Introduction
Cancer vaccines show promise as novel cancer
immunotherapies. A number of clinical trials of cancer vaccines
have been conducted (1,2), and safety (without severe adverse
events) and efficacy in certain cases of advanced disease were
demonstrated. However, marked clinical responses are rare and the
expected effect has not been observed in a number of clinical
trials (1,2). To improve the efficacy of cancer
vaccines, it is important to understand the precise immunological
mechanisms that mediate the overall immune response of vaccinated
patients.
Although the T helper (Th)1/Th2 balance in the
antitumor immune response has been appreciated as an important
factor to mediate the eradication of tumors, to the best of our
knowledge, there have been few studies of serial assessment of the
immunoglobulin (Ig)G subclass and IgE response during cancer
vaccine trials (3–6). However, it has been reported that IgG4
subclass antibodies impair antitumor immunity in melanoma patients
(7).
A phase I+II clinical vaccine trial is currently
being conducted using a complex of melanoma antigen gene-A4
(MAGE-A4) protein and cholesteryl pullulan (CHP) in patients with
MAGE-A4-expressing cancer. MAGE-A4 antigen was first identified in
the process of cloning cytotoxic T lymphocyte epitopes of melanoma
cells (8), and it also belongs to the
family of cancer testis antigens (9).
As MAGE-A4 has reportedly been expressed in various cancer types,
including uterine papillary serous carcinoma, ovarian cancer, lung
cancer, melanoma, colorectal cancer, hepatocellular cancer and
esophageal cancer (10–18), it is considered a potentially useful
target antigen. Complexes of CHP nanoparticles that contain a tumor
antigen are being used as a novel type of cancer vaccine, with a
novel antigen delivery system for the MHC class I and II pathways
(6,7,19,20).
In the current trial, complexed recombinant MAGE-A4
protein and CHP nanogel (CHP-MAGE-A4) was administered to patients
with unresectable tumors. The first 3 patients were treated with
100 µg CHP-MAGE-A4, another 3 patients with 300 µg CHP-MAGE-A4, and
another 3 patients with 300 µg CHP-MAGE-A4 plus OK-432, a compound
that has been reported to stimulate toll-like receptor-4 and to
activate antigen-presenting cells (21,22). The
primary phase I endpoint of this trial was to demonstrate the
safety of administrating 300 µg CHP-MAGE-A4 with and without
OK-432. Another aim was to clarify additional immunological
factors, particularly details of the humoral immune response, in
patients treated with CHP-MAGE-A4. The present study reports the
phase I results of this clinical trial and details of a
time-dependent transition of the IgG subclass and IgE induction by
CHP-MAGE-A4 vaccination. To the best of our knowledge, this is the
first report to include these details of the immune response during
cancer vaccination.
Materials and methods
Study design and treatment
protocol
A phase I+II clinical trial of CHP-MAGE-A4 vaccine
was designed to evaluate safety, immune response and clinical
response. The primary endpoints were to evaluate the safety and
optimal dose of the vaccine. The secondary endpoints were to
investigate the immunological and clinical responses. Toxicities
caused by the vaccination therapy were assessed using Common
Terminology Criteria of Adverse Events (CTCAE) v3.0 (23). Immunological monitoring was performed
using an enzyme-linked immunosorbent assay (ELISA) of patient sera
obtained in the pre- and post-vaccination periods, as described
below. To assess the clinical response, computed tomography (CT)
imaging was performed prior to and following vaccination. Every
measurable region was evaluated by the modified Response Evaluation
Criteria in Solid Tumors (mRECIST) (24). The study protocol was approved by the
Medical Ethics Committee of Hokkaido University (University
Hospital Medical Information Network ID: 000001999; Sapporo,
Hokkaido, Japan). Prior to every vaccination, intradermal testing
with a 10-fold dilution of CHP-MAGE-A4 was performed to confirm the
absence of an immediate-type skin reaction. The CHP-MAGE-A4 vaccine
was injected subcutaneously for a total of 6 cycles at 2-week
intervals. To evaluate vaccine safety, a dose-escalation trial was
conducted. Agents and applied doses were as follows: Group 1, 100
µg MAGE-A4 recombinant protein complexed with 1.2 mg CHP; group 2,
300 µg MAGE-A4 recombinant protein complexed with 3.6 mg CHP; and
group 3, 300 µg MAGE-A4 recombinant protein complexed with 3.6 mg
CHP and 0.5 clinical units of OK-432 (Chugai Pharmaceuticals,
Tokyo, Japan) as an immune adjuvant. Group 1 patients were dosed
first, followed by groups 2 and 3. Permission for dose escalation
was granted when no adverse events greater than grade 3 had
occurred in the preceding group during the 2 weeks following the
second dose administration.
At 4 weeks after the last dose administration, the
safety, immune response and clinical response were evaluated.
Thereafter, additional vaccine was administered according to the
patient's decision. Each of the 9 patients enrolled in the phase I
part of this study received 6–25 immunizations.
Patient eligibility
Complete written informed consent was obtained from
all patients at the time of enrollment. This study was performed in
accordance with the guidelines of the Hokkaido University
Institutional Review Board authorization. Eligibility criteria were
as follows: i) Patients with locally-advanced, recurrent or
metastatic tumors histologically confirmed as malignant and
resistant to standard therapy; ii) patients with tumors expressing
MAGE-A4 antigen, assessed by immunohistochemistry; iii) an Eastern
Cooperative Oncology Group performance status of 0–2 (25); iv) an age of ≥20 years; v)>4 months
survival expected; vi) adequate bone-marrow, cardiac, pulmonary,
hepatic and renal functions, including the following: White blood
cell count, ≥2,000/µl; hemoglobin, ≥8.0 g/dl; platelets,
≥75,000/µl; total bilirubin, <1.5 times the institutional normal
upper limit (or, in patients with hepatic metastasis, <3 times);
aspartate aminotransferase and alanine aminotransferase, <2.5
times the institutional normal upper limit (or, in patients with
hepatic metastasis, <5 times); and creatinine, <1.5 times the
institutional normal upper limit; and vii) the patient had no
desire to become pregnant. Exclusion criteria were as follows: i)
Positive for human immunodeficiency virus antibody; ii) multiple
malignant diseases; iii) concurrent autoimmune disease; iv) a past
history of anaphylaxis; v) active metastasis to the central nervous
system; vi) concurrent anticancer therapy during the 4 weeks prior
to the initiation of the trial (except with an anticancer drug that
does not require drug holidays or hormone agents), including
systemic steroids, immunosuppressive agents, irradiation or surgery
for primary lesions; vii) pregnancy or breastfeeding; and viii) a
decision by the principal investigator or physician in charge that
the patient was unsuitable. The patient accrual began in August
2009 and ended in March 2013.
