cancer research. However, previous
findings are not established enough to guide clinical treatment and
direct further effective breast cancer treatment. The current study
used a serum-free medium suspension culture method to obtain
specific polypeptide sequence binding to breast cancer stem cells
using phage display technology. As expected, a novel chemotherapy
drug targeted to breast cancer stem cells was developed using these
peptides as carriers, and this new drug may exhibit wider
application than existing drugs, including Herceptin, for curing
breast cancer from its origin. Simultaneously, this technology may
be applied to other breast cancer cell lines to enrich the stem
cells and use breast tissues in animal experiments in vivo
to validate the specificity of the polypeptide. Further research is
also necessary for the application of the current results in
clinical treatment.
In conclusion, breast cancer stem cells were
successfully enriched using serum-free suspension culture method in
the present study. Additionally, polypeptides that specifically
bound to breast cancer stem cells were identified from a phage
display random 12 peptide library. Future studies will be conducted
to identify specific surface antigens of breast cancer stem cells.
These results may provide a foundation and mechanism-based guide
for targeted breast cancer therapies.
References
|
1
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kai K, Arima Y, Kamiya T and Saya H:
Breast cancer stem cells. Breast Cancer. 17:80–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calcagno AM, Salcido CD, Gillet JP, Wu CP,
Fostel JM, Mumau MD, Gottesman MM, Varticovski L and Ambudkar SV:
Prolonged drug selection of breast cancer cells and enrichment of
cancer stem cell characteristics. J Natl Cancer Inst.
102:1637–1652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du B, Qian M, Zhou Z, Wang P, Wang L,
Zhang X, Wu M, Zhang P and Mei B: In vitro panning of a targeting
peptide to hepatocarcinoma from a phage display peptide library.
Biochem Biophys Res Commun. 342:956–962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gangopadhyay S, Nandy A, Hor P and
Mukhopadhyay A: Breast cancer stem cells: A novel therapeutic
target. Clin Breast Cancer. 13:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Zeng W, Huang Y, Zhang Q, Hu P,
Rabkin SD and Liu R: Treatment of breast cancer stem cells with
oncolytic herpes simplex virus. Cancer Gene Ther. 19:707–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simões BM, Piva M, Iriondo O, Comaills V,
López-Ruiz JA, Zabalza I, Mieza JA, Acinas O and Vivanco MD:
Effects of estrogen on the proportion of stem cells in the breast.
Breast Cancer Res Treat. 129:23–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zielske SP, Spalding AC, Wicha MS and
Lawrence TS: Ablation of breast cancer stem cells with radiation.
Transl Oncol. 4:227–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Driessens G, Beck B, Caauwe A, Simons BD
and Blanpain C: Defining the mode of tumour growth by clonal
analysis. Nature. 488:527–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schepers AG, Hugo HJ, Stange DE, van den
Born M, van Es JH, van de Wetering M and Clevers H: Lineage tracing
reveals Lgr5+ stem cell activity in mouse intestinal
adenomas. Science. 337:730–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lou H and Dean M: Targeted therapy for
cancer stem cells: The patched pathway and ABC transporters.
Oncogene. 26:1357–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoogenboom HR and Chames P: Natural and
designer binding sites made by phage display technology. Immunol
Today. 21:371–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo AJ and Cai XP: Application of
filamentous phage display technology in parasitology. Zhongguo Ji
Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 24:304–308. 2006.(In
Chinese). PubMed/NCBI
|
|
16
|
Yip YL and Ward RL: Application of phage
display technology to cancer research. Currt Pharm Biotechnol.
3:29–43. 2002. View Article : Google Scholar
|
|
17
|
Gur D, Liu S, Shukla A, Pero SC, Wicha MS
and Krag DN: Identification of single chain antibodies to breast
cancer stem cells using phage display. Biotechnol Prog.
25:1780–1787. 2009.PubMed/NCBI
|
|
18
|
Staquicini FI, Sidman RL, Arap W and
Pasqualini R: Phage display technology for stem cell delivery and
systemic therapy. Adv Drug Deliv Rev. 62:1213–1216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Zhang C, Liu G, Liu H, Zhou C, Lu
Y, Zhou C, Yuan L and Li X: A novel mouse CD133 binding-peptide
screened by phage display inhibits cancer cell motility in vitro.
Clin Exp Metastasis. 29:185–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian PG, Zhou CP, Zhang C, Yang H, Wu XJ,
Lu YX, Liu GB and Li XN: Selection and identification of specific
binding peptides for cancer stem cell surface marker CD133. Nan
Fang Yi Ke Da Xue Xue Bao. 31:761–766. 2011.(In Chinese).
PubMed/NCBI
|
|
21
|
Yang W, Luo D, Wang S, Wang R, Chen R, Liu
Y, Zhu T, Ma X, Liu R, Xu G, et al: TMTP1, a novel tumor-homing
peptide specifically targeting metastasis. Clin Cancer Res.
14:5494–5502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu ZZ, Chen P, Lu ZD, Cui SD and Dong ZM:
Enrichment of breast cancer stem cells using a keratinocyte
serum-free medium. Chin Med J (Engl). 124:2934–2936.
2011.PubMed/NCBI
|