Preparation of CHP-MAGE-A4
Full length MAGE-A4 cDNA was cloned into a pET
vector and introduced into Escherichia coli. Expression of
His-MAGE-A4 protein was induced by the addition of
isopropyl-L-thio-β-D-galactopyranoside to the bacterial cell
culture; the produced protein was recovered and highly purified
using a combination of chromatographic techniques, including metal
chelating affinity, anion exchange, size exclusion and
hydroxyapatite chromatography. CHP was synthesized by a chemical
reaction between pullulan (mean molecular weight, 100 kDa) and
cholesterol isocyanate in pyridine/dimethyl sulfoxide solution
(Nippon Oil and Fat Co., Tokyo, Japan). Subsequent to purification
by extraction and precipitation, the resultant CHP was emulsified
in water and subsequently freeze-dried. When redissolved in water
or buffers, CHP spontaneously forms nanoparticles. These
nanoparticles (20–50 nm) contain the hydrophobic domains of
cholesterol groups internally, which associate with the hydrophobic
regions of the MAGE-A4 protein, forming a stable complex in
solution. This complex of protein and CHP was used as the
CHP-MAGE-A4 vaccine. These vaccines were kindly provided by the
Department of Cancer Vaccine and Immuno-Gene Therapy of Mie
University.
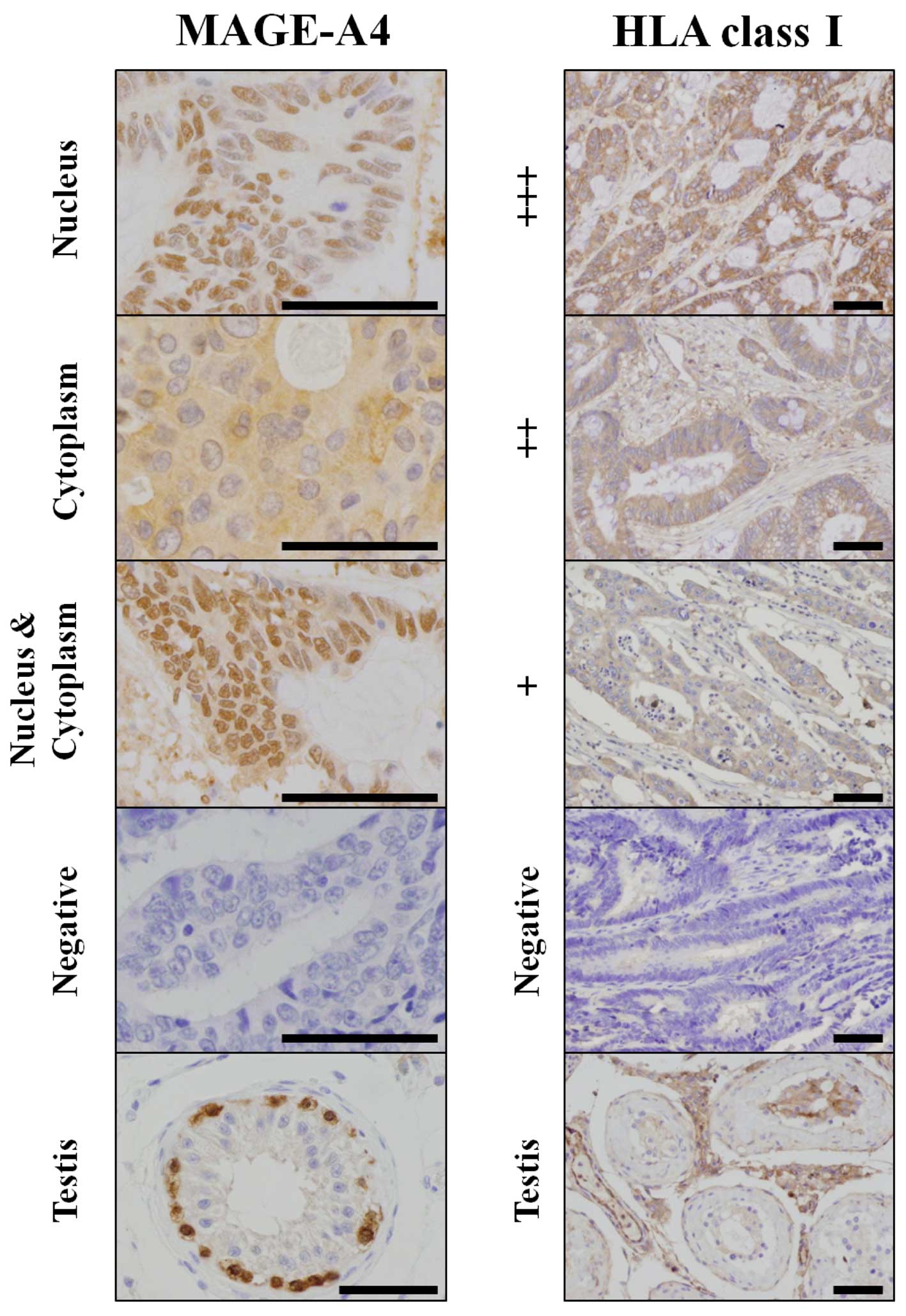
Detection of MAGE-A4 expression in
tumors
To investigate MAGE-A4 antigen expression in tumors
to determine whether patients could be enrolled in this study, each
candidate's archival, formalin-fixed, paraffin-embedded tissue
sections were subjected to immunohistochemical analysis. Patients
with even slight MAGE-A4 positivity in the tumor were eligible for
enrollment in this study. Immunohistochemical reactions were
performed using the streptavidin-biotin-peroxidase method. The
primary antibody used was MCV-1 (2.8 mg/ml; provided by Mie
University), diluted 1:2,000 in Dako Antibody Diluent and Protein
Block Serum-Free (Dako, Carpinteria, California, USA). MCV-1 is a
monoclonal antibody (mAb) generated in mice immunized with human
MAGE-A4 recombinant protein. Previous western blotting revealed
that PC10, a human lung carcinoma cell line obtained from the
Japanese Cancer Research Resources Bank (Tokyo, Japan), was
positive for MAGE-A4 (data not shown); therefore, human testis
tissue and PC10 were used as positive controls. The testis tissue
specimen was obtained from an autopsy case with consent from the
patient's family.
Archival tissue sections were deparaffinized in
xylene and rehydrated in a graded series of ethanol solutions.
Subsequent to washing in deionized water, antigens were unmasked by
incubation for 7 min with citric acid buffer (pH 6.0) in a pressure
cooker at 120°C. After washing in deionized water, endogenous
peroxidase activity was blocked by incubation for 5 min with 3%
hydrogen peroxide in methanol at room temperature. After washing in
deionized water and High-wash-phosphate buffered saline with Tween
20 (PBS-T; pH 7.7; 0.44 M NaCl, 0.1% Tween 20 in PBS; Wako Co.
Ltd., Osaka, Japan), specimens were incubated overnight at 4°C with
the aforementioned primary antibody. Following washing in
High-wash-PBS-T, sections were incubated with peroxidase-labeled
goat anti-mouse and -rabbit IgG (Fab') polyclonal antibody (catalog
no. 41435; Histfine Simple Stain MAXPO [MULTI]; Nichirei, Tokyo,
Japan) for 30 min at room temperature. After washing in
High-wash-PBS-T, immunohistochemical reactions were visualized with
freshly prepared 3,3′-diaminobenzidine tetrahydrochloride
(Histofine SAB-PO [M] kit; Nichirei). Thereafter, slides were
counterstained with hematoxylin and mounted on coverslips. Negative
control staining was performed with 1:20 dilution mouse isotype
IgG1 and IgG2a in Dako Antibody Diluent and Protein Block
Serum-Free (Dako Japan, Co., Ltd., Tokyo, Japan).
If stained cells were microscopically observed, the
specimen was labeled as ‘positive,’ regardless of the degree of
positivity, as assessed by the determination of positive ratios for
each localization (nucleus, cytoplasm, and nucleus and cytoplasm),
by counting the most abundant 5 fields; the ratio of negative cells
was also determined.
Detection of HLA class I antigen
expression in tumors
To investigate the correlation between human
leukocyte antigen (HLA) class I expression in tumors and clinical
response, archival, formalin-fixed, paraffin-embedded tissue
sections of each subject were analyzed by immunohistochemistry.
Reactions were performed using the streptavidin-biotin-peroxidase
method. The primary antibody was EMR8-5 mAb (catalog no. AB-46;
1:2,000 dilution; Hokudo, Sapporo, Japan), in a 3:1 solution of
Dako Antibody Diluent and Protein Block Serum-Free (Dako Japan,
Co., Ltd.) and 10% normal goat serum (Histofine SAB-PO [R] kit;
Nichirei). Previous western blotting with EMR8-5 revealed that PC10
was positive, and LCD was weakly positive, for HLA class I (data
not shown). Thus, PC10 was used as a positive control, and human
testis tissue as a negative control, for HLA class I
immunohistochemistry. As an index of weak HLA class I expression,
LCD, a human lung carcinoma cell line obtained from the Japanese
Cancer Research Resources Bank, was used. Immunohistochemistry was
performed as described above.
The intensity of the expression of HLA class I
antigens was evaluated by comparing normal epithelial cells with
tumor cells and was classified into the following grades: Staining
intensity denser in tumor cells than normal epithelial cells (+++),
staining intensity equal in tumor and normal epithelial cells (++),
staining intensity fainter in tumor than normal epithelial cells
(+), and tumor cells not stained (−). Endothelial cells were used
in place of normal epithelial cells when sections of normal
epithelial cells could not be obtained.
The positivity ratio for each grade was determined
by area measurement using the image processing software ImageJ
(http://rsb.info.nih.gov/ij/), as
developed by the National Institutes of Health (Bethesda, MD,
USA).
Evaluation of clinical responses
Toxicity was evaluated according to CTCAE v3.0.
Tumor responses were assessed according to mRECIST. All known sites
of disease were evaluated by CT scan after the 3rd vaccination
cycle, and 4 weeks after the 6th cycle. mRECIST differs from
existing RECIST in that all evaluable lesions with diameters >10
mm are treated as target lesions. New lesions appearing after
vaccination and initial non-target lesions growing to >10 mm are
treated as target lesions. The simple appearance of a new lesion is
not treated as progressive disease (PD).
Serum samples
To analyze antigen-specific antibody responses, sera
were collected prior to the initial vaccination and 2 weeks after
each subsequent vaccination. All sera were stored at −80°C until
analyzed.
Detection of antibody responses to the
MAGE-A4 protein
Specific antibodies in the sera were measured by
ELISA. The MAGE-A4 recombinant protein in PBS was adsorbed onto
immunoplates (442404; Nunc, Roskilde, Denmark) at a concentration
of 20 ng/50 µl/well overnight at 4°C. Plates were washed in PBS
with 0.05% Tween 20 (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) and then blocked for 2 h at room temperature with 200
µl/well of 1% bovine serum albumin (BSA)/PBS. Serum samples were
diluted in 1% BSA/PBS from 1:400 to 1:102,400. After washing, 100
µl serum/well was added as the primary antibody and incubated
overnight at 4°C. Subsequent to washing, 100 µl/well of 1:4,000
goat anti-human IgG (H+L chain)-horseradish peroxidase (HRP; MBL,
Nagoya, Japan) in 1% BSA/PBS was added as the secondary antibody
and incubated for 5 h at 4°C. Plates were washed, incubated with
100 µl/well of TMB Substrate (Pierce; Thermo Fisher Scientific
Inc., Waltham, MA, USA) for 3 min at room temperature. After that,
100 µl/well of 0.18 M H2SO4 was added and the
optical density (OD) of the sample was immediately read in a
Microplate Spectrophotometer (SPECTRA MAX 190; Molecular Devices,
Sunnyvale, CA, USA). The cutoff OD450 absorption value
was calculated according to the following equation: The mean OD
value of a 1:400 pooled serum sample from healthy donors (n=24)
plus 1.645× standard deviation; the cutoff value determined was
0.288. Similarly, healthy donor sera were used as controls to
correct errors in every examination, and patients' OD values were
corrected using the resultant calculated error ratios.
A positive reaction in seronegative patients was
defined as follows; the OD of a 1:400 serum sample that exceeded
the aforementioned cutoff value. A positive reaction in
seropositive patients was defined as follows; the OD value of a
1:400 diluted serum sample after vaccinations that was further
elevated than it had been prior to vaccinations.
Detection of IgG subclass antibody
responses to the MAGE-A4 protein
Specific IgG subclass antibodies in the sera were
measured by ELISA, as aforementioned, but using 100 µl/well of
diluted sheep anti-human IgG1, 2, 3, or 4 (H+L chain)-HRP (The
Binding Site, Birmingham, UK) in 1% BSA/PBS as the secondary
antibody, and incubations were for 5 h at 4°C. Dilutions of each
subclass were as follows: IgG1, 1:25600; IgG2, 1:12800; IgG3,
1:12800; and IgG4, 1:12800. No IgG subclass antibody cross-reacts
when used in these dilution ratios. The OD450 absorption
cutoff value for each was calculated according to the following
equation: The mean OD value of a 1:100 pooled serum sample from
healthy donors (n=24) plus 1.645× standard deviation. Cutoff values
of each IgG subclass were: IgG1, 0.192; IgG2, 0.140; IgG3, 0.076;
and IgG4, 0.004. The percentage of the IgG4 fraction in the sera of
the healthy donors was lowest. Furthermore, detection of
anti-MAGE-A4-specific IgG4 antibody in the sera of healthy donors
was difficult to obtain by ELISA. Therefore, even slight IgG4
responses following vaccination were considered to be positive.
Detection of IgE antibody responses to
the MAGE-A4 protein
Specific IgE antibodies in the sera were measured by
ELISA, as aforementioned, with differences in that as a primary
antibody, the collected serum samples of each patient were diluted
in 1% BSA/PBS from 1:40 to 1:640. As a secondary antibody, 100
µl/well of 1:1,000 rabbit polyclonal anti-human IgE (A0094; Dako
Japan, Co., Ltd.) in 1% BSA/PBS was added and incubated for 5 h at
4°C. After washing, 100 µl/well of 1:100 goat polyclonal
anti-rabbit immunoglobulin or goat polyclonal anti-mouse
immunoglobulin (as the negative control) (K1491; EnVision Kit-HRP,
Dako Japan, Co., Ltd.) in 1% BSA/PBS was added as a third antibody.
Samples were incubated for 40 min at room temperature. After
washing, OD was measured as aforementioned. There were non-specific
reactions in the negative control wells, so these OD450
absorption values were deducted from all values obtained for sample
wells.
The OD450 cutoff absorption value was
calculated according to the following equation: The mean OD value
of a 1:40 pooled serum sample from healthy donors (n=24) plus
1.645× standard deviation; the cutoff value was determined to be
0.033.
A positive reaction was defined as an OD value of a
1:40 serum sample exceeding the aforementioned cutoff value. There
were no initially seropositive patients.
Statistical analysis
The χ2 test was used to assess the
significance of the association of total IgG level with patient
clinical response. In all tests, statistical significance was set
at P<0.05. All analyses were performed using StatView
statistical software (version 5.0; SAS Institute Inc., Cary, NC,
USA).
Results
Patient characteristics
For the phase I part of this study, 9 patients with
the following cancer types were enrolled: 5 colon, 1 esophageal, 1
papilla of Vater, 1 breast and 1 pancreatic cancer case. Prior to
the vaccinations, all patients had received standard therapy for
refractory advanced, metastatic or recurrent cancer (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
|
|
| Immunohistochemical
analysis of tumor specimens |
|---|
|
|
|
|
|
|
|---|
|
|
|
|
| MAGE-A4 | HLA class I |
|---|
|
|
|
|
|
|
|
|---|
|
|
|
|
| % of positive cells
by locale |
| % of cells by
intensity |
|---|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Age/gender | Cancer type | Target lesions | N | C | N & C | Negative | +++ | ++ | + | – |
|---|
| 1 | 62/F | Colon | Lung nodule,
para-aortic LN | 0 | 100 | 0 | 0 | 0 | 37 | 63 | 0 |
| 2 | 68/M | Colon | Chest and abdominal
wall nodule | 8 | 65 | 8 | 19 | 87 | 13 | 0 | 0 |
| 3 | 61/M | Esophageal | Lung nodule, liver
nodule, supraclavicular LN | 1 | 45 | 0 | 54 | 0 | 27 | 73 | 0 |
| 4 | 63/M | Colon | Para-tracheal LN,
tracheobronchial LN | 3 | 24 | 0 | 73 | 0 | 22 | 73 | 5 |
| 5 | 68/M | Colon | Lung nodule, liver
nodule | 0 | 100 | 0 | 0 | 12 | 58 | 30 | 0 |
| 6 | 65/F | Papilla of
vater | Local
recurrence | 1 | 89 | 1 | 9 | 0 | 40 | 60 | 0 |
| 7 | 62/M | Colon | Lung nodule, liver
nodule, peritoneal nodule | 12 | 31 | 46 | 11 | 9 | 91 | 0 | 0 |
| 8 | 63/F | Breast | Lung nodule, liver
nodule, mediastinal LN | 1 | 89 | 1 | 9 | 0 | 93 | 7 | 0 |
| 9 | 48/F | Pancreatic | Primary tumor | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 |
Expression of MAGE-A4 and HLA class I
antigens in tumors
Staining regions varied from patient to patient, but
there was a trend toward prominent cytoplasmic staining in the
detection of MAGE-A4 (Table I).
Immunohistochemical staining intensity varied from patient to
patient in the detection of HLA class I. All 9 patients exhibited a
certain degree of HLA class I antigen expression in the tumors
(Table I). Representative sections
after immunohistochemical staining are shown in Fig. 1.
Adverse events
Adverse events were assessed using CTCAE v3.0. All
patients developed grade 1 local erythema at the injection sites,
which resolved without any treatment. No induration or increase in
reaction intensity was observed during sequential vaccinations.
Grade 2 blister formation was observed at the distal portion of the
injection site in patient 5 after the third vaccination. This
symptom improved with non-steroid anti-inflammatory drug treatment,
resolved after 24 days and was not observed during sequential
vaccinations. No other adverse event or delayed-type
hypersensitivity reaction associated with drug administration was
observed in any patient (Table
II).
 | Table II.Study summary. |
Table II.
Study summary.
|
|
|
|
|
| Anti-MAGE-A4
antibody response |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
| Total IgG
titers | Rise in titers of
IgG subclass and IgE Ab |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Group | Vaccination
dose | No. of
vaccinations | Adverse events
(grade) | Pre– | Post– | IgG1 | IgG2 | IgG3 | IgG4 | IgE | Tumor response | Overall
survival |
|---|
| 1 | 1 | 100 µg MAGE-A4 | 6 | ISR (1) | Negative | Negative | – | – | + | – | – | SD | 8 m
(S) |
| 2 | 1 | 100 µg MAGE-A4 | 6 | ISR (1) | Negative | >×400 | + | + | – | – | – | PD | 5 m
(S) |
| 3 | 1 | 100 µg MAGE-A4 | 6 | ISR (1) | Negative | >×400 | + | – | + | + | – | PD | 6 m
(S) |
| 4 | 2 | 300 µg MAGE-A4 | 25 | ISR (1) | Negative | >×400 | + | – | – | + | – | SD | 19 m (S) |
| 5 | 2 | 300 µg MAGE-A4 | 9 | ISR (1), blister (2) | >×400 | >×25600 | + | + | + | + | + | PD | 6 m
(S) |
| 6 | 2 | 300 µg MAGE-A4 | 6 | ISR (1) | Negative | >×400 | + | – | + | – | – | SD | 5 m
(S) |
| 7 | 3 | 300 µg MAGE-A4 +
0.5 units OK-432 | 6 | ISR (1) | Negative | >×25600 | + | + | + | + | + | PD | 3 m
(S) |
| 8 | 3 | 300 µg MAGE-A4 +
0.5 units OK-432 | 6 | ISR (1) | Negative | Negative | – | – | – | – | – | PD | 3 m
(S) |
| 9 | 3 | 300 µg MAGE-A4 +
0.5 units OK-432 | 7 | ISR (1) | Negative | >×1600 | + | – | + | – | – | SD | 5 m
(S) |
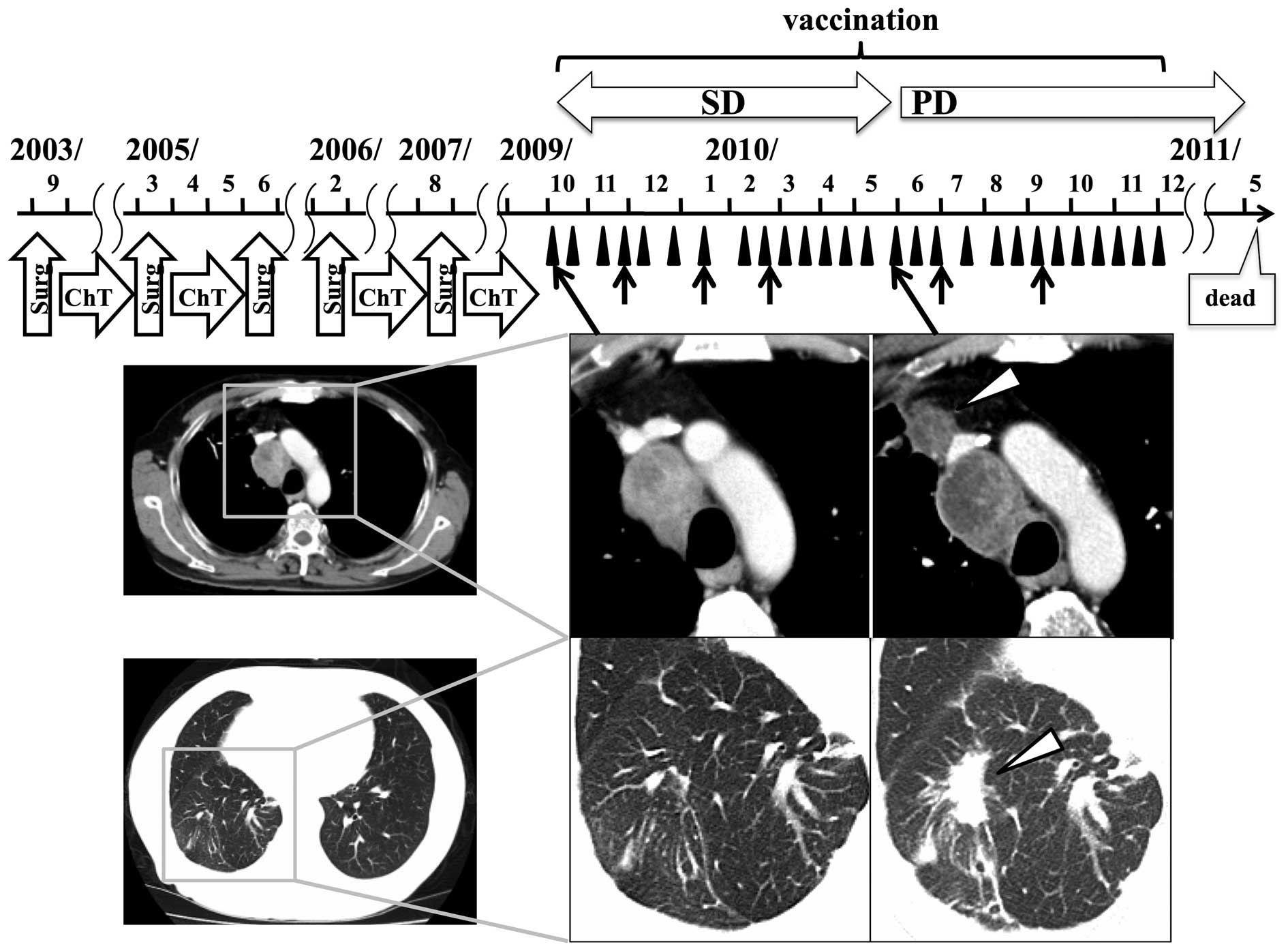
Clinical responses
Tumor responses were assessed according to mRECIST
criteria; 4 patients (patients 1, 4, 6, and 9) were assessed as
exhibiting stable disease (SD), and the other 5 as PD. The clinical
course of patient 4, who achieved the longest survival, is shown in
Fig. 2. Prolonged SD, of ~20 weeks,
was maintained by vaccination in this patient diagnosed with
frequently relapsing colon cancer. The size of the mediastinal
relapse tumor in this patient had not changed for >12 months and
necrosis had increased. However, 5 months after the first
vaccination, new and/or enlarged lesions were detected. No tumor
regression was observed in any patient.
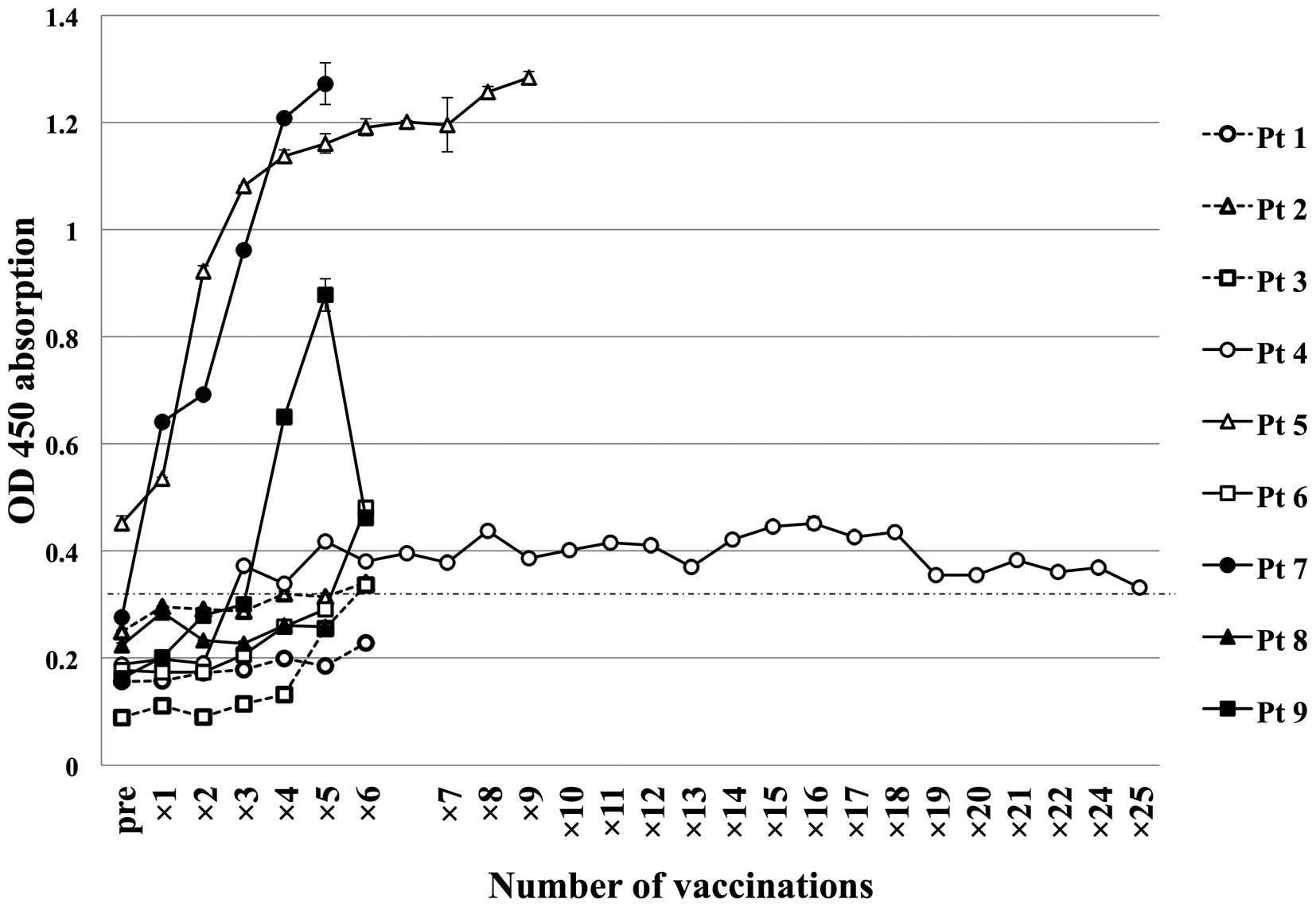
Antibody responses to the MAGE-A4
protein
Total IgG responses to the MAGE-A4 protein were
determined by ELISA. As shown in Fig.
3 and Table II, 1 of the
patients (patient 5) had pre-existing antibodies to MAGE-A4, and
was considered seropositive. The other 8 patients had no antibodies
to MAGE-A4 prior to vaccination, and were considered seronegative;
6 of these patients (patients 2, 3, 4, 6, 7 and 9) seroconverted
after vaccinations. In the patient with pre-existing anti-MAGE-A4
antibody prior to vaccination, the antibody titer increased after
the vaccinations. The anti-MAGE-A4 antibody level increased in 7 of
the 9 patients (2 in group 1, 3 in group 2 and 2 in group 3). There
was a trend toward an earlier and higher titer increase in the
patients of groups 2 and 3 compared with those of group 1. No
significant correlation (by χ2 analysis, P=0.858) was
observed between total IgG titer and clinical response (SD:
Patients 1, 4, 6 and 9 vs. PD: Patients 2, 3, 5, 7 and 8).
IgG subclass- and IgE antibody
responses to the MAGE-A4 protein
IgG1, 2 and 3 antibody responses can be driven by
Th1 and Th2 cells (26,27). On the other hand, IgG4 and IgE
antibody responses are mediated mainly by Th2 cells (26,27). In
all 7 patients who were considered positive for an antibody
response to MAGE-A4 protein, the IgG1 antibody titer rose (Fig. 4). A trend toward increasing IgG3
antibody titers was observed after the increase of IgG1 antibody
titers. Positive conversions to Th2-dominant antibody responses
(IgG4 and IgE) were also observed after vaccination in 4 patients
(patients 3, 4, 5 and 7), who had been positive for Th1-dominant
antibody (IgG1, 2 and 3) responses. In the patients who had Th2
antibody responses, 1 SD and 3 PD clinical responses were observed,
while patients without a Th2 antibody response exhibited 2 PD and 3
SD clinical responses. Total IgG4 antibody levels, including those
not specific for MAGE-A4, were measured in the sera of all 9
patients prior to and following the vaccinations, but significant
disparities were not found during the course of the study (data not
shown). As the sample size was small and statistical analysis was
difficult, trends in the results were assessed, but none were
observed when comparing tumor suppressive effects and other
factors. For example, the expression of MAGE-A4 in the tumor of
patient 4, who was the longest survivor, was only 24%. Although the
immune response of patient 4 may reflect the clinical response,
other patients (patients 5, 7 and 9), who had greater immune
responses, did not have favorable clinical responses (Fig. 4). On the other hand, among the 4
patients who had rising MAGE-A4-specific IgG4 or IgE,
characteristic clinical events were observed in 2 of them. A new
relapse lesion was observed simultaneously with the rise of
MAGE-A4-specific IgG4 in patient 4 (Fig.
4A). In patient 5, a blister was observed at the moment that
the IgG1 antibody titer rose markedly (Fig. 4B), and an immediate-type skin reaction
to the prevaccination intradermal test was observed when the IgE
antibody titer rose to the maximal level, so it was decided that
patient 5 should discontinue vaccinations (Fig. 4C).
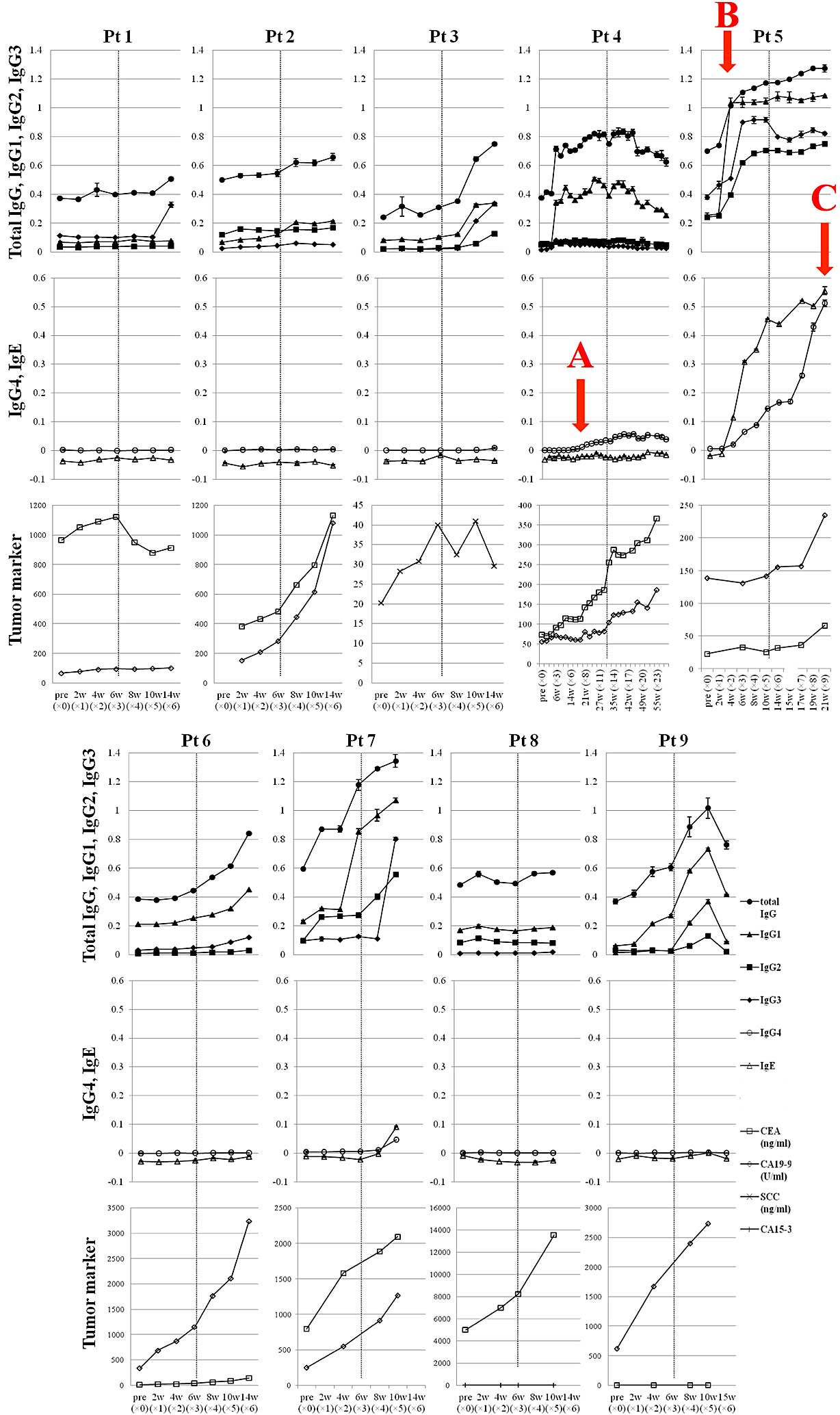 | Figure 4.Melanoma antigen gene-A4-specific
IgG1 antibody titer was elevated in 7 patients. Positive
conversions to a Th2-dominant antibody response (IgG4 and/or IgE
production) were observed after frequent vaccination in 4 patients
(patient 3, 4, 5 and 7) that had been positive for Th1-dominant
antibody responses (IgG1, 2 and 3). Pathognomonic signs observed
were as follows: A, patient 4 developed a new lesion, the tumor
marker level began to rise and an IgG4 antibody response occurred
at the same time; B, patient 5 developed a blister, evaluated as a
grade 2 adverse event at the moment that the IgG1 antibody titer
rose markedly; and C, patient 5 had an immediate-type skin reaction
to the prevaccination intradermal test at the moment that the IgE
antibody titer rose to the highest level. Ig, immunoglobulin; Th, T
helper; w, weeks; Pt, patient; CEA, carcinoembryonic antigen; CA,
cancer antigen; SCC, squamous cell carcinoma. |
Discussion
With respect to the safety of this CHP-MAGE-A4
vaccine, there were no adverse events greater than grade 3 at least
until after the sixth vaccination. Only patient 5 had grade 2
blister formation in the present trial. A previous study detailed
severe adverse events after peptide vaccination in a patient with
highly boosted cellular and humoral responses (28). Therefore, there is a possibility that
the marked rise in IgG1 antibody titer was associated with the
grade 2 blister formation in patient 5.
Cancer immunotherapy requires a relatively long
period prior to the onset of tumor response, and the tumor could
continue to grow for several weeks after the first vaccination
(27). Therefore, mRECIST was used to
precisely evaluate the efficacy of this therapy in the present
study; 4 patients were assessed as SD and the other 5 as PD.
MAGE-A4-specific antibody responses were detected in
7 of the 9 patients in this study (group 1, 2/3; group 2, 3/3;
group 3, 2/3). The observed trend was that the antibody titer rose
earlier and higher in patients of groups 2 and 3 than in those of
group 1. Therefore, the administration of 300 µg CHP-MAGE-A4 could
prove to be more effective than 100 µg for eliciting a strong
immune response. Although several studies suggested that
stereotypical immunostimulation would have certain benefits,
depending on the particular combination of vaccine agent and immune
adjuvant, in the present study, the combination with OK-432 did not
enhance the effect of CHP-MAGE-A4 vaccination. There was no
association between total IgG response and tumor response. A
possible reason proposed was that a favorable tumor response could
not be detected when there were a number of tumors or if they were
bulky. For example, although patients 5 and 7 had the highest
MAGE-A4-specific antibody titers, they did not have any tumor
responses and were evaluated as PD as they had also bulky
metastatic tumors that filled a large portion of the entire liver
(Table II).
The Th1/Th2 balance is closely associated with
antitumor immunity (29). Therefore,
the evaluation and control of the Th1/Th2 balance in patients
treated with cancer vaccines are considered to be necessary for
successful therapy. Generally, IgG1, 2 and 3 antibodies are induced
in Th1 and Th2 cytokine environments (30,31). By
contrast, IgG4 and IgE antibodies are induced in the Th2-dominant
cytokine environment (30,31). The intensity of effector function has
been regarded as follows: IgG1=IgG3>>IgG2=IgG4 (30,31).
IgE is known as an antibody produced during allergic
activity. IgG4 is known as an IgE-blocking antibody that is induced
after frequent administration of allergen during hyposensitization
therapy (32,33). In studies of humoral immunity in
patients with tumors, Th2-dominant cytokines have hardly attracted
attention, while the Th1-dominant cytokine environment has been
well investigated. There have been studies on the antitumor effects
of IgE antibody (26,27,34), but
the precise immunological mechanisms involved remain unclear.
Recently, Karagiannis et al reported that IgG4 subclass
antibodies impair antitumor immunity in melanoma (7). So there is a focused negative effect
induced by IgG4 on the antitumor immune response. There have been
few studies regarding the IgG subclasses and IgE during cancer
vaccination. To the best of our knowledge, the present study is the
first to evaluate the time-dependent transition of the IgG subclass
and IgE during cancer vaccination. In this study, the CHP-MAGE-A4
vaccine induced mainly the Th1-dominant antibody response of IgG1,
2 and 3 production. However, positive conversions to the
Th2-dominant antibody response meant that IgG4 and IgE were also
observed after several rounds of vaccination in patients who
previously had been positive for Th1-dominant antibody responses.
In total, 3 PD and 1 SD clinical responses were observed in
patients who showed the Th2 conversion in the antigen-specific
antibody response, while there were 2 PD and 3 SD clinical
responses in patients without Th2 conversion. These results suggest
a possible association between the time-dependent Th2 conversion
and the clinical benefit to the patient, although this issue must
be rigorously confirmed in later stages of clinical trials aiming
to address clinical response in a stringent manner with larger
enrollment.
Although it is unknown whether the reaction of the
Th2-dominant antibody response depends on frequent medication or
time after the first medication or superfluous Th1 reaction, in the
present study, the rise in IgG4 antibody titer was delayed compared
with the IgG1 response after frequent vaccination, confirming
similar findings of a past study (5).
IgG4 and IgE antibody responses were positive in patients 5 and 7,
who had vigorous IgG1, 2 and 3 responses. These data suggest that a
robust Th1-dominant antibody response may lead to conversion from a
Th1 to a Th2 cytokine environment. By contrast, patient 4, who was
mildly positive for a Th1-dominant antibody response, had only an
IgG4 antibody response, and prolonged survival. However, this
patient developed a new lesion, rising levels of tumor marker and
an IgG4 antibody response at the same time, suggesting that the
IgG4 antibody response may be a sensitive surrogate marker of
undesirable change in the antitumor immune response. The current
data showed that several injections of cancer vaccine were safe,
but may cause an allergic reaction that is undesirable for creation
of cancer immunity due to the similarity to conditions created
during hyposensitization therapy for allergies. In past studies,
self-antigen-derived cancer vaccines elicited allergic reactions.
Moreover, the allergic reaction resolved after elimination of
specific amino acid sequences known to evoke an allergic reaction
from studies of the peptide involved (35,36). If
characteristics of the IgG4 and IgE epitopes of MAGE-A4 were
clarified, it would be possible to avoid an allergic-like reaction
by the elimination of the pertinent IgG4 and IgE epitopes from the
vaccine agent.
In conclusion, the current results suggest that
clinicians should be aware that frequent vaccine administration may
induce a Th2 cytokine environment, and that there is a possibility
that the IgG subclass and IgE antibody responses are useful as
surrogate markers for an undesirable change in antitumor immunity,
providing an indication to discontinue vaccine administration.
Monitoring the time-dependent transitions of the IgG subclass and
IgE levels will be important during cancer vaccination therapy. It
may be necessary to reconsider protocols requiring frequent
vaccinations at relatively short intervals. Patient sera from past
cancer vaccine trials will aid in precisely addressing this
possibility and also in clarifying the precise immunological
mechanisms of the Th2 transition of the immune response induced by
cancer vaccination.
Acknowledgements
The authors would like to thank Dr. Masaki Miyamoto
(Department of Gastroenterological Surgery II, Division of Surgery,
Hokkaido University Graduate School of Medicine) for great
mentorship and contribution as a leader of the research group. The
authors would also like to thank the Department of Cancer Vaccine
and Immuno-Gene Therapy, Mie University, for providing the
CHP-MAGE-A4 vaccine and MAGE-A4-specific antibody.
References
|
1
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itoh K, Yamada A, Mine T and Noguchi M:
Recent advances in cancer vaccines: An overview. Jpn J Clin Oncol.
39:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mine T, Sato Y, Noguchi M, Sasatomi T,
Gouhara R, Tsuda N, Tanaka S, Shomura H, Katagiri K, Rikimaru T, et
al: Humoral responses to peptides correlate with overall survival
in advanced cancer patients vaccinated with peptides based on
pre-existing, peptide-specific cellular responses. Clin Cancer Res.
10:929–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ullenhag GJ, Frödin JE, Strigård K,
Mellstedt H and Magnusson CG: Induction of IgG subclass responses
in colorectal carcinoma patients vaccinated with recombinant
carcinoembryonic antigen. Cancer Res. 62:1364–1369. 2002.PubMed/NCBI
|
|
5
|
Aoki M, Ueda S, Nishikawa H, Kitano S,
HIrayama M, Ikeda H, Toyoda H, Tanaka K, Kanai M, Takabayashi A, et
al: Antibody responses against NY-ESO-1 and HER2 antigens in
patients vaccinated with combinations of cholesteryl pullulan
(CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine. 27:6854–6861.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kageyama S, Kitano S, Hirayama M, Nagata
Y, Imai H, Shiraishi T, Akiyoshi K, Scott AM, Murphy R, Hoffman EW,
et al: Humoral immune responses in patients vaccinated with 1–146
HER2 protein complexed with cholesteryl pullulan nanogel. Cancer
Sci. 99:601–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karagiannis P, Gilbert AE, Josephs DH, Ali
N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, et
al: IgG4 subclass antibodies impair antitumor immunity in melanoma.
J Clin Invest. 123:1457–1474. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Plaen E, Naerhuyzen B, De Smet C,
Szikora JP and Boon T: Alternative promoters of gene MAGE4a.
Genomics. 40:305–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immuno Rev. 188:22–32. 2002. View Article : Google Scholar
|
|
10
|
Resnick MB, Sabo E, Kondratev S, Kerner H,
Spagnoli GC and Yakirevich E: Cancer-testis antigen expression in
uterine malignancies with an emphasis on carcinosarcomas and
papillary serous carcinomas. Int J Cancer. 101:190–195. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yakirevich E, Sabo E, Lavie O, Mazareb S,
Spagnoli GC and Resnick MB: Expression of the MAGE-A4 and NY-ESO-1
cancer-testis antigens in serous ovarian neoplasms. Clin Can Res.
9:6453–6460. 2003.
|
|
12
|
Tajima K, Obata Y, Tamaki H, Tamaki H,
Yoshida M, Chen YT, Scanlan MJ, Old LJ, Kuwano H, Takahashi T, et
al: Expression of cancer/testis (CT) antigens in lung cancer. Lung
Cancer. 42:23–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad ML, Jungbluth AA, Petal SG, Iversen
K, Hoshaw-Woodard S and Busam KJ: Expression and significance of
cancer/testis antigens in primary mucosal melanoma of the head and
neck. Head Neck. 26:1053–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Yuan YH, Han Y, Liu YX, Yan L, Wang
Y and Gu J: Expression profile of cancer-testis genes in 121 human
colorectal cancer tissue and adjacent normal tissue. Clin Can Res.
11:1809–1814. 2005. View Article : Google Scholar
|
|
15
|
Gure AO, Chua R, Williamson B, Gonen M,
Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ and
Altorki NK: Cancer-testis genes are coordinately expressed and are
markers of poor outcome in non-small cell lung cancer. Clin Can
Res. 11:8055–8062. 2005. View Article : Google Scholar
|
|
16
|
Peng JR, Chen HS, Mou DC, Cao J, Cong X,
Qin LL, Wei L, Leng XS, Wang Y and Chen WF: Expression of
cancer/testis (CT) antigens in Chinese hepatocellular carcinoma and
its correlation with clinical parameters. Cancer Lett. 219:223–232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barrow C, Browning J, MacGregor D, Davis
ID, Sturrock S, Jungbluth AA and Cebon J: Tumor antigen expression
in melanoma varies according to antigen and stage. Clin Can Res.
12:764–771. 2006. View Article : Google Scholar
|
|
18
|
Akcakanat A, Kanda T, Tanabe T, Komukai S,
Yajima K, Nakagawa S, Ohashi M and Hatakeyama K: Heterogeneous
expression of GAGE, NY-ESO-1, MAGE-A and SSX proteins in esophageal
cancer: Implications for immunotherapy. Int J Cancer. 118:123–128.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kageyama S, Wada H, Muro K, Niwa Y, Ueda
S, Miyata H, Takiguchi S, Sugino SH, Miyahara Y, Ikeda H, et al:
Dose-dependent effects of NY-ESO-1 protein vaccine complexed with
cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and
survival benefits of esophageal cancer patients. J Transl Med.
11:2462013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu XG, Schmitt M, Hiasa A, Nagata Y, Ikeda
H, Sasaki Y, Akiyoshi K, Sunamoto J, Nakamura H, Kuribayashi K and
Shiku H: A novel hydrophobized polysaccharide/oncoprotein complex
vaccine induces in vitro and in vivo cellular and
humoral immune responses against HER2-expressing murine sarcomas.
Cancer Res. 58:3385–3390. 1998.PubMed/NCBI
|
|
21
|
Okamoto M, Furuichi S, Nishioka Y,
Oshikawa T, Tano T, Ahmed SU, Takeda K, Akira S, Ryoma Y, Moriya Y,
et al: Expression of toll-like receptor 4 on dendritic cells is
significant for anticancer effect of dendritic cell-based
immunotherapy in combination with an active component of OK-432, a
streptococcal preparation. Cancer Res. 64:5461–5470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakahara S, Tsunoda T, Baba T, Asabe S and
Tahara H: Dendritic cells stimulated with a bacterial product,
OK-432, efficiently induce cytotoxic T lymphocytes specific to
tumor rejection peptide. Cancer Res. 63:4112–4118. 2003.PubMed/NCBI
|
|
23
|
No authors listed, . Japanese translation
of common terminology criteria for adverse events (CTCAE), and
instructions and guidelines. Int J Clin Oncol. 9:(Suppl 3). 1–82.
2004.(In Japanese).
|
|
24
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nigro EA, Brini AT, Soprana E, Ambrosi A,
Dombrowicz D, Siccardi AG and Vangelista L: Antitumor IgE
adjuvanticity: Key role of Fc epsilon RI. J Immunol. 183:4530–4536.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reali E, Greiner JW, Corti A, Gould HJ,
Bottazzoli F, Paganelli G, Schlom J and Siccardi AG: IgEs targeted
on tumor cells: Therapeutic activity and potential in the design of
tumor vaccines. Cancer Res. 61:5517–5522. 2001.PubMed/NCBI
|
|
28
|
Yoshida K, Noguchi M, Mine T, Komatu N,
Yutani S, Ueno T, Yanagimoto H, Kawano K, Itoh K and Yamada A:
Characteristics of severe adverse events after peptide vaccination
for advanced cancer patients: Analysis of 500 cases. Oncol Rep.
25:57–62. 2011.PubMed/NCBI
|
|
29
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
30
|
Romagnani S: Th1 and Th2 in human
diseases. Clin Immunol Immunopathol. 80:225–235. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaw DR, Khazaeli MB and LoBuglio AF:
Mouse/human chimeric antibodies to a tumor-associated antigen:
Biologic activity of the four human IgG subclasses. J Natl Cancer
Inst. 80:1553–1559. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jutel M and Akdis CA: Immunological
mechanisms of allergen-specific immunotherapy. Allergy. 66:725–732.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aalberse RC, Stapel SO, Schuurman J and
Rispens T: Immunoglobulin G4: An odd antibody. Clin Exp Allergy.
39:469–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riemer AB, Untersmayr E, Knittelfelder R,
Duschl A, Pehamberger H, Zielinski CC, Scheiner O and
Jensen-Jarolim E: Active induction of tumor-specific IgE antibodies
by oral mimotope vaccination. Cancer Res. 67:3406–3411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada A, Yano H, Takao Y, Ono T,
Mastumoto T and Itoh K: Nonmutated self-antigen-derived cancer
vaccine peptides elicit an IgE-independent but mast cell-dependent
immediate-type skin reaction without systemic anaphylaxis. J
Immunol. 176:857–863. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohkouchi S, Yamada A, Imai N, Mine T,
Harada K, Shichijo S, Maeda Y, Saijo Y, Nukiwa T and Itoh K:
Non-mutated tumor-rejection antigen peptides elicit type-I allergy
in the majority of healthy individuals. Tissue Antigens.
59:259–272. 2002. View Article : Google Scholar : PubMed/NCBI
|